Play all audios:
ABSTRACT Inflammatory breast cancer (IBC) is the most aggressive form of breast cancer. Although it is a rare subtype, IBC is responsible for roughly 10% of breast cancer deaths. In order to
obtain a better understanding of the genomic landscape and intratumor heterogeneity (ITH) in IBC, we conducted whole-exome sequencing of 16 tissue samples (12 tumor and four normal samples)
from six hormone-receptor-positive IBC patients, analyzed somatic mutations and copy number aberrations, and inferred subclonal structures to demonstrate ITH. Our results showed that
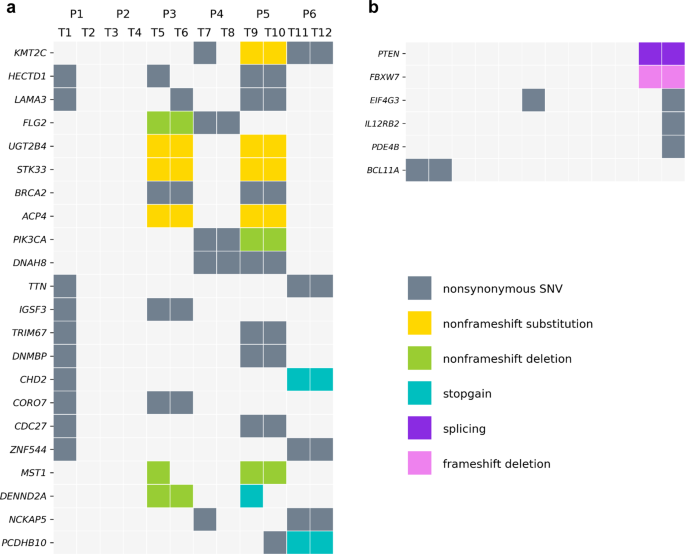
_KMT2C_ was the most frequently mutated gene (42%, 5/12 samples), followed by _HECTD1_, _LAMA3_, _FLG2_, _UGT2B4_, _STK33_, _BRCA2_, _ACP4_, _PIK3CA_, and _DNAH8_ (all nine genes tied at 33%
frequency, 4/12 samples). Our data indicated that _PTEN_ and _FBXW7_ mutations may be considered driver gene mutations for IBC. We identified various subclonal structures and different
levels of ITH between IBC patients, and mutations in the genes _EIF4G3_, _IL12RB2_, and _PDE4B_ may potentially generate ITH in IBC. SIMILAR CONTENT BEING VIEWED BY OTHERS SOMATIC MUTATIONAL
LANDSCAPE ACROSS INDIAN BREAST CANCER CASES BY WHOLE EXOME SEQUENCING Article Open access 12 August 2024 SOMATIC GENETIC ABERRATIONS IN BENIGN BREAST DISEASE AND THE RISK OF SUBSEQUENT
BREAST CANCER Article Open access 12 June 2020 INTRATUMOR GENETIC HETEROGENEITY AND CLONAL EVOLUTION TO DECODE ENDOMETRIAL CANCER PROGRESSION Article Open access 10 February 2022
INTRODUCTION Inflammatory breast cancer (IBC) is an aggressive form of breast cancer defined by the rapid onset of inflammatory signs (such as erythema, edema, warmth, and induration)
involving more than one-third of the breast1,2,3. IBC accounts for 1–6% of breast cancer cases2,4,5 yet causes roughly 10% of breast cancer deaths6,7. The prognosis in patients with IBC is
worse than in non-IBC, with the 3‐year survival rate for IBC patients far lower (around 40%) than patients with other types of breast carcinoma (around 85%)5,8. Although treatment approaches
based on hormone-receptor (HR) or HER2 status are available, there are no treatments that are specifically recommended for tumors with an IBC phenotype. The scarcity of data from IBC
patients and the poor understanding at the molecular level has hindered the development of specific therapeutic interventions. In order to develop potential IBC-specific targeted therapies,
obtaining more genomic information is crucial. Intratumor heterogeneity (ITH) arises from heritable and stochastic genetic and epigenetic changes, as well as environmental variations within
the tumor9. Since tumors with ITH have subclones with distinct mutations that may relate to cancer-specific phenotypes, ITH is intricately related to cancer progression, resistance to
therapy, and recurrences10. It is clear that a better understanding of ITH is very important to the development of genome-informed precision medicine11. The rapidly evolving technology of
next-generation sequencing (NGS) has made it possible to analyze genomic characteristics of tumor samples at an unprecedented speed. Since 2015, eight NGS-based studies on IBC tumors have
been published. Among them, six out of eight used targeted sequencing12,13,14,15,16,17,18, and two conducted whole-exome sequencing (WES)19,20. These studies reported frequently mutated
genes in IBC, such as _TP53_ (43–75%), _PIK3CA_ (13–42%), _BRCA2_ (13–26%), _ARID1A_ (10–21%), _RB1_ (11–16%), and _PTEN_ (11–15%)12,13,14,15,16,17,18. Frequent _HER3_ hotspot mutations were
also found in IBC tumors and cell line studies confirmed a role for mutant _HER3_ in IBC cell proliferation15. Frequent genomic alterations in the PI3K/AKT/mTOR pathway have been seen15,
and somatic activation of this pathway (i.e., _PIK3CA_ activating mutation or gain14, _ERBB2_ activating mutation, _PTEN_ deletion, _AKT1_ activating mutation) was significantly associated
with shorter progression-free survival (PFS) in trastuzumab-naïve HER2-positive IBC patients19. However, most of these studies were based on targeted sequencing panels, and none of them
provided information for intratumor subclonal structures or evaluated ITH. In the current study, we performed whole-exome sequencing in 16 tissue samples (12 tumor and 4 normal samples) from
six IBC patients to obtain a comprehensive understanding of the IBC genomic landscape. Based on the mutation calls and somatic copy number alterations, we characterized ITH and subclonal
structures, identified primary and secondary driver genes for the tumor and subclone formation, which could shed light on potential new treatment strategies for IBC. RESULTS PATIENT AND
SAMPLE DESCRIPTION Clinical and pathological information of the six IBC patients (P1–P6) are provided in Supplementary Table 1. The median age at sample collection time was 56 years (ranging
from 36 to 72 years). All six patients had HR+ tumors, with 5/6 (83.3%) patients having estrogen-receptor-positive (ER+) tumors, and the other had a progesterone-receptor positive (PR+)
tumor. By only considering HR+ IBC tumors, our study eliminated additional confounding introduced by differences in HR subtypes seen in previous studies. Details of the tumor and normal
tissue samples obtained from the six IBC patients are found in Supplementary Table 2. The samples from P2 were obtained from an incisional biopsy, which limited the volume of tissue
obtained, and these samples were subsequently found to be insufficient for conducting subclone identification. SEQUENCING QUALITY VALIDATION We achieved a mean sequencing depth of ~170×
(ranging from 133 to 210×, Supplementary Table 3), with mapping rates exceeding 99% in all 16 samples. After stringent filtering criteria (see Methods), we obtained a total of 1477 somatic
mutations. We called 293, 15, 261, 120, 495, and 293 somatic mutations, respectively, in patients P1–P6 (Supplementary Data 1). Four of the six patients (P1, P2, P4, and P6) had matched
normal samples, allowing us to validate the stringency of our mutation calling pipeline (see Methods). We identified artifactual mutations in one, six, one, and four instances, respectively,
in patients P1, P2, P4, and P6. Artifactual mutations in normal samples also had much lower allele frequencies (AFs) and tended to be obtained at lower depths compared to tumor mutation
calls, which indicated that FFPE-induced artifacts had negligible effects to the data presented in our study (Supplementary Fig. 1). SOMATIC MUTATION IDENTIFICATION We used a somatic
mutation classification system as previously described21. Five of six patients exhibited mutational signatures characterized predominantly by C > T transitions, with the sixth patient P6
showing a mix of C > G and C > T transitions (Supplementary Fig. 2). These results were consistent with previous reports for breast cancer, which have also found C > T transitions
to constitute the majority of somatic mutations21,22. In total, we found 787 mutated genes from the 12 tumor samples in six patients. In these samples, _KMT2C_ was the most frequently
mutated gene (5/12 samples, 42%). Nine mutated genes were found in four samples (33%, including _HECTD1_, _LAMA3_, _FLG2_, _UGT2B4_, _STK33_, _BRCA2_, _ACP4_, _PIK3CA_, and _DNAH8_), and 12
genes were mutated in three different samples (25%, including _TTN_, _IGSF3_, _TRIM67_, _DNMBP_, _CHD2_, _CORO7_, _CDC27_, _ZNF544_, _MST1_, _DENND2A_, _NCKAP5_, and _PCDHB10_). Figure 1a
shows the 22 most frequently mutated genes. In addition, mutations in 244 genes were found in two tumor samples, with the remaining gene mutations (in 521 genes) private to single tumor
samples. We also analyzed the gene mutations at the patient-level. _KMT2C_, _HECTD1_, and _LAMA3_ were the most frequently mutated genes as they were shared by three of six patients (50%).
Histone methyltransferase _KMT2C_ is a tumor suppressor gene reported to be a driver gene for breast cancer23,24. There were 57 mutated genes identified within two patients (2/6, 33%), and
the rest of the mutated genes were not common to multiple patients. All counted mutations were nonsynonymous (i.e., frameshift/non-frameshift indel, stop-gain/stop-loss, splicing, or
nonsynonymous SNV). COPY NUMBER ABERRATION (CNA) INFERENCE We obtained ~25,000 germline variants in each patient with matched normal samples (P1, P2, P4, and P6). We used TITAN, a
probabilistic model that simultaneously infers CNA and loss of heterozygosity (LOH) segments from read depth and digital allele ratios at germline heterozygous SNP loci across the exome from
tumor WES data25. Figure 2 shows the profiles of CNAs for the four patients with matched normal samples. We observed that patient P2 had a relatively low tumor cell fraction. Patient P6 had
the best sample quality and showed extensive LOH. SUBCLONE IDENTIFICATION Using CNA information, we conducted PyClone analysis to estimate cancer cell fractions (CCFs) of all mutations and
then assigned each mutation to different subclones (see Methods). For each patient, we obtained the subclone CCF density (represented as violin plots) and plotted CCFs in one tumor sample
against the other tumor sample (as a scatter plot) (Fig. 3). Major subclones from the density plots are labelled in the same color in the scatter plot. For patient P6 (Fig. 3a, b), we
observed six distinct subclones with different cluster CCFs. Subclone 4, 5, and 11 all had very low subclone CCFs in one of the two samples (but high CCFs in the other sample), indicating
clear ITH. Subclone 9 had cluster CCFs of greater than 0.7 in both samples, suggesting a high possibility of this subclone containing driver genes. This subclone also contained mutations in
_PTEN_ and _FBXW7_, both tumor suppressor genes previously reported26,27 as driver genes for breast cancer. Subclone 11 contained _EIF4G3_, _IL12RB2_, and _PDE4B_ mutations, and all three
mutations had zero allele frequencies in the tumor sample P6_T11, indicating the possibility of secondary driver genes for this subclone. We used Integrative Genomics Viewer (IGV) to check
and confirm that the high CCFs of these genes were not caused by duplication. _EIF4G3_, _IL12RB2_, and _PDE4B_ genes are all located in chromosome 1. Figure 4 shows the phylogenetic tree for
P6. In order to further explore the relationships between different subclones in patient P6, we constructed the subclonal architecture based on cluster CCFs (see Methods). Supplementary
Figure 3 depicts the deduced linear and/or branching relationships of subclones in P6. For example, in architecture c (one of the four possible subclonal architectures of sample T11),
subclone 9 represented the subclonal trunk mutations, with subclone 3, 1, and 5 all derived from it (i.e., they were all linear in relationship to subclone 9). Subclone 5 was derived from
subclone 3, but subclone 3 and 1 occupied different subpopulations of cells (i.e., subclones 3 and 1 were diverging branches). Three major subclones were found in patient P4 (Fig. 3c, d),
and their subclone CCFs had little differences between the two samples, indicating high similarity between tumor samples from P4. Six major subclones were identified in patient P1 (Fig. 3e,
f). Most of the mutations had CCFs below 0.2 (subclone 0 and 1), while subclone 2 and 4 reflected ITH. Also, a mutation of the driver gene _BCL11A_28 was found in subclone 3. Figure 1b shows
these important functional genes. DISCUSSION To obtain a better understanding of the genomic alterations and ITH in inflammatory breast cancer, we applied WES to matched normal and tumor
samples of IBC patients. Herein, we report the frequently mutated genes, varying levels of ITH, subclonal structures and possible driver genes in different patients. Our study is one of the
few attempts using WES to analyze IBC19 and investigate ITH with subclonal structures in IBC. Previous studies have reported the proportion of positive receptors in IBC tumors. The
prevalence of overexpressed or amplified HER2 was about 40% (compared with 25% in non-IBCs), and the prevalence of HR positivity is lower, about 30% (compared with 60–80% in non-IBCs)29. The
HR+ percentage of IBC tumors in recent NGS-based studies was about 39% (ranging from 29 to 54%)12,13,14,15,16,17,18. However, since HR+ IBC patients tend to have worse clinical outcomes
than HR+ non-IBC patients29, this study sought to explore the genomic landscape of HR+ IBC tumors. This strategy also prevents potential confounding effects from HR subtypes, in contrast to
previous IBC studies. We found a frequently mutated gene _KMT2C_, which has been reported as frequently altered in other IBC30 (15% mutation rate) and non-IBC16 cases (11% mutation rate). As
a reported driver gene, _KMT2C_ had the highest genetic mutation rate among histone methyltransferases in breast cancer and was most frequently mutated in Luminal A breast cancer31.
Previous works demonstrated that _KMT2C_ mediated ER-independent growth of HR+ breast cancer cell lines24,32 and _KMT2C_ loss promoted hormone-independent ER+ breast cancer cell
proliferation32. Thus, the HR positivity of our samples could be an important factor for the enrichment of _KMT2C_ mutation found in our study (a 42% mutation rate). The deletion of _KMT2C_
is significantly associated with shorter PFS32, and amplification/gain of this gene was significantly associated with longer survival, compared with patients who had no change in copy
number32. In patient P6, _PTEN_ and _FBXW7_ mutations were detected at high CCFs, thus they may be driver mutations for this patient. The lipid phosphatase _PTEN_ is a major negative
regulator of the PI3K/Akt/mammalian target of rapamycin (mTOR) pathway26. PI3K inhibitors, such as alpelisib, have been approved for treatment of _PIK3CA_-mutant ER+ breast cancers33.
Everolimus (a rapamycin analog and an inhibitor of the mTOR pathway) has also been approved for ER+ breast cancer34. _FBXW7_ is a critical tumor suppressor, which controls the
proteasome-mediated degradation of mTOR27. Human breast cancer cell lines harboring deletions or mutations in _FBXW7_ are particularly sensitive to rapamycin treatment27. Finally, breast
cancer patients with lower _FBXW7_ mRNA expression had poorer survival35. Also in patient P6, _EIF4G3_, _IL12RB2,_ and _PDE4B_ mutations only occurred in sample T12 and formed a subclone
with relatively high CCF (>0.6). This was an interesting finding as it indicated that this subclone was newly generated only in a specific area of the tumor. These genes seemed to have a
strong positive selection in specific environment and conditions, as well as a potential to drive secondary tumor progression. Phosphodiesterase type IV (PDE4) degrades the intracellular
second messenger cyclic AMP in many cell types. As PDE4s regulate many active processes such as immune cell proliferation and inflammatory mediators releasing, PDE4 inhibitors are potent
inhibitors of inflammation, and they have been approved for the treatment of many inflammatory diseases including asthma, arthritis and chronic obstructive pulmonary disease36,37. Previous
works showed that _PDE4B_ is a potential therapeutic target as well as prognostic molecular marker in colorectal cancer38,39. Further study is needed to investigate if _PDE4B_ could also be
a therapeutic target or marker for IBC patients. _IL12RB2_, which encodes for one chain of the interleukin-12 (IL-12) receptor, is involved in several inflammatory diseases40. IL-12 is a
heterodimeric proinflammatory cytokine. Overexpression of IL-12 can cause persistent inflammation41, thus contributing to the aggressive nature of IBC29. Genetic polymorphisms in _IL12RB2_
are associated with increased risk of chronic inflammatory disease42. Also, hyperactivation of the IL-6 pathway is frequently observed in IBC, and associated with poor prognosis29. In our
samples, we observed a high percentage of tumor cells harboring _IL12RB2_ mutations (i.e., high CCF), though it remains unclear whether the _IL12RB2_ mutations play any functional roles in
influencing the inflammatory pathways. The presence of ITH in patients with IBC or other cancers indicates that an individual tissue biopsy may be insufficient to evaluate the genomic
profile of an entire tumor, which could introduce bias in the selection of personalized therapies. For example, the gene coding for the estrogen receptor, _ESR1_, is often found to be
mutated in metastatic ER+ breast cancers previously treated with estrogen therapy43. The high _ESR1_ mutational prevalence in previously treated tumors, juxtaposed with the rarity of _ESR1_
mutations in treatment-naïve primary tumors, suggest the development of resistance subclones during treatment, and thus has raised much interest in understanding ITH43. Furthermore, several
landmarks of disease progression in breast cancer, such as resistance to chemotherapy and metastases, arose within detectable subclones in the primary tumor44. These findings highlight the
importance of subclonal structure analysis. In this study, conducting WES on multiple samples from each IBC tumor allowed us to investigate many more genes than using targeted sequencing,
and thus we were able to identify specific subclonal structures and ITH. However, the main limitation of our study is the small sample size. Given the rarity of IBC, many genomic studies on
this disease subtype face challenges in acquiring enough samples. In this study, the tumor tissues without matched normal specimens further reduced the number of available samples. Moreover,
although we demonstrated extensive ITH in HR+ IBC, the limited sample size prevented us from reaching more definitive conclusions on the role of clonal expansion in IBC. One interesting
aspect is the genomic level comparison between IBCs and non-IBCs, which remains underexplored. A previous study using immunohistochemistry suggested overexpression of E-cadherin to be a key
difference45, but large-scale nonbiased approaches are also needed. Further research comparing IBC and non-IBC samples with matched clinical characteristics may uncover the genomic origin of
IBC. To definitively answer the effects of clonal expansion on the inflammatory phenotype of IBC, non-IBC patients who have inflammatory recurrence during follow-up could be enrolled, to
compare primary non-IBC tumor tissues with tumor tissue at recurrence. Another limitation of this study is the lack of information regarding treatments prior to sample collection for some
patients. Patient P6 received chemotherapy before sample collection, which could possibly influence the genomic signature and result in significant ITH. In conclusion, we conducted WES on
multiple samples of human IBC tumors with matched normal samples, and our results revealed the high frequency and diversity of somatic mutations, subclonal structures, differing levels of
ITH, and potential driver genes in IBC patients. These findings encourage future studies and clinical trials for developing targeted therapies that could benefit IBC patients. METHODS
PATIENT SAMPLES Sixteen samples were collected from six IBC patients, including 12 tumors (two from each patient) and 4 matched normal samples (in four out of six patients). The six patients
P1–P6 were enrolled between 1993 and 2012. This study was based on detecting archived tissue samples and reviewing archived medical/pathologic reports. Patient consent was waived by the
Institutional Review Board of the Office of Human Research at Thomas Jefferson University under an approved protocol. IDENTIFICATION OF MOLECULAR SUBTYPE Immunohistochemical (IHC) staining
of paraffin-embedded tissue sections with monoclonal antibodies were used to determine patients’ ER and PR status as part of a routine diagnostic procedure. HR status was positive if the
patients were either ER or PR positive. HER2 status was also determined by IHC staining following standard guidelines at the time of diagnosis. The FDA approved DAKO guidelines were used for
scoring patient P5 (2004)46,47. The 2007 ASCO/CAP guideline48 was used for patient P6 (2012). There were no standard guidelines before the FDA approval, therefore we matched the old scoring
systems49,50 with modern standards for those early patients (P1–P4). The percentage of ER- and PR-positive cells and HER2 status scores were obtained from pathological reports and shown in
Supplementary Table 1. DNA EXTRACTION AND WES For all tumor samples, IBC diagnosis was confirmed by two independent pathologists and the tumor regions were macro-dissected under a
microscope. For each sample, we extracted total DNA from approximately ten 14-um sections of formalin-fixed, paraffin-embedded (FFPE) blocks (tissue surface area, 100–150 mm2) using the
AllPrep DNA/RNA FFPE kit (Qiagen), with a protocol we empirically optimized. The AllPrep kit is well-validated on long-term preserved FFPE samples51,52. Before library construction, all DNA
samples were assessed using a NanoDrop spectrophotometer for OD 260/280 and OD 260/230, a Qubit fluorometer for concentration, and a 2100 Bioanalyzer (Agilent) for peak analysis. We then
performed WES (using SeqCap EZ Exome 2.0 kit from Nimblegen for library construction) on Illumina HiSeq 2000 paired-end sequencing system. The human genome GRCh37 was used as a reference and
the raw reads were aligned using BWA-0.7.1753. The BAM files were generated through samtools-1.9, then further processed through duplicates marking, Base Quality Score Recalibration (BQSR),
gVCF generating, joint genotyping and Variant Quality Score Recalibration (VQSR) by GATK-4.1.0.054. The sequencing quality assessment was evaluated by QPLOT55. MUTATION CALLING AND QUALITY
CONTROL Based on the best practice procedures for sequencing alignment and quality control56, somatic mutations were called by MuTect2 using genomic references from the Broad Institute57. We
created a Panel of Normals (PoN) by aggregating all the normal samples so that we could remove common germline variants as well as commonly noisy sites (e.g., mapping artifacts or other
somewhat random but systematic artifacts of sequencing). This PoN also served as the normal sample for P3 and P5 since they did not have matched normal samples for somatic calling. We
applied the default filter to conservatively select somatic calls with confidence. Final mutation calls were selected through a stringent filtering process and functionally annotated by
ANNOVAR58. We applied the following filtering criteria for somatic mutation calling: (1) read depth > 25; (2) mutant AF > 0.05 in tumor samples; (3) corresponding allele frequency
<0.01 in matched normal samples (if present); (4) mutations listed in 1000 Genomes Project59 or Exome Sequencing Project60 removed. The following filtering criteria were applied for
germline variant calling: (1) read depth ≥ 50; (2) genotype quality score ≥ 30; (3) allele fractions ≥0.3 and ≤0.7; (4) multiple-allele variants removed; (5) variant quality score
recalibration (VQSR) ≤ 97.00; (6) variants in segmental duplication removed61. We validated the quality of our somatic mutation calls using methods that we have previously established61.
Briefly, when running Mutect2 in patients with matched normal samples (P1, P2, P4, and P6), we performed the same pipeline and filtering criteria but switched the normal and tumor samples.
The mutation calls that passed the criteria are declared as artifactual mutations. If there were major artifacts in FFPE samples, we would be able to call artifactual mutations in matched
normal samples since they were also FFPE samples. COPY NUMBER ABERRATION (CNA) INFERENCE CNAs were inferred using TITAN-1.26.025 based on the called germline heterozygous variants
information. CNA analysis was only performed on tumor samples with matched normal. First, we used HMMcopy-0.99.062 to count the number of reads in nonoverlapping windows of 10 kb directly
from BAM files. Then we obtained corrected read depth using mappability and GC content. CNAs were inferred by the ratios of tumor/normal, mutant/reference depths at the germline heterozygous
variants sites. We set the maximum copy number to 5 and the number of clonal clusters to 2 in the TITAN settings. SUBCLONE INFERENCE Finally, we inferred subclones using PyClone-0.13.163
based on the obtained CNA information. PyClone is a hierarchical Bayes statistical model that uses the measurement of allelic prevalence in deep sequencing data to estimate the proportion of
tumor cells harboring a mutation (referred to herein as ‘cancer cell fraction’ (CCF))63. We first computed the CCF for each mutation, and then performed hierarchical clustering to assign
each mutation to one cluster (subclone). In the PyClone settings, the number of iterations was set to 50,000 and the density model was chosen to be Beta Binomial emission. In order to obtain
a better result, we optimized the input parameters and custom-built the yaml mutations files. CONSTRUCTION OF SUBCLONAL ARCHITECTURE We deduced linear and/or branching evolutionary
relationships of all subclones in patient P6 based on their cluster CCFs using established methods61. A linear relationship between two subclones would indicate that the one with smaller CCF
was derived from the one with larger CCF, suggesting that the mutations in the derived subclone occurred later in the same ancestral cells, which already carried the mutations in the larger
subclone. A branching relationship between two subclones would indicate that the mutations in each of the subclones occurred in different ancestral cells and the subclones occupied
different portions of the tumor cells. REPORTING SUMMARY Further information on research design is available in the Nature Research Reporting Summary linked to this article. DATA
AVAILABILITY The data generated and analyzed during this study are described in the following data record: https://doi.org/10.6084/m9.figshare.1453825264. Release of full genetic sequencing
data was not included in the IRB protocol. Thus, only sequencing data related to this paper have been released, and these data have been deposited in NCBI Sequence Read Archive (SRA) with
the accession code https://identifiers.org/ncbi/bioproject:PRJNA71335965. Additional files underlying the figures and supplementary figures are available as part of the figshare data record.
CODE AVAILABILITY The codes for Fig. 1 and Fig. 3, which were written in Python and R languages are available upon request. REFERENCES * Ellis, D. L. & Teitelbaum, S. L. Inflammatory
carcinoma of the breast. A pathologic definition. _Cancer_ 33, 1045–1047 (1974). Article CAS PubMed Google Scholar * Jaiyesimi, I. A., Buzdar, A. U. & Hortobagyi, G. Inflammatory
breast cancer: a review. _J. Clin. Oncol._ 10, 1014–1024 (1992). Article CAS PubMed Google Scholar * Robertson, F. M. et al. Inflammatory breast cancer: the disease, the biology, the
treatment. _CA Cancer J. Clin._ 60, 351–375 (2010). Article PubMed Google Scholar * Anderson, W. F., Schairer, C., Chen, B. E., Hance, K. W. & Levine, P. H. Epidemiology of
inflammatory breast cancer (IBC) 1. _Breast Dis._ 22, 9–23 (2006). Article Google Scholar * Levine, P. H., Steinhorn, S. C., Ries, L. G. & Aron, J. L. Inflammatory breast cancer: the
experience of the surveillance, epidemiology, and end results (SEER) program. _J. Natl Cancer Inst._ 74, 291–297 (1985). CAS PubMed Google Scholar * Hance, K. W., Anderson, W. F., Devesa,
S. S., Young, H. A. & Levine, P. H. Trends in inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program at the National Cancer
Institute. _J. Natl Cancer Inst._ 97, 966–975 (2005). Article PubMed Google Scholar * Brewer, T. et al. Statin use in primary inflammatory breast cancer: a cohort study. _Br. J. Cancer_
109, 318–324 (2013). Article CAS PubMed PubMed Central Google Scholar * Chang, S., Parker, S. L., Pham, T., Buzdar, A. U. & Hursting, S. D. Inflammatory breast carcinoma incidence
and survival: The Surveillance, Epidemiology, and End Results program of the National Cancer Institute, 1975‐1992. _Cancer Interdiscip. Int. J. Am. Cancer. Soc._ 82, 2366–2372 (1998). CAS
Google Scholar * Tabassum, D. P. & Polyak, K. Tumorigenesis: it takes a village. _Nat. Rev. Cancer_ 15, 473–483 (2015). Article CAS PubMed Google Scholar * Stanta, G. & Bonin,
S. Overview on clinical relevance of intra-tumor heterogeneity. _Front. Med._ 5, 85 (2018). Article Google Scholar * Neelakantan, D., Drasin, D. J. & Ford, H. L. Intratumoral
heterogeneity: clonal cooperation in epithelial-to-mesenchymal transition and metastasis. _Cell Adhes. Migr._ 9, 265–276 (2015). Article CAS Google Scholar * Matsuda, N. et al.
Identification of frequent somatic mutations in inflammatory breast cancer. _Breast Cancer Res. Treat._ 163, 263–272 (2017). Article CAS PubMed Google Scholar * Ross, J. S. et al.
Comprehensive genomic profiling of inflammatory breast cancer cases reveals a high frequency of clinically relevant genomic alterations. _Breast Cancer Res. Treat._ 154, 155–162 (2015).
Article CAS PubMed Google Scholar * Liang, X. et al. Targeted next-generation sequencing identifies clinically relevant somatic mutations in a large cohort of inflammatory breast cancer.
_Breast Cancer Res._ 20, 1–12 (2018). Article CAS Google Scholar * Hamm, C. A. et al. Genomic and immunological tumor profiling identifies targetable pathways and extensive CD8+/PDL1+
immune infiltration in inflammatory breast cancer tumors. _Mol. Cancer Ther._ 15, 1746–1756 (2016). Article CAS PubMed Google Scholar * Bertucci, F. et al. NOTCH and DNA repair pathways
are more frequently targeted by genomic alterations in inflammatory than in non‐inflammatory breast cancers. _Mol. Oncol._ 14, 504–519 (2020). Article CAS PubMed PubMed Central Google
Scholar * Winn, J. S. et al. Genetic variants detected using cell-free DNA from blood and tumor samples in patients with inflammatory breast cancer. _Int. J. Mol. Sci._ 21, 1290 (2020).
Article CAS PubMed Central Google Scholar * Bingham, C. et al. Mutational studies on single circulating tumor cells isolated from the blood of inflammatory breast cancer patients.
_Breast Cancer Res. Treat._ 163, 219–230 (2017). Article CAS PubMed PubMed Central Google Scholar * Goh, G. et al. Clonal evolutionary analysis during HER2 blockade in HER2-positive
inflammatory breast cancer: a phase II open-label clinical trial of afatinib+/-vinorelbine. _PLoS Med._ 13, e1002136 (2016). Article PubMed PubMed Central CAS Google Scholar * Doebar,
S. et al. Progression of ductal carcinoma in situ to invasive breast cancer: comparative genomic sequencing. _Virchows Arch._ 474, 247–251 (2019). Article CAS PubMed Google Scholar *
Alexandrov, L. B. et al. Signatures of mutational processes in human cancer. _Nature_ 500, 415–421 (2013). Article CAS PubMed PubMed Central Google Scholar * Nik-Zainal, S. et al.
Landscape of somatic mutations in 560 breast cancer whole-genome sequences. _Nature_ 534, 47–54 (2016). Article CAS PubMed PubMed Central Google Scholar * Pereira, B. et al. The somatic
mutation profiles of 2,433 breast cancers refine their genomic and transcriptomic landscapes. _Nat. Commun._ 7, 1–16 (2016). Google Scholar * Bertucci, F. et al. Genomic characterization
of metastatic breast cancers. _Nature_ 569, 560–564 (2019). Article CAS PubMed Google Scholar * Ha, G. et al. TITAN: inference of copy number architectures in clonal cell populations
from tumor whole-genome sequence data. _Genome Res._ 24, 1881–1893 (2014). Article CAS PubMed PubMed Central Google Scholar * Chalhoub, N. & Baker, S. J. PTEN and the PI3-kinase
pathway in cancer. _Annu. Rev. Pathol._ 4, 127–150 (2009). Article CAS PubMed PubMed Central Google Scholar * Mao, J.-H. et al. FBXW7 targets mTOR for degradation and cooperates with
PTEN in tumor suppression. _Science_ 321, 1499–1502 (2008). Article CAS PubMed PubMed Central Google Scholar * Khaled, W. T. et al. BCL11A is a triple-negative breast cancer gene with
critical functions in stem and progenitor cells. _Nat. Commun._ 6, 1–10 (2015). Article CAS Google Scholar * Lim, B., Woodward, W. A., Wang, X., Reuben, J. M. & Ueno, N. T.
Inflammatory breast cancer biology: the tumour microenvironment is key. _Nat. Rev. Cancer_ 18, 485–499 (2018). Article CAS PubMed Google Scholar * Cheasley, D. et al. Molecular
comparison of interval and screen‐detected breast cancers. _J. Pathol._ 248, 243–252 (2019). Article CAS PubMed Google Scholar * Liu, L., Kimball, S., Liu, H., Holowatyj, A. & Yang,
Z.-Q. Genetic alterations of histone lysine methyltransferases and their significance in breast cancer. _Oncotarget_ 6, 2466 (2015). Article PubMed Google Scholar * Gala, K. et al. KMT2C
mediates the estrogen dependence of breast cancer through regulation of ERα enhancer function. _Oncogene_ 37, 4692–4710 (2018). Article CAS PubMed PubMed Central Google Scholar * André,
F. et al. Alpelisib for PIK3CA-mutated, hormone receptor–positive advanced breast cancer. _N. Engl. J. Med._ 380, 1929–1940 (2019). Article PubMed Google Scholar * Hidalgo, M. &
Rowinsky, E. K. The rapamycin-sensitive signal transduction pathway as a target for cancer therapy. _Oncogene_ 19, 6680–6686 (2000). Article CAS PubMed Google Scholar * Ibusuki, M.,
Yamamoto, Y., Shinriki, S., Ando, Y. & Iwase, H. Reduced expression of ubiquitin ligase FBXW7 mRNA is associated with poor prognosis in breast cancer patients. _Cancer Sci._ 102, 439–445
(2011). Article CAS PubMed Google Scholar * Manning, C. D. et al. Suppression of human inflammatory cell function by subtype‐selective PDE4 inhibitors correlates with inhibition of
PDE4A and PDE4B. _Br. J. Pharmacol._ 128, 1393–1398 (1999). Article CAS PubMed PubMed Central Google Scholar * Lai, S. H., Zervoudakis, G., Chou, J., Gurney, M. E. & Quesnelle, K.
M. PDE4 subtypes in cancer. _Oncogene_ 39, 3791–3802 (2020). * Kim, D. U., Kwak, B. & Kim, S.-W. Phosphodiesterase 4B is an effective therapeutic target in colorectal cancer. _Biochem.
Biophys. Res. Commun._ 508, 825–831 (2019). Article CAS PubMed Google Scholar * Tsunoda, T. et al. Inhibition of phosphodiesterase-4 (PDE4) activity triggers luminal apoptosis and AKT
dephosphorylation in a 3-D colonic-crypt model. _Mol. Cancer_ 11, 1–12 (2012). Article CAS Google Scholar * Mizuki, N. et al. Genome-wide association studies identify IL23R-IL12RB2 and
IL10 as Behçet’s disease susceptibility loci. _Nat. Genet._ 42, 703–706 (2010). Article CAS PubMed Google Scholar * Gee, K., Guzzo, C., Che Mat, N. F., Ma, W. & Kumar, A. The IL-12
family of cytokines in infection, inflammation and autoimmune disorders. _Inflamm. Allergy Drug Targets_ 8, 40–52 (2009). Article CAS PubMed Google Scholar * Chognard, G. et al. The
dichotomous pattern of IL-12r and IL-23R expression elucidates the role of IL-12 and IL-23 in inflammation. _PLoS ONE_ 9, e89092 (2014). Article PubMed PubMed Central CAS Google Scholar
* Reinert, T., Saad, E. D., Barrios, C. H. & Bines, J. Clinical implications of ESR1 mutations in hormone receptor-positive advanced breast cancer. _Front. Oncol._ 7, 26 (2017).
Article PubMed PubMed Central Google Scholar * Yates, L. R. et al. Subclonal diversification of primary breast cancer revealed by multiregion sequencing. _Nat. Med._ 21, 751–759 (2015).
Article CAS PubMed PubMed Central Google Scholar * Kleer, C. G., van Golen, K. L., Braun, T. & Merajver, S. D. Persistent E-cadherin expression in inflammatory breast cancer. _Mod.
Pathol._ 14, 458–464 (2001). Article CAS PubMed Google Scholar * Varga, Z., Noske, A., Ramach, C., Padberg, B. & Moch, H. Assessment of HER2 status in breast cancer: overall
positivity rate and accuracy by fluorescence in situ hybridization and immunohistochemistry in a single institution over 12 years: a quality control study. _BMC Cancer_ 13, 615 (2013).
Article PubMed PubMed Central CAS Google Scholar * Lebeau, A. et al. Her-2/neu analysis in archival tissue samples of human breast cancer: comparison of immunohistochemistry and
fluorescence in situ hybridization. _J. Clin. Oncol._ 19, 354–363 (2001). Article CAS PubMed Google Scholar * Wolff, A. C. et al. American Society of Clinical Oncology/College of
American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. _Arch. Pathol. Lab Med._ 131, 18–43 (2007). Article CAS PubMed
Google Scholar * Slamon, D. J. et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. _Science_ 244, 707–712 (1989). Article CAS PubMed Google Scholar *
Cobleigh, M. A. et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has
progressed after chemotherapy for metastatic disease. _J. Clin. Oncol._ 17, 2639–2648 (1999). Article CAS PubMed Google Scholar * Ludyga, N. et al. Nucleic acids from long-term preserved
FFPE tissues are suitable for downstream analyses. _Virchows Arch._ 460, 131–140 (2012). Article CAS PubMed Google Scholar * Patel, P. G. et al. Reliability and performance of
commercial RNA and DNA extraction kits for FFPE tissue cores. _PLoS ONE_ 12, e0179732 (2017). Article PubMed PubMed Central CAS Google Scholar * Li, H. & Durbin, R. Fast and
accurate short read alignment with Burrows–Wheeler transform. _Bioinformatics_ 25, 1754–1760 (2009). Article CAS PubMed PubMed Central Google Scholar * McKenna, A. et al. The Genome
Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. _Genome Res._ 20, 1297–1303 (2010). Article CAS PubMed PubMed Central Google Scholar * Li, B.
et al. QPLOT: a quality assessment tool for next generation sequencing data. _BioMed Res. Int._ 2013, 865181 (2013). * Van der Auwera, G. A. et al. From FastQ data to high‐confidence variant
calls: the genome analysis toolkit best practices pipeline. _Curr. Protoc. Bioinformatics_ 43, 11.10.1–11.10.33 (2013). * Cibulskis, K. et al. Sensitive detection of somatic point mutations
in impure and heterogeneous cancer samples. _Nat. Biotechnol._ 31, 213–219 (2013). Article CAS PubMed PubMed Central Google Scholar * Wang, K., Li, M. & Hakonarson, H. ANNOVAR:
functional annotation of genetic variants from high-throughput sequencing data. _Nucleic Acids Res._ 38, e164–e164 (2010). Article PubMed PubMed Central CAS Google Scholar * Consortium,
G. P. A global reference for human genetic variation. _Nature_ 526, 68–74 (2015). Article CAS Google Scholar * Fu, W. et al. Analysis of 6,515 exomes reveals the recent origin of most
human protein-coding variants. _Nature_ 493, 216–220 (2013). Article CAS PubMed Google Scholar * Wei, Q. et al. Multiregion whole-exome sequencing of matched primary and metastatic
tumors revealed genomic heterogeneity and suggested polyclonal seeding in colorectal cancer metastasis. _Ann. Oncol._ 28, 2135–2141 (2017). Article CAS PubMed PubMed Central Google
Scholar * Lai, D. & Shah, S. HMMcopy: Copy number prediction with correction for GC and mappability bias for HTS data. https://rdrr.io/bioc/HMMcopy/ (2012). * Roth, A. et al. PyClone:
statistical inference of clonal population structure in cancer. _Nat. Methods_ 11, 396–398 (2014). Article CAS PubMed PubMed Central Google Scholar * Luo, R. et al. Metadata record for
the article: Whole-exome sequencing identifies somatic mutations and intratumor heterogeneity in inflammatory breast cancer. _figshare_ https://doi.org/10.6084/m9.figshare.14538252 (2021). *
Rui Luo. _Sequence Read Archive_ https://identifiers.org/ncbi/bioproject:PRJNA713359 (2021). Download references ACKNOWLEDGEMENTS This study was funded by The Jamie Lieberman Memorial
Endowment Fund and The Inflammatory Breast Cancer Network Foundation, and in part by the National Cancer Institute Grant (R01CA207468) and the Pennsylvania Department of Health Grant (SAP#
4100062221). Research reported in this publication utilized the Circulating Tumor Cell Core Facility at the Sidney Kimmel Cancer Center at Jefferson Health and was supported by the National
Cancer Institute of the National Institutes of Health under Award Number P30CA056036. The funding agencies were not involved in the design, conduct, analysis, or interpretation of the study.
Publication made possible in part by support from the Thomas Jefferson University Open Access Fund. AUTHOR INFORMATION Author notes * These authors contributed equally: Rui Luo, Weelic
Chong. AUTHORS AND AFFILIATIONS * Department of Medical Oncology, Sidney Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA, USA Rui Luo, Weelic Chong, Zhenchao Zhang, Chun
Wang, Zhong Ye, Maysa M. Abu-Khalaf, Daniel P. Silver, Ronald E. Myers & Hushan Yang * Department of Molecular Physiology and Biophysics, Vanderbilt University, Nashville, TN, USA Qiang
Wei & Bingshan Li * Department of Pathology, Sidney Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA, USA Robert T. Stapp & Wei Jiang * Division of Hematology
Oncology, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA Massimo Cristofanilli Authors * Rui Luo View author publications You can also search for this author inPubMed
Google Scholar * Weelic Chong View author publications You can also search for this author inPubMed Google Scholar * Qiang Wei View author publications You can also search for this author
inPubMed Google Scholar * Zhenchao Zhang View author publications You can also search for this author inPubMed Google Scholar * Chun Wang View author publications You can also search for
this author inPubMed Google Scholar * Zhong Ye View author publications You can also search for this author inPubMed Google Scholar * Maysa M. Abu-Khalaf View author publications You can
also search for this author inPubMed Google Scholar * Daniel P. Silver View author publications You can also search for this author inPubMed Google Scholar * Robert T. Stapp View author
publications You can also search for this author inPubMed Google Scholar * Wei Jiang View author publications You can also search for this author inPubMed Google Scholar * Ronald E. Myers
View author publications You can also search for this author inPubMed Google Scholar * Bingshan Li View author publications You can also search for this author inPubMed Google Scholar *
Massimo Cristofanilli View author publications You can also search for this author inPubMed Google Scholar * Hushan Yang View author publications You can also search for this author inPubMed
Google Scholar CONTRIBUTIONS R.L., W.C., and H.Y. conceived the study. Z.Z. and Z.Y. prepared tissue samples. R.T.S. and W.J. provided patient clinical information and pathology reports.
R.L., Q.W., and W.C. performed bioinformatics analysis. M.M.A., D.P.S., B.L., R.E.M., and M.C. reviewed all analyzed data. R.L., W.C., and C.W. prepared the manuscript. All authors discussed
the results, revised, and approved the paper. R.L. and W.C. contributed equally to this study. CORRESPONDING AUTHOR Correspondence to Hushan Yang. ETHICS DECLARATIONS COMPETING INTERESTS
M.M.A. received honorarium for a consultant/advisory role from AstraZeneca, Immunomedics, PUMA, Biothera, Biotheranostics, Agendia, Norvartis, and Lilly. M.C. received honorarium from Lilly,
Menarini, Foundation Medicine, CytoDyn, G1 Therapeutics, and Sermonix. H.Y. is on the SAB of Oriomics Inc., a shareholder of Illumina, Pfizer, and Oriomics, and serves as a NIH study
section reviewer. The above reported activities were not related to the research reported in this article. The remaining authors declare no competing interests. ADDITIONAL INFORMATION
PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION
SUPPLEMENTARY DATA 1 REPORTING SUMMARY RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,
adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Luo, R., Chong, W., Wei, Q. _et al._ Whole-exome sequencing identifies somatic mutations and intratumor heterogeneity in inflammatory breast cancer. _npj
Breast Cancer_ 7, 72 (2021). https://doi.org/10.1038/s41523-021-00278-w Download citation * Received: 23 September 2020 * Accepted: 11 May 2021 * Published: 01 June 2021 * DOI:
https://doi.org/10.1038/s41523-021-00278-w SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative