Play all audios:
ABSTRACT Tumours expressing human chorionic gonadotropin (hCG), the majority of which are difficult to biopsy due to their vascularity, have disparate prognoses depending on their origin. As
optimal management relies on accurate diagnosis, we aimed to develop a sensitive cell free DNA (cfDNA) assay to non-invasively distinguish between cases of gestational and non-gestational
origin. Deep error-corrected Illumina sequencing of 195 common single nucleotide polymorphisms (SNPs) in cfDNA and matched genomic DNA from 36 patients with hCG-secreting tumours (serum hCG
5 to 3,042,881 IU/L) and 7 controls with normal hCG levels (≤4 IU/L) was performed. cfDNA from confirmed gestational tumours with hCG levels ranging from 1497 to 700,855 IU/L had multiple
(_n_ ≥ 12) ‘non-host’ alleles (i.e. alleles of paternal origin). In such cases the non-host fraction of cfDNA ranged from 0.3 to 40.4% and correlated with serum hCG levels. At lower hCG
levels the ability to detect non-host cfDNA was variable, with the detection limit dependent on the type of causative pregnancy. Patients with non-gestational tumours were identifiable by
the absence of non-host cfDNA, with copy number alterations detectable in the majority of cases. Following validation in a larger cohort, our sensitive assay will enable clinicians to better
inform patients, for whom biopsy is inappropriate, of their prognosis and provide optimum management. SIMILAR CONTENT BEING VIEWED BY OTHERS THREE YEARS OF CLINICAL EXPERIENCE WITH A
GENOME-WIDE CFDNA SCREENING TEST FOR ANEUPLOIDIES AND COPY-NUMBER VARIANTS Article Open access 17 March 2021 SINGLE CIRCULATING FETAL TROPHOBLASTIC CELLS ELIGIBLE FOR NON INVASIVE PRENATAL
DIAGNOSIS: THE EXCEPTION RATHER THAN THE RULE Article Open access 17 June 2020 THE VALUE OF INCREASING SEQUENCING DEPTH FOR NONINVASIVE PRENATAL SCREENING FOR WHOLE CHROMOSOMAL ANEUPLOIDY
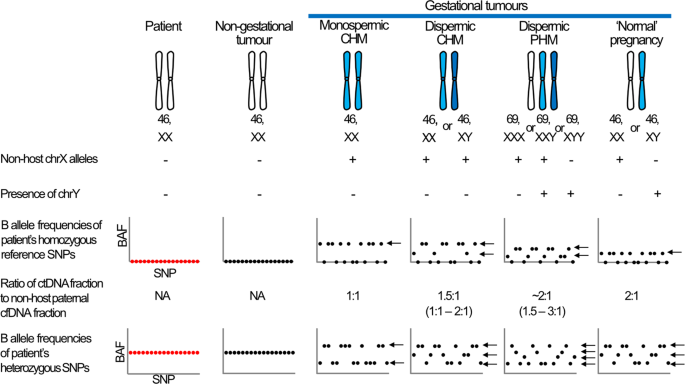
Article Open access 21 January 2025 INTRODUCTION Gestational trophoblastic tumours (GTTs) comprise a group of malignancies that arise from products of conception. The tumours, which include
invasive mole, choriocarcinoma and the rarer placental site and epithelioid trophoblastic tumours (PSTT and ETT), may arise from pre-malignant hydatidiform moles (abnormal pregnancies caused
by an excess of paternal DNA1) or from normal, ectopic, miscarried, or aborted pregnancies (Fig. 1)2. The majority of GTTs arising from molar pregnancies are diagnosed during routine
monitoring of serum human chorionic gonadotrophin (hCG) levels in the months following evacuation of the molar pregnancy. However, GTTs arising from molar and other types of pregnancy may
occur years after the causative pregnancy, by which point the disease has, in many cases, metastasised to the lungs, brain or other tissues3. Although hCG is a highly sensitive marker of
trophoblastic disease that correlates with tumour volume3, this marker is also expressed by some non-gestational malignancies4. Determining the origin of hCG producing tumours in women is
crucial for optimal patient management and counselling as, although GTTs are highly curable, the prognosis for patients with non-gestational tumours is invariably poorer5. Furthermore,
patients with hCG-secreting non-gestational tumours can avoid inappropriate intensive anti-cancer therapy if they can be reliably identified. Short-tandem repeat (STR) genotyping of tumour
and normal tissue is typically used to distinguish between the two entities; gestational tumours uniquely have alleles from the paternal component of the causative pregnancy, but the alleles
of non-gestational tumours match those of the patient6. Due to the vascular nature of trophoblastic tumours, biopsies may result in life-threatening haemorrhage and are not routinely
performed when a gestational tumour is suspected. Previous studies have demonstrated that circulating tumour DNA (ctDNA) is detectable in cell-free DNA (cfDNA) circulating in GTT patients’
plasma, but the approaches have limitations which preclude their use for diagnostics7,8,9. STR genotyping can be applied to any case but lacks sensitivity, failing to detect ctDNA when serum
hCG is lower than ~15,000 international units per litre (IU/L) and with variable detection up to 60,000 IU/L7. The highly sensitive duplex sequencing approach used by Lavoie et al. requires
DNA from the father of the pregnancy and the design of custom patient-specific probes targeting rare single nucleotide polymorphisms (SNPs) identified in a prior sequencing experiment9. In
addition to the limitation that the father of the pregnancy may not be known or be available for testing, these requirements substantially increase expense and turnaround time, precluding
its routine use. To overcome the limitations of both methods we devised a common SNP-based assay that is sensitive, applicable to the majority of patients, and only requires their blood
sample making it suitable for routine diagnostics. RESULTS VALIDATION USING CTDNA OF KNOWN ORIGIN As gestational tumours uniquely contain DNA from the paternal component of the causative
pregnancy (Fig. 1), we screened 195 common autosomal SNPs (minor allele frequencies ranging from 0.26 to 0.71) to determine if ‘non-host’ alleles were present in plasma cfDNA of female
cancer patients with elevated serum hCG. Illumina sequencing of gDNA was used to identify homozygous SNPs in the patient and simultaneous ultradeep sequencing (median read depth ~8500×) of
the cfDNA samples (Supplementary Table 1)) was used to detect the presence or absence of non-host alleles at these loci. The use of unique molecular identifiers facilitated error correction
and quantification of the number of molecules assessed (by generating consensus reads; median 635 per cfDNA sample), which is important to ensure that sufficient theoretical sensitivity is
achieved for samples such as cfDNA which typically have low input amounts (~10–20 ng) and may have low proportions of the DNA population of interest (<10%)10,11,12. To assess the
sensitivity and specificity of the assay, we screened gDNA and cfDNA samples from gestational tumour cases with serum hCG levels ranging from 59 to 700,855 IU/L (_n_ = 19) and compared
results to those from females with non-gestational tumours (hCG range 5–239,171 IU/L) (_n_ = 5) and females with normal hCG levels (≤4 IU/L) (_n_ = 7). Whilst the genotype of cfDNA from a
non-gestational tumour is expected to match that of the patient, cfDNA from a gestational tumour is expected to carry non-host (i.e. paternal) alleles at approximately half of the SNPs where
the patient is homozygous (Fig. 1). As expected, no non-host alleles were detected in the cases with normal hCG levels (Fig. 2). Four of the five non-gestational cases had no non-host
alleles and one case (GTD018) had a single SNP with a low-frequency variant (2/411 consensus reads) that did not match the patient. Thus, in total, only 1 of the 1211 homozygous SNPs across
the 12 patients (median of 101 per patient) had an allele in cfDNA that did not match the respective gDNA sample. In gestational cases where the hCG was ≥824 IU/L, multiple SNPs (mean 36;
range 12–62) had non-host alleles, but when the hCG ≤ 328 IU/L the number of SNPs with non-host alleles was in the range of non-gestational cases (0–2 SNPs) (Fig. 2). IU/L A genomic DNA
sample from the father of the causative pregnancy was available for four gestational cases. All of the non-host alleles identified in the patients’ cfDNA were present in the respective
paternal sample, confirming their gestational origin (Supplementary Fig. 1). Although allele frequencies for each independent SNP varied within a patient, analysis of separate cfDNA aliquots
of the same sample extracted four years apart showed that allele frequencies for each SNP were consistent (Supplementary Fig. 1 A, B), demonstrating that targeting multiple SNPs gives a
reliable method of detection and estimation of the proportion of non-host cfDNA. The non-patient alleles detected in samples from two distinct timepoints for CFD007 were also consistent
(Supplementary Fig. 1C). Probes targeting seven chrX loci (including four common SNPs) and six chrY loci were used to determine the sex of the tumour. Reads aligning to chrY were detected in
the single case known to have originated from a normal male pregnancy (CFD031)7 and in three of the 17 cases derived from molar pregnancies (CFD003, CFD010, CFD015), in cases with non-host
fractions as low as 0.5%. As dispermic XY or XXY molar pregnancies harbour paternally inherited X chromosomes but normal XY pregnancies do not, it is possible to distinguish between the two
entities using chrX SNPs (Fig. 1). Even though the assay was underpowered to identify such occurrences as only four common chrX SNPs were analysed, non-host chrX alleles were detected in two
of the three dispermic XY cases (CFD003 and CFD010) (Table 1). Analysis of B-allele frequencies confirmed the monospermic molar origin of CFD007 and suggested CFD002 may have a similar
origin (Supplementary Fig. 2). COPY NUMBER ALTERATIONS IN NON-GESTATIONAL CASES The absence of non-host alleles in a cfDNA sample from a patient with elevated hCG could be due to failure to
detect very low levels of gestational ctDNA (e.g. in cases with low hCG levels) or due to the tumour being non-gestational. The presence of chromosomal copy number alterations (CNAs) has
previously been reported in some non-gestational tumours6,13 and in cfDNA from CFD008 by STR genotyping7. We proposed that CNAs may provide an alternative means to confirm the presence of
non-gestational ctDNA in samples which lack evidence of non-host alleles and that these could be detected by identifying deviation of the B allele frequency of SNPs from the heterozygous
state. The B allele profiles of heterozygous SNPs in the cfDNA from CFD008 (hCG 239,171 IU/L) and GTD018 (hCG 10,953 IU/L) had evidence of CNAs at multiple chromosomes (Fig. 3), but these
were not found in the three cases with low hCG levels less than 1000 IU/L. CASES WITHOUT A PRIOR DIAGNOSIS After demonstrating the validity of the assay, we subsequently screened a further
twelve patients with a hCG-secreting tumour (range 242–3,042,881 IU/L) of unknown origin i.e. patients who at the time of sampling did not have a tissue biopsy, only two of which had a known
history of molar pregnancy. A single blood sample was taken from each patient within the first 16 days of undergoing chemotherapy and three patients (NP01, NP02, NP03) had longitudinal
sampling (Table 2 and Supplementary Table 1). A DNA sample was available from the partner of only two patients (NP08 and NP09). All patients except GTD009, GTD028 and NP09 (discussed below)
had multiple SNPs (median 39; range 15–50) with non-host alleles in their primary plasma cfDNA samples (Table 2 and Supplementary Fig. 3). The gestational nature was supported by the
presence of chrY reads in three cases (NP01, NP02, NP03) and a B-allele frequency profiles in concordance with those of monospermic and dispermic molar pregnancies in NP08 and GTD002,
respectively (Supplementary Fig. 2). Patients NP04 and NP07 subsequently had tumour tissue available for STR genotyping, which confirmed the gestational origin (Supplementary Fig. 4). To
investigate the sensitivity of the assay, we analysed additional samples from NP01, NP02 and NP03 when the patients’ tumour biomarker (hCG) levels had dropped by 1–3 orders of magnitude to
242, 437, and 1174 IU/L, respectively. None of the non-host alleles previously identified at 41 SNPs in NP02 were detected and only 3/50 and 3/44 non-host alleles were detected for NP01 and
NP03, respectively (Table 2). As only ~25% of each sample library was pooled for probe capture of the SNP regions, we subsequently pooled, captured, and sequenced the remainder of the
libraries for these three samples with the aim of increasing the number of unique molecules and thus the sensitivity. Despite lowering the limit of detection (0.23%, 0.12% and 0.18% for
NP01b, NP02b and NP03, respectively), the number of SNPs with non-host alleles only marginally increased to four and five for NP01b and NP03b, respectively, while non-host alleles remained
undetectable for NP02b (Supplementary Table 1). This suggests that majority of the non-host alleles in these samples with low hCG levels were present at levels below 0.2% of the total cfDNA.
Overall, the serum hCG level of gestational samples correlated with the fraction of non-host cfDNA (Spearman’s rho = 0.814, _P_ = 2.4 × 10−6) (Fig. 4). Based on their hCG levels, the ctDNA
fraction in GTD028 and NP09 is expected to be >10%, but non-host alleles were not detected at any of their homozygous SNPs, consistent with non-gestational tumours. GTD009 had a
relatively low hCG level (3840 IU/L) and had a single SNP with non-host alleles at 0.5% allele frequency (3/639 consensus reads). All three patients subsequently had tumour tissue removed;
STR genotyping confirmed their non-gestational origin with presence of copy number alterations (data not shown), indicating that the single SNP with a non-host allele in GTD009 was due to a
low-frequency error or a somatic mutation. These and other copy number alterations were identifiable as allelic imbalances in all three cfDNA samples and the only available tumour sample
(Supplementary Fig. 5). Although direct comparison between gestational and non-gestational ctDNA fractions could not be made, the amount of cfDNA per ml of plasma was generally relatively
higher in non-gestational cases (Supplementary Fig. 6). DISCUSSION In addition to the clinical benefit due to the risks associated with taking tumour biopsies and the difference in prognosis
and management compared to non-gestational tumours, GTT are ideally suited for ctDNA diagnosis due to their unique genetic make-up. The presence of paternal DNA from the causative pregnancy
provides a wide and highly-specific target for detection, circumventing the typical requirement of knowledge of tumour-specific recurrent mutations or patient-specific mutations for other
solid tumours14,15 and avoiding confounding events such as clonal haematopoiesis16. Our assay confidently detected non-host cfDNA with fractions as low as 0.28% (Fig. 4), the lowest reported
to date in trophoblastic tumour samples. In gestational cases derived from molar pregnancies, ≥12 SNPs with non-host alleles were detected in cases with hCG levels as low as 824 IU/L.
However, in the cases without a history of molar pregnancy (thus likely originating from normal pregnancies), when the hCG was 1174 IU/L, or lower, non-host cfDNA was only detectable at
<10% of the expected SNPs. The difference in molar and normal pregnancy cases can be attributed in part to the different relative contributions of paternal DNA (two paternal genomes in
molar pregnancies and one in normal pregnancies) (Fig. 1) as demonstrated by molar pregnancies typically having relatively higher non-host cfDNA fractions (Fig. 4). Although the duplex
sequencing assay appeared to have greater sensitivity than our assay based on hCG levels (732 IU/L) the fraction of non-host cfDNA was ~0.8%, which would have been detectable with our assay.
Thus it is likely that other factors such as tumour type, that can vary in their hCG secretion17, tumour site, and time since treatment could also affect the ratio between hCG and non-host
cfDNA fraction. Across seven normal hCG and eight non-gestational samples, only two out of a total of 1502 homozygous SNPs had a false positive non-host allele present at low levels,
indicating that the error rate of this consensus-read based assay is low. We conservatively set the detection threshold of 12 SNPs with non-host alleles, which had consistent results when
hCG levels were ≥1497 IU/L, but further sampling of gestational and non-gestational cases will likely reduce this threshold and determine whether other informative features (e.g. amount of
cfDNA per ml (Supplementary Fig. 6)) can be used to distinguish between gestational and non-gestational cases when hCG levels are low. Although copy number alterations detected using the B
allele profile of the heterozygous SNPs can confirm the presence of ctDNA in non-gestational cases (Fig. 3), such events were not detectable in the cases with hCG levels <1000 IU/L.
Despite the technical limitations at low hCG levels, using the first sample taken after admission we could determine the origin of all 12 of the test cases (Table 2) without the need for a
partner’s DNA sample. In all seven patients who were clinically treated as gestational cases and did not have surgery or a biopsy during their treatment, non-host alleles were detected.
Their assumed gestational origin was further supported by the detection of a Y chromosome in 3 cases (Table 2), and B-allele frequency profiles indicative of molar origin in two cases
(Supplementary Fig. 2). In the absence of tissue to confirm the diagnosis, sequencing cfDNA from a distinct plasma sample or using digital PCR or alternative techniques18 to screen a subset
of the identified informative SNPs could be utilised to independently confirm the findings. Identifying the causative pregnancy may help to further stratify patients as the type and interval
since the causative pregnancy is a factor in the FIGO/WHO risk scoring of gestational trophoblastic tumours19, with the latter being the most important prognostic factor for PSTT and
ETT20,21. Owing to the rarity of fresh tumour material, there have been few genome-wide studies of GTT22,23 particularly at base-pair resolution24 and no recurrent or tumour subtype-specific
mutations have been identified. Thus, although we demonstrate that ctDNA can be effectively used for diagnosis of gestational tumours, specifying the tumour subtype remains elusive. In
conclusion we have developed a sensitive, reproducible, and affordable (~$220 per case) non-invasive diagnostic technique for identifying GTT which can be used for any patient presenting
with a malignancy and raised hCG, with reliable detection of GTT when hCG is ≥1497 IU/L. This technology can ensure that suspected GTT cases are confirmed without the need for potentially
dangerous biopsies and patients with non-GTT malignancies can be spared aggressive non-curative therapies. METHODS SAMPLE COLLECTION AND EXTRACTION The human samples, collected with informed
written consent, used in this research project were obtained from the Imperial College Healthcare Tissue Bank (ICHTB), which is approved by Wales REC3 to release human material for research
(17/WA/0161), and the samples for this project (R14021, R19029) were issued from sub-collection reference numbers ONC_MS_11_003, MED_RF_19_013 and CAN_GM_19_034. Cases with tumours of known
origin refer to those 1) whose tumour arose during hCG monitoring following a molar pregnancy, 2) with genetic diagnosis from their tumour tissue, 3) with non-host alleles previously
identified by STR genotyping of cfDNA (Table 1). Cases of unknown origin lacked all of the above, although in some cases they subsequently had tumour tissue removed and a genetic diagnosis
was made. Blood samples were stored in EDTA tubes on ice for a maximum of 2 h before plasma was isolated: after centrifugation at 1000 _g_ for 10 min at 4 °C the supernatant was isolated and
centrifuged at 2000 _g_ for 10 min at 4 °C25. The supernatant was removed and then stored at −80 °C until cfDNA was extracted from 3 ml of plasma using the QIAamp circulating nucleic acid
kit (Qiagen) in accordance with the manufacturer’s instructions. Genomic DNA was extracted from the buffy coat of the centrifuged sample or from a separate whole blood sample using the
QIAamp DNA Blood Mini kit (Qiagen). Where available tumour tissue was manually dissected from formalin-fixed paraffin embedded (FFPE) tissue sections and DNA was extracted using a using a
QIAamp DNA FFPE Tissue Kit (Qiagen, UK) according to the manufacturer’s instructions. DNA was quantified using Qubit fluorometry (Thermo Fisher). DNA LIBRARY PREPARATION Typically, 20 ng
(range 10–100 ng) of cfDNA or gDNA was used to prepare a DNA library in accordance with the manufacturer’s instructions, using Cell3™Target Library Preparation kit (Nonacus) or Lotus DNA
Library Prep Kit (Integrated DNA Technologies), respectively. Illumina dual-indexed adaptors containing nine nucleotide unique molecular identifiers (UMIs) were ligated, before PCR
amplification with the number of cycles adjusted to input DNA (see Supplementary Table 1 for library preparation details). Following purification with Target Pure NGS Clean-up Beads
(Nonacus), quality control using Tapestation High Sensitivity D5000 ScreenTape (Agilent Technologies) and quantification using Qubit fluorometry, samples were pooled (100–150 ng of cfDNA
libraries and 40–50 ng of gDNA libraries). Pooled DNA libraries totalling ~1500 ng were hybridised to a 60 kb custom capture library, which contained 195 common autosomal SNPs (median minor
allele 0.49; range 0.26–0.71; minimum of 3 and median of 8.5 SNPs per chromosome) from the Cell3 Target Paternity Panel (Nonacus), 7 chrX targets and 6 chrY targets. The regions of interest
were captured and amplified (14 cycles) using the Cell3 Target Capture Enrichment Reagents kit (Nonacus). Amplified libraries were purified (Target Pure NGS Clean-up Beads), evaluated
(Tapestation High Sensitivity D5000 ScreenTape), quantified (Qubit) and, if necessary, pooled before sequencing on Illumina NextSeq500 mid output using the following read lengths: R1 (71),
R2 (71), i7 (17); i5 (8). DATA PROCESSING AND SNP GENOTYPING Fastq files for R1, R2 (UMI), R3, I1 and I2 were generated using bcl2fastq. Reads were aligned to hg38, using bwa-mem v0.7.1326
fgbio’s toolset was used to annotate the RX tag of the BAM file with the UMI (http://fulcrumgenomics.github.io/fgbio/tools/latest/AnnotateBamWithUmis.html). The ‘pileups snps’ tool in
amplimap27 was used to generate counts for all reference and alternate autosomal SNPs. To remove PCR duplicates and to correct PCR and sequencing errors, reads with a minimum mapping quality
of Q20 which had identical UMIs and mapping location were grouped. Consensus calls were generated for each group: only bases with a minimum quality of Q20 comprising >80% of the total
family of at least two reads were counted. Non-host alleles (alleles present in cfDNA that did not match the homozygous alleles in the patient) with a minimum count of two non-host bases per
SNP were identified. The non-host cfDNA fraction was calculated as the mean fraction of consensus base counts for non-host SNP divided by the sum of the patient and non-host consensus base
counts. For cases with detectable non-host DNA, read counts for sex chromosome regions were analysed to determine the sex of the tumour. B allele frequencies for SNPs that were heterozygous
in the patients were used to identify features of the causative pregnancy in gestational cases and evidence of copy number alterations in non-gestational cases. STR GENOTYPING Fifteen
autosomal STR loci on 13 chromosomes and the Amelogenin (sex chromosome) locus were amplified from 1–4 ng of DNA using the AmpFlSTR Identifiler Plus or Globalfiler IQC kits (Applied
Biosystems, Warrington, UK) and resolved by capillary electrophoresis using an ABI 3130 Genetic Analyzer. Genotypes were analysed using GeneMapper version 5.0 software (Applied Biosystems,
Warrington, UK). CORRELATION OF SERUM HCG LEVELS AND NON-HOST CFDNA Spearman’s rank correlation coefficient was calculated for all gestational samples with ≥12 SNPs with non-host alleles
using the cor.test() function in R. REPORTING SUMMARY Further information on research design is available in the Nature Research Reporting Summary linked to this article. DATA AVAILABILITY
Sequencing data (.bam files) are available from the European Nucleotide Archive (accession number PRJEB49976). CODE AVAILABILITY Custom codes used in this study can be obtained upon a
reasonable request to the corresponding author. REFERENCES * Lawler, S. D., Fisher, R. A. & Dent, J. A prospective genetic study of complete and partial hydatidiform moles. _Am. J.
Obstet. Gynecol._ 164, 1270–1277 (1991). Article CAS Google Scholar * Hui, P. et al. Gestational trophoblastic disease. in _WHO Classifications of Tumours of Female Reproductive Organs_
(eds. Kurman, R., Carcangiu, M., Herrington, C. & Young, R.) vol. 6 155–168 (IARC Press, 2014). * Seckl, M. J. et al. Gestational trophoblastic disease: ESMO Clinical Practice Guidelines
for diagnosis, treatment and follow-up. _Ann. Oncol._ 24, vi39–vi50 (2013). Article Google Scholar * Iles, R. K., Delves, P. J. & Butler, S. A. Does hCG or hCGβ play a role in cancer
cell biology? _Mol. Cell. Endocrinol._ 329, 62–70 (2010). Article CAS Google Scholar * Fisher, R. A. et al. The impact of molecular genetic diagnosis on the management of women with
hCG-producing malignancies. _Gynecol. Oncol._ 107, 413–419 (2007). Article CAS Google Scholar * Fisher, R. A. & Kaur, B. Molecular genotyping in the diagnosis of trophoblastic
tumours. _Diagn. Histopathol._ 25, 66–76 (2019). Article Google Scholar * Openshaw, M. R. et al. Circulating cell free DNA in the diagnosis of trophoblastic tumors. _EBioMedicine_ 4,
146–152 (2016). Article Google Scholar * Kristiansen, M. K. et al. Cell-free DNA in pregnancy with choriocarcinoma and coexistent live fetus: a case report. _Medicines_ 95, e4721 (2016).
Google Scholar * Lavoie, J.-M. et al. Targeted error-suppressed detection of circulating paternal DNA to establish a diagnosis of gestational trophoblastic neoplasm. _JCO Precis. Oncol_.
1–6 (2017). https://doi.org/10.1200/PO.17.00154. * Zhang, J. et al. Non-invasive prenatal sequencing for multiple Mendelian monogenic disorders using circulating cell-free fetal DNA. _Nat.
Med._ 25, 439–447 (2019). Article CAS Google Scholar * Che, H. et al. Noninvasive prenatal diagnosis by genome-wide haplotyping of cell-free plasma DNA. _Genet. Med_. (2020).
https://doi.org/10.1038/s41436-019-0748-y. * Mattox, A. K. et al. Applications of liquid biopsies for cancer. _Sci. Transl. Med_. 11, eaay1984 (2019).
https://doi.org/10.1126/scitranslmed.aay1984. * Fisher, R. A. & Maher, G. J. Genetics of gestational trophoblastic disease. _Best Pract. Res. Clin. Obstet. Gynaecol_. S1521693421000110
(2021). https://doi.org/10.1016/j.bpobgyn.2021.01.004. * Phallen, J. et al. Direct detection of early-stage cancers using circulating tumor DNA. _Sci. Transl. Med._ 9, eaan2415 (2017).
Article Google Scholar * Abbosh, C. et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. _Nature_ 545, 446–451 (2017). Article CAS Google Scholar * Abbosh, C.,
Swanton, C. & Birkbak, N. J. Clonal haematopoiesis: a source of biological noise in cell-free DNA analyses. _Ann. Oncol._ 30, 358–359 (2019). Article CAS Google Scholar * Horowitz, N.
S., Goldstein, D. P. & Berkowitz, R. S. Placental site trophoblastic tumors and epithelioid trophoblastic tumors: Biology, natural history, and treatment modalities. _Gynecol. Oncol._
144, 208–214 (2017). Article Google Scholar * Vessies, D. C. L. et al. Performance of four platforms for KRAS mutation detection in plasma cell-free DNA: ddPCR, Idylla, COBAS z480 and
BEAMing. _Sci. Rep._ 10, 8122 (2020). Article CAS Google Scholar * Ngan, H. Y. S. et al. Update on the diagnosis and management of gestational trophoblastic disease. _Int. J. Gynaecol.
Obstet. Organ Int. Fed. Gynaecol. Obstet._ 143, 79–85 (2018). Article Google Scholar * Schmid, P. et al. Prognostic markers and long-term outcome of placental-site trophoblastic tumours: a
retrospective observational study. _Lancet_ 374, 48–55 (2009). Article CAS Google Scholar * Froeling, F. E. M. et al. Intensified therapies improve survival and identification of novel
prognostic factors for placental-site and epithelioid trophoblastic tumours. _Br. J. Cancer_ 120, 587–594 (2019). Article CAS Google Scholar * Poaty, H. et al. Genome-wide high-resolution
aCGH analysis of gestational choriocarcinomas. _PLoS ONE_ 7, e29426 (2012). Article CAS Google Scholar * Mello, J. B. et al. Genomic profile in gestational and non-gestational
choriocarcinomas. _Placenta_ 50, 8–15 (2017). Article CAS Google Scholar * Savage, P. et al. A case of intraplacental gestational choriocarcinoma; characterised by the methylation pattern
of the early placenta and an absence of driver mutations. _BMC Cancer_ 19, 744 (2019). Article Google Scholar * Page, K. et al. The importance of careful blood processing in isolation of
cell-free DNA. _Ann. N.Y. Acad. Sci._ 1075, 313–317 (2006). Article CAS Google Scholar * Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. _ArXiv13033997
Q-Bio_ (2013). * Koelling, N. et al. amplimap: a versatile tool to process and analyze targeted NGS data. _Bioinformatics_ 35, 5349–5350 (2019). Article CAS Google Scholar Download
references ACKNOWLEDGEMENTS We are grateful to Rosetrees Trust and The Stoneygate Trust who partly funded this work (Seedcorn2020\100003 to GJM). Infrastructure support was provided by
Imperial Experimental Cancer Medicine Centre and we thank Naina Patel, Lottie Ion and Kelly Gleason for support and access to equipment. We are grateful to Katerina Rekopoulou, Avik Datta
and the Imperial BRC Genomics Facility (supported by NIHR funding to the Imperial Biomedical Research Centre) who have provided resources and support that have contributed to the research
results reported within this paper. We thank Lee Silcock and Jane Hayward (Nonacus Ltd) for technical support and Prof Syndercombe-Court and Lesley Nott at the DNA Forensics Unit at King’s
College London for capillary electrophoresis and Jingky Lozano-Kuehne for helpful discussions. Tissue samples were provided by the Imperial College Healthcare NHS Trust Tissue Bank funded by
the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London. The views expressed are those of the
author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. AUTHOR INFORMATION Author notes * These authors contributed equally: Mark R. Openshaw, Michael J.
Seckl. AUTHORS AND AFFILIATIONS * Trophoblastic Tumour Screening & Treatment Centre, Imperial College London, Charing Cross Campus, Fulham Palace Road, London, W6 8RF, UK Geoffrey J.
Maher, Rosemary A. Fisher, Baljeet Kaur, Xianne Aguiar, Preetha Aravind, Natashia Cedeno, James Clark, Debbie Damon, Ehsan Ghorani, Adam Januszewski, Ravindhi Murphy, Rajat Roy, Naveed
Sarwar, Mark R. Openshaw & Michael J. Seckl * Department of Surgery and Cancer, ICTEM Building, Hammersmith Hospitals Campus of Imperial College London, Du Cane Road, London, W12 0NN, UK
Geoffrey J. Maher, Foteini Kalofonou, Rajat Roy & Michael J. Seckl Authors * Geoffrey J. Maher View author publications You can also search for this author inPubMed Google Scholar *
Rosemary A. Fisher View author publications You can also search for this author inPubMed Google Scholar * Baljeet Kaur View author publications You can also search for this author inPubMed
Google Scholar * Xianne Aguiar View author publications You can also search for this author inPubMed Google Scholar * Preetha Aravind View author publications You can also search for this
author inPubMed Google Scholar * Natashia Cedeno View author publications You can also search for this author inPubMed Google Scholar * James Clark View author publications You can also
search for this author inPubMed Google Scholar * Debbie Damon View author publications You can also search for this author inPubMed Google Scholar * Ehsan Ghorani View author publications
You can also search for this author inPubMed Google Scholar * Adam Januszewski View author publications You can also search for this author inPubMed Google Scholar * Foteini Kalofonou View
author publications You can also search for this author inPubMed Google Scholar * Ravindhi Murphy View author publications You can also search for this author inPubMed Google Scholar * Rajat
Roy View author publications You can also search for this author inPubMed Google Scholar * Naveed Sarwar View author publications You can also search for this author inPubMed Google Scholar
* Mark R. Openshaw View author publications You can also search for this author inPubMed Google Scholar * Michael J. Seckl View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS G.J.M. designed the assay, performed the experimental and analytical work, and drafted the manuscript. R.A.F. and M.R.O. designed the study, collected
and extracted DNA from samples, and critically evaluated the manuscript. B.K., X.A., P.A., N.C., J.C., D.D., E.G., A.J., F.K., R.M., R.R., N.S. consulted the patients and/or arranged sample
collection. M.J.S. designed the study and critically evaluated the manuscript. M.R.O. and M.J.S. equally contributed to the work. CORRESPONDING AUTHOR Correspondence to Geoffrey J. Maher.
ETHICS DECLARATIONS COMPETING INTERESTS The authors have no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims
in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTAL FIGURES SUPPLEMENTAL TABLE 1 REPORTING SUMMARY RIGHTS AND PERMISSIONS OPEN ACCESS This article is
licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in
this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative
Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a
copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Maher, G.J., Fisher, R.A., Kaur, B. _et al._ Sensitive
screening of single nucleotide polymorphisms in cell free DNA for diagnosis of gestational tumours. _npj Genom. Med._ 7, 26 (2022). https://doi.org/10.1038/s41525-022-00297-x Download
citation * Received: 12 October 2020 * Accepted: 04 March 2022 * Published: 08 April 2022 * DOI: https://doi.org/10.1038/s41525-022-00297-x SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative