Play all audios:
ABSTRACT The corrosion of alloy steels with different amounts of Cr was studied using electrochemical tests, wet–dry cycle corrosion, X-ray diffraction, and Kelvin probe force microscopy.
The results show that the content of Cr is positively correlated with the corrosion resistance of bare steel, but the corrosion resistance of atmospheric corrosion does not show the same
pattern. The atmospheric corrosion resistance of Cr-containing steel exhibits three different stages with the change of Cr element content. When the Cr content is in the range of 1–4%, the
corrosion rate is high and does not change within the Cr content. As the Cr content was further increased from 4 to 7%, the corrosion rate exhibited a linear decrease and then drops rapidly
when the Cr content reaches 8%. These three different corrosion rate stages are related to the influence of Cr content on Fe3O4 content in the rust layer. SIMILAR CONTENT BEING VIEWED BY
OTHERS EFFECT OF NITROGEN CONTENT ON CORROSION BEHAVIOR OF HIGH-NITROGEN AUSTENITIC STAINLESS STEEL Article Open access 21 September 2023 EFFECT OF NANO-GRAIN CARBIDE FORMATION ON
ELECTROCHEMICAL BEHAVIOR OF 316L STAINLESS STEEL Article Open access 15 June 2021 THE CORROSION BEHAVIOR OF LOW CARBON STEEL (AISI 1010) INFLUENCED BY GRAIN SIZE THROUGH MICROSTRUCTURAL
MECHANICAL Article Open access 01 March 2024 INTRODUCTION It is well known that the corrosion resistance of materials can be improved by adding corrosion-resistant alloying elements1,2,3. In
recent years, a method to improve the corrosion resistance of materials is to form a dense and stable rust layer. One of the typical examples is weathering steel. By adding certain amounts
of corrosion-resistant elements, a dense rust layer is formed on the steel surface, which can impede further corrosion process of the substrate to achieve improvement of the steel corrosion
resistance4,5,6,7,8. The recent researches have suggested that addition of Cr, Cu, or P alloying elements can significantly improve the rust layer structure stability of the weathering
steel, thereby improving its applicability in severe industrial and marine environments9,10,11,12,13,14,15. Several studies have focused on the effect of alloying elements on the corrosion
resistance. A study by Ma et al.16 on the atmospheric corrosion behavior of weathering steel in the tropical marine environment found that the weathering resistance of weathering steel is
related to the deposition of chloride ions and the enrichment of Cr in the rust layer and that the latter can significantly improve the corrosion resistance of weathering steels in
environments with low chloride ion deposition. Several studies17,18,19,20,21 found that Cu can enhance the stability of the rust layer when used together with Cr, thus improving the
corrosion resistance of weathering steel. However, a minimum of 0.10 wt% Cu is required in order to significantly improve the corrosion resistance of the steel22. The rust layer of
corrosion-resistant steel after long-term exposure to the atmosphere has a two-layer construction, and a significant difference Cr content presents in the inner and outer layers,
respectively23. A Cr-enriched inner layer mainly consists of α-(Fe1−_x_,Cr_x_)OOH, a Cr substituted FeOOH, which acts as a final stable protective layer. Other studies also found that a
pseudo-passivation film formed on some Cr-containing steels in the CO2 corrosion environment, and this film is consisted of Cr(OH)324,25. After analyzing the rust layer structure of
Cr-containing steel, Jiang et al.26 found that Cr is mainly in the forms of Cr(OH)3, CrOOH, or FeCr2O4 in the rust layer, that is, Cr mainly exists in the form of Cr3+ among the participants
during the iron oxide process. Yamashita et al.27 believed that the existence of Cr element can replace the nano-sized goethite particles to create a denser protective rust layer in the
inner rust layer. Although all the existing studies so far used several alloying elements together28,29, it is not difficult to see from these studies that the Cr content and the corrosion
resistance have a close correlation. However, the study of the influence of chromium on the initial stages of the atmospheric corrosion of weathering steels by Kamimura and Stratmann21 found
that the positive effect of chromium is not only not apparent in saline environments but also seems to be even harmful by accelerating weathering steels corrosion in coastal environments.
Other researchers30,31,32 also pointed out that the presence of 0.60–1.30 wt% Cr was sometimes slightly detrimental, especially in industrial atmospheres. Therefore, the effect of the Cr
content on the formation of the weather-resistant rust layer in steel, as well as the relationship between the Cr content and the density of the rust layer, has not yet reached a consentient
understanding. In this work, the steel samples with eight different Cr contents were studied to identify the relationship between the Cr content and the atmospheric corrosion resistance. A
dry–wet cyclic corrosion test was used to study the effect of the Cr content on the corrosion resistance of the corrosion layer during the atmospheric corrosion process. The purpose of this
study is to provide an insight into the influence of the Cr content on atmospheric corrosion resistance. In addition, this study may provide useful data for the development of micro-alloyed
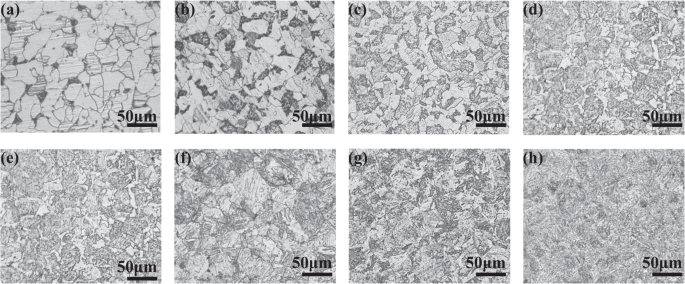
corrosion-resistant materials. RESULTS MICROSTRUCTURE The metallographic structure of the eight experimental materials is shown in Fig. 1. It can be seen from the figure that Cr changes the
microstructure of the matrix. The Cr content primarily affects the proportion of bainite in the steel. The W1 structure is essentially ferrite with a very small amount of lath bainite. W2,
W3, and W4 are both granular bainite and ferrite. As the Cr content increases, the proportion of granular bainite increases gradually. This occurs because the addition of Cr to a steel can
delay the pearlite transformation and promote the bainite transformation, making it easier to form the bainite structure at a slow cooling rate after austenitizing. When the Cr content
reaches ≥5%, the structure is all bainite, and the size of bainite becomes finer with the increase of Cr content; the W8 has the smallest grain size. WET–DRY CYCLES CORROSION RESULTS To
study the atmospheric corrosion behavior of the steels with different Cr contents, dry and wet cycle experiments were carried out on the eight steel samples. Figure 2 shows that, as the
corrosion cycles increased, the corrosion rate gradually stabilizes. At the initial stage of the experiment, the main reaction is the corrosion of the steel substrate. Therefore, the
corrosion rate gradually decreases with the increase of Cr content. The Cr content showed a certain positive effect on the corrosion resistance in the early stage of corrosion. However,
after the 72-h dry–wet cycle accelerated experiment, the corrosion rate change started to show two different trends for the samples with different Cr contents. The effect of Cr on corrosion
rate of the samples with low Cr contents (W1–W4) decreased gradually and trended to be the same, while the effect of Cr on corrosion resistance in those with high Cr content (W5–W8) still
showed a positive correlation with the Cr content. This is mainly due to the gradual formation of the rust layer on the steel surface during the prolonged experiment time, where the main
factor that affects the corrosion resistance has shifted from the corrosion resistance of the steel substrate to the density of the rust layer. The results after 120-h experiment show no
more significant difference in the corrosion rate when the Cr content is the steel is <4%. The result from long-period cycling corrosion test indicated that 4% Cr content appeared to be a
threshold for its role in affecting the corrosion rate. After the 168-h dry and wet cycle corrosion experiment (Fig. 2d), a stable rust layer structure has been formed on the surface of the
material, and the corrosion rate at this time can be divided into 3 groups: (1) The corrosion rates of W1–W4 are basically the same, at ~5.5 mm y−1. (2) The corrosion resistance of W4–W7
gradually increases, and the corrosion rate decreases linearly from 5.5 to 2.7 mm y−1; (3) The corrosion rate of W8 is only 0.3 mm y−1, which is merely one-eighteenth of W1. The macroscopic
morphology (Supplementary Fig. 1) of the eight experimental materials after wet–dry cycles corrosion test shows that, at 24 h, the surface of the experimental steel features a thin layer of
corrosion products, which is relatively flat and does not bubble. The surface rust layers of W1–W6 are predominantly dark brown, with some yellow-brown rust spots; W7 has a small amount of
brick red rust on the surface, and W8 exhibits only a very slight corrosion and the substrate is visible. At 72 h, the presence of corrosion products is clearly more noticeable compared with
24-h samples but still not dense. There is obvious surface bubbling for W1–W4. The surface rust layers for W5–W7 are relatively flat. W8 is evenly covered with yellowish brown rust and is
very dense. After 120 h, bubbling was observed for W5–W7, and there was no change in the surface appearance for W8. After 168 h, the degree of corrosion had obviously increased, the rust
layer morphology for W1–W4 exhibited no difference, the corrosion extent for W5–W7 gradually reduced, and for W8 the surface was still covered in the yellow-brown rust layer, and the
corrosion was trivial. Furthermore, the microscopic morphology of corrosion products after 168 h (Supplementary Fig. 2) show that the surfaces of W1–W7 feature more corrosion products,
significant surface bubbling, and many cracks. The surface of W8, however, is covered by fine and uniform corrosion products, which is very dense with no cracks, thus protecting the
substrate. Figure 3 shows the microscopic morphologies of the experimental steels after the removal of the rust. The surfaces of W1–W4 exhibit many pitting pits and severe uniform corrosion
morphology. W5–W7 present many localized corrosion features. There are virtually no obvious corrosion marks on the surface of W8, but a small number of small pits. These results show that,
in the Cr content range from 1 to 4%, the uniform corrosion decreases and the local corrosion increases as the Cr content increases. In the Cr content range from 5 to 7%, the depth and the
number of pitting pits decrease with increasing Cr content. When the Cr content reaches 8%, the corrosion behavior of the surface changes suddenly, resulting in improved corrosion resistance
and less surface corrosion. These results are consistent with the other studies, which had a conclusion that the local corrosion of steel becomes more obvious, and the interface of the base
rust layer becomes uneven after adding Cr to steel33. The scanning electron microscopic (SEM) observation results of the rust layer cross-section are shown in Fig. 4, and energy-dispersive
spectroscopic (EDS) line scan analysis was performed on the marked areas in Fig. 4. It can be seen from the cross-sectional topography that there is no obvious delamination in the rust
layer. For W1–W4, the number of the pitting pits increases gradually as the Cr content increases, the rust layer cross-section is uneven, and there are many microscopic cracks and holes.
Samples W5–W7 exhibit significant local corrosion, the bonding between the rust layer and the substrate is weak, and large cracks exist in the rust layer. The rust layer of W8 is the
thinnest, with a thickness only ~20 μm. The rust layer structure is very dense and exhibits good bonding with the matrix. It can be seen from the EDS line scan that the rust layer near the
substrate is enriched in Cr. Cl− exists in the entire rust layer and is slightly higher in the inner layer than that in the outer rust layer. This indicates that the rust layer is an ion
conduction layer, and Cl− can penetrate through the rust layer to the metal interface, causing corrosion reaction therewith; this gradually increases the density of the rust layer. Cracks
and holes also appear in the cross-section of the rust layer. This is because the rust layer contains various corrosion products, and the densities of different corrosion products are
different and smaller than that of the matrix. During the continuous accumulation of corrosion products on the surface of the steel substrate, stress is generated at the interface between
the rust layer and the substrate, and corrosion products themselves have poor ductility, resulting in cracks and holes. The X-ray diffraction (XRD) analysis on the rust layers of the samples
after the wet–dry cycles corrosion test was performed, and the results are shown in Fig. 5. It can be seen from figure that, for all the samples analyzed, the rust layers contain α-FeOOH,
γ-FeOOH, and Fe3O4. However, the content of each substance is significantly different in the different samples. Therefore, the _K_ value method was used in order to semi-quantitatively
analyze the rust layers to calculate the proportion of different phases in the rust layers. The results are shown in Table 1. It can be seen from the table that, during the initial 24 h of
corrosion, the rust layers of W1–W6 contain a large amount of Fe3O4, while those of W7 and W8 only contain α-FeOOH. At 72 and 120 h, surface corrosion of the samples became more extensive,
and the proportion of Fe3O4 is detectably higher, which is consistent with the increase in the black rust amount, as evidenced by the macroscopic morphology results (Supplementary Fig. 1).
After 168 h, the proportion of Fe3O4 becomes relatively small. Most of the corrosion products for W1–W7 are Fe3O4, while most of the corrosion products for W8 are α-FeOOH and γ-FeOOH with
almost no Fe3O4. The proportion of α-FeOOH increases with the increase of number of corrosion cycles. The XRD results show that increasing the Cr content has little effect on the γ-FeOOH
content in the rust layer, which remains at a low level. Therefore, for each experimental steel we analyzed the relationship between the corrosion rate and the ratio of Fe3O4 in the rust
layer for different corrosion cycles (Fig. 6). For each corrosion cycle, the ratio of Fe3O4 in the corrosion product of W8 is consistent with its corrosion rate. After 168 h, it can be seen
clearly that the Fe3O4 ratio can be categorized into three groups based on the Cr content. For W1–W4, the ratio of Fe3O4 remains stable at ~92%. For W4–W7, the ratio of Fe3O4 decreases
linearly from 92 to 73%, while the rust layer of W8 contains only 0.9% Fe3O4. This change of Fe3O4 content in the rust layer is consistent with the results from the corrosion rate change
curve. DISCUSSION The polarization curves (Supplementary Fig. 3) of the eight experimental materials in the 3.5% NaCl solution shown that the corrosion resistance of the steel matrix
improves as the Cr content increases. This is consistent with the traditional understanding of the role of Cr element but not with the results observed in the atmospheric corrosion process.
In order to further investigate this difference, the microscopic structure change of the substrate caused by Cr was studied to further clarify the reason for improving the corrosion
resistance of the substrate. Many studies34,35,36 investigated the possibility that the steel structure affects the corrosion resistance, and the addition of alloying elements changes the
microstructure of the steel matrix, which in turn can affect its corrosion resistance. Our work showed that, when Cr was added as an alloying element to low-carbon steel, it delayed the
pearlite transformation and promoted the bainite transformation, allowing the bainite structure to form more easily after austenitization. It can also be seen from Fig. 1 that the addition
of Cr does changes the original phase structure of the material. When the Cr content is <4%, the material was composed of two phases of ferrite and bainite. When the Cr content was
increased to 8%, the grain size further improved the corrosion resistance. Therefore, it is considered that the difference of the corrosion resistance in the experiment might be mainly
contributed by the structural changes caused by the addition of different amount of Cr. To validate the hypothesis, W3 sample was selected for Kelvin probe force microscopy (KPFM) analysis.
(Supplementary Fig. 4 and Fig. 7). Supplementary Fig. 4 shows the metallographic structure of the W3 steel containing 3% Cr. The structure is composed of ferrite and granular bainite. The
locus marked in the figure was where the KPFM testing performed. The KPFM result is shown in Fig. 7. Figure 7a–d are the height map, the potential map, the three-dimensional map of the
potential, and the potential change curve of the section, respectively. It can be seen from Fig. 7d that the potential of the granular bainite is much higher than the potential of the matrix
ferrite, and the difference between the two is ~≥80 mV. The existence of this potential difference may have two effects. (1) The corrosion resistance of the steel matrix improves with more
phase content that has better corrosion resistance, as shown in Fig. 2a and Supplementary Fig. 3 that display an early corrosion rate decrease. The corrosion resistance of the steel matrix
continues to improve with the addition of more Cr content29,37,38,39. (2) The galvanic difference between the two phases caused the variations of the morphology and structure during the
atmospheric corrosion process. Due to the difference of the potential between the two phases, the corrosion process is promoted and the corrosion morphology changes from uniform corrosion to
local pitting, as shown in Fig. 3. At the later stage after a long-period dry–wet alternate corrosion process, the major factor that influence the corrosion rate became the structure of the
rust layer. Therefore, a detailed analysis of the changes in the structure of the rust layer is also required. The rust layer structure plays an important role in the corrosion resistance
of materials40,41. In atmospheric corrosion, the main process on the material surface is the oxidation of Fe and the transformation of the rust layer. In Evans model42, it is considered that
a carbon steel undergoes strong cathodic depolarization in the rust layer at the beginning of corrosion and then an anodic reaction in the rust layer. This process occurs at the metal/Fe3O4
interface: $${\mathrm{Fe}} \to {\mathrm{Fe}}^{2 + } + 2{\mathrm{e}}^ -$$ (1) The cathodic reaction occurs at the Fe3O4/FeOOH interface: $${\mathrm{Fe}}^{2 + } + 8{\mathrm{FeOOH}} +
2{\mathrm{e}} \to 3{\mathrm{Fe}}_3{\mathrm{O}}_4 + 4{\mathrm{H}}_2{\mathrm{O}}$$ (2) $$3{\mathrm{Fe}}_3{\mathrm{O}}_4 + 3/4{\mathrm{O}}_2 + 9/2{\mathrm{H}}_2{\mathrm{O}} \to
9{\mathrm{FeOOH}}$$ (3) It is considered that α-FeOOH is an insulating inactive substance, which is the most stable hydroxy iron oxide and a main constituent of the protective rust layer.
Tanaka et al.43 identified that Fe3O4 plays an important role in reducing the corrosion resistance of rust layers, while α-FeOOH and γ-FeOOH promote the formation of Fe3O4. Fe3O4 serves as a
conductor in the rust layer. Its conductivity is similar to that of pure metal. The electrons in the metal can be transferred by Fe3O4 as carriers. Therefore, when the rust layer contains a
large amount of Fe3O4, it does not provide a protective effect. Instead, Fe3O4 acts as an active point on the surface and provides carriers for the electrochemical process, thus promoting
the corrosion process of metals. Addition of Cr can have two effects on the alteration of low-carbon steel rust layer. On the one hand, Cr often exists in the form of Cr3+, which replaces
Fe3+ ions in FeOOH44. This breaks the balance process in Eq. (3) above, thus accelerating the conversion of Fe3O4 into FeOOH and promoting the formation of FeOOH (Fig. 5 and Table 1). To
validate this, the content of the rust layers of the samples after 168 h of the wet–dry cycles corrosion is plotted in Fig. 8 along with their Cr content. The figure shows that, as Cr
content increases, the content of Fe3O4 decreases continuously, while FeOOH increases. On the other hand, as a general passivation element, the addition of Cr will inevitably increase the
passivation tendency of the material’s surface, thereby suppressing the reaction process. In summary, Cr addition affects the passivation and rust layer structure of steels, thereby
suppressing the corrosion process. In the present work, the effect of Cr addition on the corrosion resistance of low-corrosion-resistance steels was investigated using KPFM and dry–wet cycle
corrosion testing. The following conclusions were drawn from the present study: * The corrosion resistance for the long-period atmospheric corrosion can be significantly improved when the
Cr content is >4%. For the low-carbon steel with 1–8% Cr content, the effect of Cr content on the atmospheric corrosion rate can be divided into three ranges: almost no effect <4%, a
negative correlation at 4–7%, and a step point at 8% content. * When the Cr content is <4%, it has almost no effect on the Fe3O4 content in the atmospheric corrosion rust layer. When the
Cr content is >4%, the Cr content can promote the conversion of Fe3O4 to FeOOH in the rust layer, and this conversion and Cr content present positive correlation. METHODS MATERIALS
PREPARATION Eight groups of experimental steel samples with different Cr contents marked as W1, W2, W3, W4, W5, W6, W7, and W8 were prepared. The compositions of these samples are shown in
Table 2. They were prepared by vacuum melting and then casting into round ingots, followed by 1-h homogenization at 1200 °C. The steel ingots were rolled into 6-mm-thick strips with a final
rolling temperature of approximately 900 °C, followed by water quenching to 520–540 °C and then air cooled to room temperature. In order to eliminate internal stress from the rolling
process, the samples were normalized at 850 °C for 0.5 h. The detailed process is described in Supplementary Fig. 5. The final samples were cut into 10 mm × 10 mm × 3 mm. All the samples
were sanded to 2000#, polished, and etched with 4% nitric acid alcohol solution to observe the microstructure. WET–DRY CYCLIC CORROSION TEST The dry–wet cycle experiment is generally
considered as one of the more efficient methods for simulating atmospheric corrosion because of its similarity with the actual outdoor corrosion45,46. Each cycle of the wet/dry corrosion
test is 1 h, which consists of two stages: (1) immersing the specimens in a 0.5% NaCl solution with pH = 3.0 at 40 °C for 45 min and (2) drying the specimens with four heating lamps for 15
min. The relative humidity inside the testing container was maintained at 70% (±5%) during the experiment. The samples have a size of 50 mm × 25 mm × 3 mm, and each experimental group was
made of three samples. Prior to the experiments, all of the samples were processed as follows: sanding (to 1500#) → pure water cleaning → acetone degreasing → anhydrous ethanol dehydration →
24-h drying. The experimental periods were 24, 72, 120, and 168 h. For each sample, the initial weight was recorded as _W_0 (accurate to 0.0001 g), and the sample size was measured using a
Vernier caliper. After the experiment, all the samples were placed in an ultrasonic cleaner with a solution of 500 mL HCl + 500 mL H2O + 3.5 g hexamethylenetetramine at 25 °C for about 15
min to remove the corrosion product47. Then the samples were rinsed using deionized water, followed by anhydrous alcohol and dried in cold air. The final masses _W_t were recorded (accurate
to 0.0001 g). Meanwhile, an untreated sample also went through the same processes for each steel to calibrate the mass loss produced during the process of removing corrosion products. The
corrosion rates (_R_, mm y−1) were calculated as follows: $$R = 8.76 \times 10^4\frac{{W_0 - W_{\mathrm{t}}}}{{S\rho t}},$$ (4) where (_W_0 − _W_t) represents the weight loss of the sample
after the dry–wet cyclic corrosion test, measured in grams; _S_ represents the exposed area of the sample and is in cm2; _ρ_ represents the sample’s density and was taken as 7.8 g cm−3; and
_t_ is the test time in years. CORROSION MORPHOLOGY AND RUST ANALYSIS Surface and cross-sectional morphologies of the samples were observed using a KEYENCE VX-200 stereomicroscope and a FEI
Quanta250 SEM. Their rust layers were analyzed with an EDS. The phases in the corrosion products were determined by XRD on a Rigaku analyzer over a 10–90° range with a scanning rate of 4 °
min−1. The parameters of the generator were set to 40 kV and 40 mA. ELECTROCHEMICAL TEST Electrochemical tests were performed using a M2273 electrochemical workstation and a three-electrode
system, where the samples were used as the working electrodes, a Pt plate as the counter electrode, and a saturated calomel electrode (SCE) as the reference electrode. The test solution was
0.5% NaCl (pH = 3). The samples were cut to 10 mm × 10 mm × 3 mm in size and were then covered with epoxy resin with a 1.0-cm2 exposed area. Prior to each test, the open circuit potential
(OCP) was monitored for at least 20 min until it reached a stable state. Potentiodynamic polarization tests ranged from −0.25 V (versus OCP) to −0.3 VSCE, and the scanning rate was 0.5 mV
s−1. All of the measurements were performed at room temperature (∼25 °C) and were repeated three times to ensure reproducibility. KPFM TEST Atomic force microscope (Multimode 8, Bruker) is
used in the KPFM measurements. The equipment was located in a clean room at a constant temperature of 25 °C and an ambient relative humidity of approximately 20%. The probes used in the KPFM
measurements were PFQNE-AL with a force constant of 1.5 N m−1 and a resonant frequency of 300 kHz. The dual-scan mode was used to record the second signal in addition to the surface
topographic signal. In a KPFM measurement, when a potential difference exists between the tip and the sample, an AC voltage is applied to the tip to induce harmonic oscillation of the
cantilever. Afterwards, a voltage of the same magnitude is applied to the tip to stop the oscillation and reduce the potential difference. Using this method, the work function of the sample
can be derived. Some studies have shown that the work function is closely related to the corrosion potential. The lower the work function of a material, the lower the corrosion potential and
the easier the corrosion48,49. DATA AVAILABILITY The data of this study are available from the corresponding authors upon reasonable request. REFERENCES * Cui, Z. et al. Passivation
behavior and surface chemistry of 2507 super duplex stainless steel in artificial seawater: influence of dissolved oxygen and pH. _Corros. Sci._ 150, 218–234 (2019). Article CAS Google
Scholar * Yao, J., Macdonald, D. D. & Dong, C. Passive film on 2205 duplex stainless steel studied by photo-electrochemistry and ARXPS methods. _Corros. Sci._ 146, 221–232 (2019).
Article CAS Google Scholar * Liu, M., Cheng, X., Li, X., Pan, Y. & Li, J. Effect of Cr on the passive film formation mechanism of steel rebar in saturated calcium hydroxide solution.
_Appl. Surf. Sci._ 389, 1182–1191 (2016). Article CAS Google Scholar * Tewary, N. K., Kundu, A., Nandi, R., Saha, J. & Ghosh, S. Microstructural characterisation and corrosion
performance of old railway girder bridge steel and modern weathering structural steel. _Corros. Sci._ 113, 57–63 (2016). Article CAS Google Scholar * Hao, L., Zhang, S., Dong, J. &
Ke, W. Atmospheric corrosion resistance of MnCuP weathering steel in simulated environments. _Corros. Sci._ 53, 4187–4192 (2011). Article CAS Google Scholar * Cheng, X., Jin, Z., Liu, M.
& Li, X. Optimizing the nickel content in weathering steels to enhance their corrosion resistance in acidic atmospheres. _Corros. Sci._ 115, 135–142 (2017). Article CAS Google Scholar
* Thee, C. et al. Atmospheric corrosion monitoring of a weathering steel under an electrolyte film in cyclic wet-dry condition. _Corros. Sci._ 78, 130–137 (2014). Article CAS Google
Scholar * Morcillo, M., Chico, B., Díaz, I., Cano, H. & De la Fuente, D. Atmospheric corrosion data of weathering steels. A review. _Corros. Sci._ 77, 6–24 (2013). Article CAS Google
Scholar * Li, X. et al. Share corrosion data. _Nature_ 527, 441–442 (2015). Article CAS Google Scholar * Fu, G., Zhu, M. & Gao, X. Rust layer formed on low carbon weathering steels
with different Mn, Ni contents in environment containing chloride ions. _Mater. Sci._ 22, 501–505 (2016). Google Scholar * Zhou, Y., Chen, J., Xu, Y. & Liu, Z. Effects of Cr, Ni and Cu
on the corrosion behavior of low carbon microalloying steel in a CF-containing environment. _J. Mater. Sci. Technol._ 29, 168–174 (2013). Article CAS Google Scholar * Cheng, X., Tian, Y.,
Li, X. & Zhou, C. Corrosion behavior of nickel‐containing weathering steel in simulated marine atmospheric environment. _Mater. Corros._ 65, 1033–1037 (2014). Article CAS Google
Scholar * Wu, W., Cheng, X., Hou, H., Liu, B. & Li, X. Insight into the product film formed on Ni-advanced weathering steel in a tropical marine atmosphere. _Appl. Surf. Sci._ 436,
80–89 (2018). Article CAS Google Scholar * Morcillo, M., Díaz, I., Cano, H., Chico, B. & De, lF. D. Atmospheric corrosion of weathering steels. Overview for engineers. Part I: Basic
concepts. _Constr. Build. Mater._ 213, 723–737 (2019). Article Google Scholar * Cano, H. et al. Characterization of corrosion products formed on Ni 2.4wt%-Cu 0.5wt%-Cr 0.5wt% weathering
steel exposed in marine atmospheres. _Corros. Sci._ 87, 438–451 (2014). Article CAS Google Scholar * Ma, Y., Li, Y. & Wang, F. Weatherability of 09CuPCrNi steel in a tropical marine
environment. _Corros. Sci._ 51, 1725–1732 (2009). Article CAS Google Scholar * Song, L., Chen, Z. & Hou, B. The role of the photovoltaic effect of γ-FeOOH and β-FeOOH on the corrosion
of 09CuPCrNi weathering steel under visible light. _Corros. Sci._ 93, 191–200 (2015). Article CAS Google Scholar * Yamashita, M., Miyuki, H., Matsuda, Y., Nagano, H. & Misawa, T. The
long term growth of the protective rust layer formed on weathering steel by atmospheric corrosion during a quarter of a century. _Corros. Sci._ 36, 283–299 (1994). Article CAS Google
Scholar * Díaz, I. et al. Five-year atmospheric corrosion of Cu, Cr and Ni weathering steels in a wide range of environments. _Corros. Sci._ 141, 146–157 (2018). Article CAS Google
Scholar * Kamimura, T., Hara, S., Miyuki, H., Yamashita, M. & Uchida, H. Composition and protective ability of rust layer formed on weathering steel exposed to various environments.
_Corros. Sci._ 48, 2799–2812 (2006). Article CAS Google Scholar * Kamimura, T. & Stratmann, M. The influence of chromium on the atmospheric corrosion of steel. _Corros. Sci._ 43,
429–447 (2001). Article CAS Google Scholar * Larrabee, C.P. & Coburn, S. k. The atmospheric corrosion of steels as influenced by changes in chemical composition, Proc. 1st
International Congress on Metallic Corrosion, London, 279–285 (1961). * Nishimura, T., Katayama, H., Noda, K. & Kodama, T. Effect of Co and Ni on the corrosion behavior of low alloy
steels in wet/dry environments. _Corros. Sci._ 42, 1611–1621 (2000). Article CAS Google Scholar * Wang, B., Xu, L., Zhu, J., Xiao, H. & Lu, M. Observation and analysis of
pseudopassive film on 6.5%Cr steel in CO2 corrosion environment. _Corros. Sci._ 111, 711–719 (2016). Article CAS Google Scholar * Zhu, J. et al. Essential criterion for evaluating the
corrosion resistance of 3Cr steel in CO2 environments: prepassivation. _Corros. Sci._ 93, 336–340 (2015). Article CAS Google Scholar * Jiang, S., Chai, F., Su, H. & Yang, C. Influence
of chromium on the flow-accelerated corrosion behavior of low alloy steels in 3.5% NaCl solution. _Corros. Sci._ 123, 217–227 (2017). Article CAS Google Scholar * Yamashita, M., Shimizu,
T., Konishi, H., Mizuki, J. & Uchida, H. Structure and protective performance of atmospheric corrosion product of Fe-Cr alloy film analyzed by Mssbauer spectroscopy and with synchrotron
radiation X-rays. _Corros. Sci._ 45, 381–394 (2003). Article Google Scholar * Qian, Y., Ma, C., Niu, D., Xu, J. & Li, M. Influence of alloyed chromium on the atmospheric corrosion
resistance of weathering steels. _Corros. Sci._ 74, 424–429 (2013). Article CAS Google Scholar * Qian, Y., Niu, D., Xu, J. & Li, M. The influence of chromium content on the
electrochemical behavior of weathering steels. _Corros. Sci._ 71, 72–77 (2013). Article CAS Google Scholar * Guo, S., Xu, L., Zhang, L., Chang, W. & Lu, M. Corrosion of alloy steels
containing 2% chromium in CO2 environments. _Corros. Sci._ 63, 246–258 (2012). Article CAS Google Scholar * Morcillo, M., Diaz, I., Chico, B., Cano, H. & Fuente, D. D. L. Weathering
steels: from empirical development to scientific design. A review. _Corros. Sci._ 83, 6–31 (2014). Article CAS Google Scholar * Xu, L., Wang, B., Zhu, J., Li, W. & Zheng, Z. Effect of
Cr content on the corrosion performance of low-Cr alloy steel in a CO2 environment. _Appl. Surf. Sci._ 379, 39–46 (2016). Article CAS Google Scholar * Tahara, A. & Shinohara, T.
Influence of the alloy element on corrosion morphology of the low alloy steels exposed to the atmospheric environments. _Corros. Sci._ 47, 2589–2598 (2005). Article CAS Google Scholar *
Wang, C., Wang, T., Cao, L. & Zhang, G. The effect of phase structure on the corrosion behavior of Al100-xMox alloy thin films. _J. Alloy. Compd._ 790, 563–571 (2019). Article CAS
Google Scholar * Cano, H. Atmospheric corrosion of weathering steels. Overview for engineers. Part II: Testing, inspection, maintenance. _Constr. Build. Mater._ 222, 750–765 (2019). Article
Google Scholar * Gong, W. et al. Effects of ausforming temperature on bainite transformation, microstructure and variant selection in nanobainite steel. _Acta Mater._ 61, 4142–4154
(2013). Article CAS Google Scholar * Uhlig, H. H. & King, C. Corrosion and corrosion control. _J. Electrochem. Soc._ 119, 327C (1972). Article Google Scholar * Ha, H. Y., Lee, T.
H., Lee, C. G. & Yoon, H. Understanding the relation between pitting corrosion resistance and phase fraction of S32101 duplex stainless steel. _Corros. Sci._ 149, 226–235 (2019). Article
CAS Google Scholar * Zhang, Y., Huang, F., Hu, Q., Peng, Z. & Liu, J. Effect of micro-phase electrochemical activity on the initial corrosion dynamics of weathering steel. _Mater.
Chem. Phys._ 241, 122045 (2020). Article CAS Google Scholar * Tamura, H. The role of rusts in corrosion and corrosion protection of iron and steel. _Corros. Sci._ 50, 1872–1883 (2008).
Article CAS Google Scholar * Rocca, E., Faiz, H., Dillmann, P., Neff, D. & Mirambet, F. Electrochemical behavior of thick rust layers on steel artefact: mechanism of corrosion
inhibition. _Electrochim. Acta_ 316, 219–227 (2019). Article CAS Google Scholar * Evans, U. R. & Taylor, C. A. J. Mechanism of atmospheric rusting. _Corros. Sci._ 12, 227–246 (1972).
Article CAS Google Scholar * Tanaka, H., Mishima, R., Hatanaka, N., Ishikawa, T. & Nakayama, T. Formation of magnetite rust particles by reacting iron powder with artificial α-, β-
and γ-FeOOH in aqueous media. _Corros. Sci._ 78, 384–387 (2014). Article CAS Google Scholar * Mancio, M., Kusinski, G., Monteiro, P. J. M. & Devine, T. M. Electrochemical and in-situ
SERS study of passive film characteristics and corrosion performance of 9%Cr microcomposite steel in highly alkaline environments. _J. ASTM Int._ 6, 1–10 (2009). Article Google Scholar *
Lyon, S. B., Thompson, G. E. & Johnson, J. B. Accelerated atmospheric corrosion testing using a cyclic wet/dry exposure test: aluminum, galvanized steel, and steel. _Corrosion_ 43,
719–726 (1987). Article CAS Google Scholar * Zhu, F., Persson, D. & Thierry, D. Formation of corrosion products on open and confined metal surfaces exposed to periodic wet/dry
conditions-a comparison between zinc and electrogalvanized steel. _Corrosion_ 57, 582–590 (2001). Article CAS Google Scholar * British Standards Institute Staff. _ISO 8407. Corrosion of
Metals and Alloys. Removal of Corrosion Products from Corrosion Test Specimens_ (B S I Standards, 2009). * Lacroix, L., Laurence, R., Christine, B. & Georges, M. Combination of AFM,
SKPFM, and SIMS to study the corrosion behavior of S-phase particles in AA2024-T351. _J. Electrochem. Soc._ 155, C131 (2008). Article CAS Google Scholar * Li, M., Guo, L. Q., Qiao, L. J.
& Bai, Y. The mechanism of hydrogen-induced pitting corrosion in duplex stainless steel studied by SKPFM. _Corros. Sci._ 60, 76–81 (2012). Article CAS Google Scholar Download
references ACKNOWLEDGEMENTS The authors acknowledge the support of the National Natural Science Foundation of China (No. 51671028) and the National Key Research and Development Program of
China (No. 2016YFB0300604) AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Corrosion and Protection Centre, University of Science and Technology Beijing, 100083, Beijing, China Baozhuang Sun,
Xiaomei Zuo, Xuequn Cheng & Xiaogang Li * Key Laboratory for Corrosion and Protection (MOE), 100083, Beijing, China Xuequn Cheng & Xiaogang Li Authors * Baozhuang Sun View author
publications You can also search for this author inPubMed Google Scholar * Xiaomei Zuo View author publications You can also search for this author inPubMed Google Scholar * Xuequn Cheng
View author publications You can also search for this author inPubMed Google Scholar * Xiaogang Li View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS X.C. and X.Z. conceived the project and designed the experiments. B.S. and X.Z. performed the experiments and analyses. B.S. wrote the paper. X.L. and X.C. revised the paper.
All the authors contributed to the interpretation of the experimental data and discussed the results. CORRESPONDING AUTHORS Correspondence to Xuequn Cheng or Xiaogang Li. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published
maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the
source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative
Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Sun, B., Zuo, X., Cheng, X. _et al._ The role of chromium content in the long-term
atmospheric corrosion process. _npj Mater Degrad_ 4, 37 (2020). https://doi.org/10.1038/s41529-020-00142-5 Download citation * Received: 15 June 2020 * Accepted: 19 October 2020 * Published:
17 November 2020 * DOI: https://doi.org/10.1038/s41529-020-00142-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative