Play all audios:
ABSTRACT To improve the corrosion resistance of TiNi alloy fabricated by laser powder bed fusion (LPBF), a porous oxidation layer was synthesized by micro-arc oxidation in a sodium aluminate
and sodium silicate electrolyte. The influences of the applied voltage and the processing time on the morphology of oxidation layer were investigated, and the corrosion behavior of the
oxidation layer in artificial saliva was evaluated and compared with that of the as-fabricated LPBF alloy. The results indicate that, as increasing the applied voltage and the processing
time, the oxidation layer becomes uniform and integrated. The optimum parameters are with an applied voltage of 450 V and processing time of 40 min. The oxidation layer primarily contains
α-Al2O3 and consists of two layers, i.e., a thin, compact and uniform inner layer and a porous outer layer. The formation of stable α-Al2O3 phase in the coating and its almost non-porous
dense structure reduce the channels for corrosion ions to penetrate into the substrate through coating, thereby improving the corrosion resistance of TiNi alloy. SIMILAR CONTENT BEING VIEWED
BY OTHERS IMPROVING WEAR RESISTANCE OF PLASMA-SPRAYED CALCIA AND MAGNESIA-STABILIZED ZIRCONIA MIXED COATING: ROLES OF PHASE STABILITY AND MICROSTRUCTURE Article Open access 11 December 2020
INFLUENCE OF SILICON MORPHOLOGY ON DIRECT CURRENT PLASMA ELECTROLYTIC OXIDATION PROCESS IN ALSI10MG ALLOY PRODUCED WITH LASER POWDER BED FUSION Article Open access 22 August 2022 EFFECT OF
NICKEL-COATED CARBON NANOTUBES ON THE PREPARATION AND WEAR RESISTANCE OF MICROARC OXIDATION CERAMIC COATING ON ZL109 ALUMINUM ALLOY Article Open access 30 June 2022 INTRODUCTION TiNi alloys
possess shape memory or superelastic property, excellent mechanical property, and high damping property; therefore, they have been widely applied in the medical field1,2,3. Compared with
other developed new type metallic biomaterials, such as biodegradable magnesium and its alloys, TiNi alloys possess higher strength and multifunction, showing great potential in biomedical
applications4,5,6. In order to ensure the long-term safe application of an implant in the human body, implant materials are required to have excellent corrosion resistance and
biocompatibility7. For both properties, high corrosion resistance is one of the main prerequisites for good biocompatibility, as it minimizes the release of metal ions. The surface of TiNi
alloys is covered by a dense oxide film (TiO2) in human body fluids, and the properties of the oxide film determine its corrosion behavior. However, previous work indicated that this layer
of the spontaneously formed oxide film is insufficient to resist the cyclic and complex loads over long-term applications, and this may cause cracks and increase the amount of nickel ions
released from the newly exposed TiNi under the damaged titanium dioxide layer8. Excessive Ni ions can lead to DNA damage, inflammation, tissue death adjacent to TiNi implants9. The high Ni
content of TiNi possesses a potential threat to its safe application in vivo, so it is particularly important to understand its corrosion resistance performance and many studies have been
performed. For example, Li et al. investigated the anti-corrosion properties of Ti44Ni47Nb9 shape memory alloy in simulated body fluids including 0.9% NaCl. They found that the addition of
Nb lead to the formation of Nb2O5 in the passive film and improve the electrochemical corrosion behavior of TiNi alloy10. Zhang et al. reported that the TiNi alloy exhibited considerably
greater erosion resistance than 304 stainless steel by the recovery of the passive film formed on TiNi alloy11. Churakova et al.12 reported that the rate of the corrosion process for the
ultrafine-grained Ti49.0Ni51.0 alloy is much lower than the coarse-grained state because of the much dense passive film formed on ultrafine-grained samples. The reported results demonstrated
that the performance of the surface passivation film of the alloys is an important factor affecting its anti-dissolution performance. Usually, the healing of the damaged passivation film is
a difficult and relatively slow process. In order to ensure the safe use of TiNi alloys in corrosive environments, fabricating a protective coating on the alloy surface is a preferential
option13. Several surface treatment techniques have been developed for this purpose, including anodic oxidation14, ion implantation15 and micro-arc oxidation (MAO)16 etc. Among these
techniques, thin film layer formed on the substrate by anodic oxidation and ion implantation technique cannot effectively inhibit the release of nickel ions, and the bonding between the
coating and the substrate is poor14,15. MAO can manufacture oxide ceramic coatings on the surface of valve metals (e.g., aluminum, magnesium, zirconium, titanium, etc.) with excellent
properties17, such as high hardness, excellent bonding strength to the substrate, high wear and corrosion resistance. In addition, the surface of MAO produced oxide film is rough and porous,
which is conducive to cell adhesion, reproduction and growth16,18,19. However, the high Ni content of the isoatomic ratio in TiNi makes it impossible to form an anodic film on the surface
like conventional titanium alloys, which presents difficulties for its surface micro-arc oxidation coating20. During the past decades, endeavors have been made in fabricating micro-arc oxide
coatings on TiNi surfaces. Liu et al.21 used MAO to prepare ceramic coating on the NiTi alloy in a sodium aluminate solution and investigated the wear resistance and apatite-forming ability
of micro-arc oxide film layers. Sukuroglu et al.22,23 formed TiO2 coatings on TiNi surfaces, resulting in non-cytotoxic coatings and significantly superior corrosion resistance compared
with the uncoated samples. These pioneer studies indicated the possibility of forming the micro-arc oxidation coating on TiNi alloy surface to obtain improved wear and bioactivity of the
coatings. However, the influencing factors and the formation mechanism of the micro-arc oxidation coating are still not clear. Further investigation and understanding on TiNi micro-arc
oxidation are required to advance the development of TiNi micro-arc oxidation. It is known that laser powder bed fusion (LPBF) is a category of additive manufacturing (AM) technology that
employs a laser beam as the heat source to heat and melt the powder material, which constructs 3D parts in a layer-by-layer manner24,25,26. Due to the crucial use of TiNi alloys in the
medical field, TiNi alloys and their properties have attracted extensive attentions in additive manufacturing27,28. Owing to the rapid solidification and repeated heating in LPBF process,
the microstructure of LPBF TiNi samples is composed of many fine equiaxed grains. The passivation film formed on the LPBF TiNi sample surface in normal saline is more uniform and denser,
which results in better corrosion resistance compared to the wrought TiNi samples29. Since the MAO process is closely related to the surface morphology and compositions of the base
materials30,31,32,33,34,35, the micro-arc oxidation process for TiNi alloy fabricated by LPBF technique with unique microstructure may be different from those of wrought samples. However, so
far, no related studies have been reported on such a topic. It is known that Al2O3 is a kind of chemical bioinert ceramic, and the release of Al ions is very low, which has the great
potential to block the metallic ions from the substrate and improve the corrosion resistance of the metallic materials36. In addition, aluminum oxide ceramics have been widely used as
implant materials for bone joints, so they have good biocompatibility37. Therefore, in this work, the Al2O3 coating is fabricated on the surface of LPBF TiNi alloys using micro-arc oxidation
technology, with aim to investigate the influence of electrification time and voltage on the microstructure, and the electrochemical corrosion and ion release behavior of the coating formed
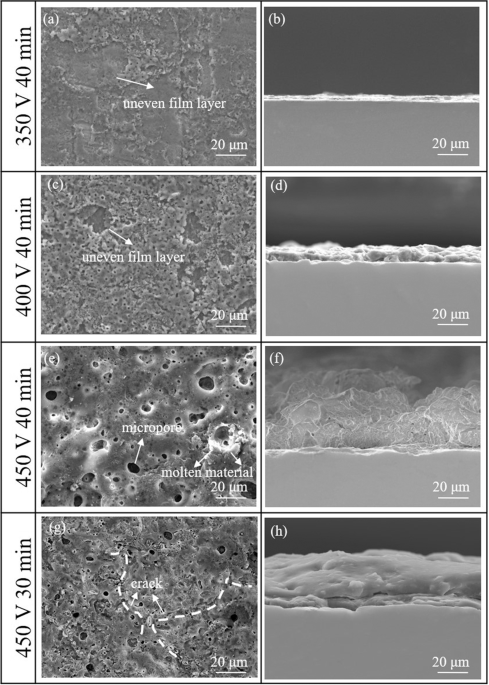
on the surface of TiNi alloys fabricated by LPBF. RESULTS SURFACE MORPHOLOGY OF THE MICRO-ARC-OXIDIZED SAMPLES Figure 1 shows the surface and transversal morphologies of LPBF TiNi samples
after micro-arc oxidation treatment at different times and voltages. It is shown that the discharge channels (i.e., surface micropores) are present on the surface of the film fabricated
under all parameters. The surface and transvers morphologies of the samples with low voltage (350 V) contain only a few micro-pores with a diameter of about 0.74 ± 0.22 μm and a film
thickness of about 0.69~2.05 μm (Fig. 1a, b). As the voltage rises (400 V), the number of micro-pores in the oxide layer increases, but the distribution is not uniform and the average
micropore size is 1.52 ± 0.80 μm (Fig. 1c); the layer thickness of the oxide layer is between 3.2 and 9.8 μm (Fig. 1d). When the voltage rises to 450 V, the average micropore size is 8.63 ±
2.99 μm (Fig. 1e) and there is a buildup of obvious small-sized molten material around it. The film thickness is between 22.5 and 35.6 μm (Fig. 1f). The oxide film and the substrate have a
good metallurgical bonding, and there are no large holes between the film and the substrate. These results indicated that the pore size in the surface and the thickness of the micro-arc
oxidation film of the LPBF samples gradually increase as increasing the voltage. This may be attributed to the two reasons as follows. The one is the increase in current density per unit
area of the film, which promotes the growth of the film. The other is the rise of voltage causing the film to be discharged by breakdown, which enhances the driving force for micro-arc
oxidation and film growth. Besides the oxidation voltage, the oxidation time may also influence the morphology of the layer. Due to the very lower thermal conductivity of the ceramic layer,
thermal stresses are generated by the temperature difference at various places on the surface of the coated sample during the shorter oxidation time, resulting in small cracks on the coating
surface (Fig. 1g, h). With the increase of voltage and oxidation time, the plasma discharge intensity on the surface of the sample and the sintering temperature of the coating increases,
which is beneficial to the metallurgical chemical reaction between the matrix and the coating interface, and improve the interface bonding strength. In addition, the 40 min-sample has a
larger pore size (8.63 ± 2.99 μm) and lower porosity (13.8%) than those of 30 min-sample (4.49 ± 0.98 μm, 16.1%). Longer oxidation time results in the increase in the plasma spark discharge
size, which leads to larger pores in the most outer part of the coating. However, the inner layer of the coating becomes more compact, which is attributed to the more complete hydrolysis and
deposition reactions of aluminate into the interior of coating. SURFACE COMPOSITION OF THE MICRO-ARC-OXIDIZED SAMPLES Figure 2 displays the EDS images of the micro-arc oxidation layer
fabricated by different processing parameters. The oxidized layers are mainly composed of Al, O, Ti and Ni. Small amounts of silicon and carbon are also detected. Numerical results of the
EDS analyses of micro-arc oxide coatings formed on LPBF TiNi alloy are shown in Table 1. The results show that with increase in the oxidation voltage and time, the contents of Al and Si
increase whereas the contents of Ti and Ni decrease, which indicates that the formation of alumina and silicon oxide is promoted by increasing the oxidation voltage and time. In addition,
when the voltage is lower, the element distribution on the layer surface is heterogeneous, with some Ti and Ni concentrations near the micro-pores (Fig. 2a, b). After the voltage reaches 450
V, the Ti and Ni contents increase near the micropores (Fig. 2c). Shorter oxidation time results in less effective film composition and poorer film quality (Fig. 2d). EDS analysis on the
cross-sections of the MAO samples indicates remarkably higher Al content than Si and Ti in the coating, slightly higher Ti content in the dense layer than the porous layer, but slightly
higher Al and Si contents in the porous layer than the dense layer (Fig. 3). The XRD results indicate that the oxide layer formed on the MAO samples is mainly composed of Al2O3, SiO2 and
rutile TiO2 (Fig. 4). Al2O3 ceramic coatings are mainly composed of α-Al2O3 and γ-Al2O3, where γ-Al2O3 is the main constituent phase with a face-centered cubic structure, and α-Al2O3 is a
rhombohedral hexahedron and a high-temperature stable phase. As increasing the voltage, the oxide layer becomes more crystalized, as evidenced by the α-Al2O3 diffraction peak at 2θ = 37.53°
as well as γ-Al2O3 and a small amount of SiO2 and TiO2 phases in 450 V sample compared to those in 350 V and 400 V samples. The contents of Al2O3 and TiO2 and SiO2 in 450 V sample analyzed
by XRD are 93.6%, 3.6% and 2.8% respectively, which suggests that the main composition in the coating is Al2O3. Shorter oxidation time leads to the lower crystallinity of Al2O3 in oxide
layer in 450 V 30 min sample. ELECTROCHEMICAL CORROSION BEHAVIOR The open-circuit potential curves of MAO coated and uncoated LPBF alloy in artificial saliva solution with a fluoride ion
concentration of 0.5% at pH 4 are presented in Fig. 5. At the beginning of the immersion of the uncoated LPBF sample in artificial saliva solution, the open-circuit potential (_E_ocp) shifts
in a positive direction, suggesting a protective oxide film formed on the electrode surface. Subsequently, significant fluctuations are observed in the uncoated LPBF sample _E_ocp, which is
attributed to the passivation film on the surface of the sample reacting with the solution. The _E_ocp of coated sample is less fluctuated and becomes stable completely at around −0.1 V
after approximately 1200 s, while the LPBF sample continuously shifts negatively and is stabilized at around −0.4 V at the end of the OCP test. The significantly higher _E_ocp of coated
samples demonstrates superior protection and corrosion resistance by oxide film on the surface of the sample. The results of the OCP tests imply that the passivation film formed
spontaneously on the surface of the TiNi sample is unstable, while the Al2O3 film fabricated by micro-arc oxidation on TiNi sample is effective in preventing corrosion reactions at high
fluoride ion concentrations and low pH of artificial saliva solution. Figure 6 shows the electrochemical impedance spectra of MAO coated and uncoated LPBF alloy in artificial saliva solution
at 37 ± 1 °C with a fluoride ion concentration of 0.5% at pH 4. According to the Nyquist diagram (Fig. 6a), the radius of capacitive arc resistance of the coated sample is evidently larger
than that of the uncoated samples. As displayed in the magnitude Bode plot (Fig. 6d), the coated sample has significantly larger impedance value than the pre-treatment sample throughout the
frequency variation range. In the low and medium frequency region, a curve with a slope of approximately −1 has an impedance modulus of 107 Ω cm2 order of magnitude at the lowest
frequencies. For the uncoated samples, there is a flat portion of the curve because of the solution resistance in the high frequency (102–105 Hz). In the middle-frequency ranges (100–102
Hz), a curve with a slope of −1 is observed. In addition, the modulus of impedance in low-frequency ranges maintains the order of magnitude of 104 Ω cm2. The variation of bode plot of the
phase angle with frequency also shows better passivation film stability on coated sample than on uncoated sample (Fig. 6e). From the viewpoint of EIS results, the maximum phase angle appears
in the low-frequency region (10−2–10−1 Hz) after oxidation, while the maximum phase angle appears in the medium frequency region (101–102 Hz) for the uncoated samples. The maximum phase
angle significantly shifts towards the low frequency after the micro-arc oxidation treatment, as its corresponding frequency range is widened. It has been reported that the relationship
between the maximum phase angle and frequency in the phase angle-frequency plot reflect the electrochemical reaction rate of the measured samples38,39. The closer the maximum phase angle
appears to the low frequency, the better the corrosion resistance of the sample. Thus, above results indicated the coated samples possessed good corrosion resistance than that of uncoated
samples. Equivalent electric circuits are available to describe the electrochemical corrosion behavior of coated and uncoated samples. The Rs (Q1R1) (Q2Rct) system can better simulate the
Al2O3 ceramic layer corrosion process (Fig. 6b). In the equivalent electric circuit, Rs represents the solution resistance; Q1 and R1 denote the capacitance of the porous layer surface/media
interface and porous layer resistance, respectively; Q2 refers to the capacitance of the compact layer surface/media interface; Rct is the charge transfer resistance. Due to the uneven and
rough nature of the layer, the capacitive element of the coating is represented by a Constant Phase Element (CPE), which accounts for the physical quantity of capacitance
deviation40,41,42,43. Table 2 summarizes the EIS fitting results. The EIS spectrum of LPBF TiNi sample was fitted using the Rs (QbRb) circuit (Fig. 6c) with the electrochemical data obtained
as shown in Table 3. In the equivalent circuit, Qb denotes the non-Faraday impedance, reflecting the performance of the electrode bilayer capacitance deviating from the ideal capacitance44;
Rb refers to the Faraday impedance, which represents the charge-transfer resistance associated with electrode processes. It reflects the ability of charge transfer at the interface between
the solution and the electrode. A smaller Rb value indicates easier charge transfer at the interface. For coated electrodes, Rct represents the difficulty of charge transfer at the interface
between the aggressive medium and the compact layer45. Rb ((2.585 ± 0.594) × 103 Ω cm2) for the LPBF sample represents the resistance of the passivation film comparable to the resistance of
porous layer of the MAO coated sample R1 ((7.42 ± 0.93) × 103 Ω cm2). However, the high resistance Rct of the compact layer in the coating indicates the dominant role of this compact layer
in increasing the denseness of the film. In addition, the polarization resistance (Rp) obtained from the following polarization tests (Table 4) reveals that the Rp of the LPBF sample is at
103 order of magnitude and the Rp of the coated sample is at 106 order of magnitude. This demonstrates that the presence of the compact layer within the coating effectively inhibits the
transfer of charge between the substrate and the corrosive solution, protecting the film layer and the substrate from corrosion. Figure 7 shows the potentiodynamic polarization results of
uncoated and MAO coated LPBF sample in artificial saliva solution at 37 ± 1 °C with a fluoride ion concentration of 0.5% at pH 4. Both samples have evident anodic and cathodic polarization
processes. The whole polarization curve of coated sample indicates a tendency of shifting towards the region of lower current density and the higher potential compared to those of uncoated
sample, which means an improvement of the corrosion resistance of Al2O3 coated sample. Because of the existence of a dissolution reaction in conjunction, the self-corrosion potential
(_E_corr) and self-corrosion current density (_I_corr) were determined by the cathodic Tafel extrapolation method46, as shown in Table 4. _E_corr of coated sample (−0.24 ± 0.02 V) is
slightly higher than that of the uncoated sample (−0.38 ± 0.01 V), indicating less tendency to corrosion. _I_corr is a kinetic parameter representing the corrosion velocity of the sample.
The _I_corr of coated sample ((1.78 ± 0.73) × 10−8 A cm−2) is almost three orders lower than that of uncoated sample ((2.11 ± 0.05) × 10−5 A cm−2), which indicates that micro-arc oxidation
treatment can significantly reduce the corrosion rate of LPBF samples. During the anodic polarization, the current density of the coated samples varies between 10−8 and 10−6 A cm−2, while
the current density of the uncoated samples is with the order of magnitude of 10−4 A cm−2, this further confirms that micro-arc oxidation treatment could significantly improve the corrosion
resistance of LPBF samples. IMMERSION EXPERIMENTS Coated and uncoated LPBF samples were immersed in artificial saliva solution with 0.5% F− at pH 4 for 28 days and the concentration of Ti
and Ni release from LPBF alloys was tested. The results are shown in Fig. 8 and the specific release concentration values are summarized in Table 5. The ion concentrations of Ti and Ni
precipitated from the coated and uncoated samples gradually increase with the increase in immersion time (Fig. 8). As measured by the slope of soaking time versus ion release, the coated
TiNi sample releases ions at much lower concentration and rate than the uncoated sample, which suggests that micro-arc oxidation of LPBF sample can effectively inhibit iron release. The
release of Ni ions is greater than that of Ti ions after immersion in the corrosive medium for both coated and uncoated LPBF samples, and such disparity is more evident with increase in
immersion time. It should be noted that the artificial saliva solution used in this work is much corrosive than that of human body solution, such as blood, tissue fluid etc., in which Ni
ions can be much more easily released47,48. Even in such corrosive solution, the Ni ion released from the coated LPBF TiNi alloy is 0.93 ± 0.03 mg L−1 in 24 h, which is much lower than the
range of 3–30 mg L−1 reported by Messer et al.49 that could alter cell metabolic functions. Since the Al2O3 is a kind of bioinert ceramic36,37, no Al ions were detected in this work.
CORROSION MORPHOLOGY Figure 9 shows the surface morphologies of the coated and uncoated LPBF samples after immersion in artificial saliva solution for 28 days. A small amount of micron
cracking is observed in coated TiNi sample (Fig. 9a). For the uncoated TiNi sample, some corrosion pits appear on the surface of the sample (Fig. 9b). The energy spectrum analysis of coated
LPBF samples after immersion shows that the main composition of the layer is Al2O3 and SiO2, while small amounts of Ti and Ni are distributed around the micro-pores (Fig. 10). Due to the
corrosion solution entering the pores and the damage to the film layer inside the pores, a small amount of Ti and Ni are released. Microcracks can also facilitate the transfer of ions.
DISCUSSION FORMATION MECHANISM OF THE MICRO-ARC OXIDE LAYER ON LPBF SAMPLE Micro-arc oxidation, also known as plasma electrolytic oxidation (PEO), is a surface treatment technology developed
based on anodic oxidation. It forms an oxide ceramic film in situ on the surface of the metallic materials through the instantaneous sintering effect of the high-temperature plasma
micro-region generated due to liquid phase discharge50. Usually, it is divided into three stages, i.e., (i) anodic oxidation stage, (ii) spark discharge stage, and (iii) arc discharge stage.
At the anodic oxidation stage (0–400 V, Fig. 11a), the substrate titanium alloys, may lose electrons and become ions, then combine with oxygen-containing anions in the aqueous solution to
form a TiO2 film51. As shown in Fig. 1a–d, the formed oxide film is very thin and hardly nonporous, which is near to the substrate and improve the bonding strength between the substate and
the MAO coating. $${\rm{Ti}}+2{{\rm{H}}}_{2}{\rm{O}}\to {{\rm{TiO}}}_{2}+4{{\rm{H}}}^{+}+4{{\rm{e}}}^{-}$$ (1) At the spark discharge stage (400–450 V, Fig. 11b, c), as the thickness of the
oxide film layer increases, the growth of the TiO2 oxide film is hindered. The elements in the electrolyte begin to enter the film layer and form new compounds. A large number of aluminate
ions and SiO32− aggregate on the surface of the anode, leading to the deposition of Al(OH)3, Al2SiO5 and SiO2 on the surface of the anode and causing the chemical reactions as follows52.
$${[{\rm{Al}}{({\rm{OH}})}_{4}]}^{-}{({{\rm{H}}}_{2}{\rm{O}})}_{{\rm{x}}}\to {\rm{Al}}{({\rm{OH}})}_{3}+{{\rm{OH}}}^{-}+{{\rm{XH}}}_{2}{\rm{O}}$$ (2) $${\rm{Al}}{{({\rm{OH}})}_{4}}^{-}\to
{\rm{Al}}{({\rm{OH}})}_{3}+{{\rm{OH}}}^{-}$$ (3) $${[{{\rm{Al}}}_{2}{\rm{O}}{({\rm{OH}})}_{6}]}^{2-}\to 2{\rm{Al}}{({\rm{OH}})}^{3}+{{\rm{O}}}^{2-}$$ (4)
$$2{{{\rm{SiO}}}_{3}}^{2-}-4{{\rm{e}}}^{-}\to 2{{\rm{SiO}}}_{2}+{{\rm{O}}}_{2}$$ (5) The above reactions lead to the gradually decrease of Al and Si and the increase in Ti from porous to
compact layer in the coating, which can be confirmed by the EDS profiles shown in Fig. 3. At the micro-arc discharge stage (~450 V, 40 min, Fig. 11d), the electrical breakdown occurs under
high temperature and high pressure, the Al(OH)3 colloidal deposit layer dehydrates under high temperature and forms Al2O3, and the substance and plasma eject and form a molten substance,
while rough and porous surface pits structure53. $$2{\rm{Al}}{({\rm{OH}})}_{3}\to {{\rm{Al}}}_{2}{{\rm{O}}}_{3}+3{{\rm{H}}}_{2}{\rm{O}}$$ (6) Therefore, the formed Al2O3 (ceramic film) is
uniform, and the micro-arc oxidation film layer is gradually formed in the dynamic balance of formation and dissolution. The micro-arc induces high temperature melts the oxide film
instantly, and Al(OH)3 first generates γ-Al2O3 under the action of the temperature and energy during the discharge process. When the temperature reaches 1150–1200 °C, a small amount is
converted into α-Al2O3. In this work, a high-quality micro-arc oxidation film is manufactured with an oxidation time of 40 min and a voltage of 400–450 V. EDS and XRD results indicated that
the Al2O3 is the main composition in the coating. In the course of microarc oxidation under constant voltage (450 V, 40 min), the electrochemical reaction driving force decreases and the
oxidation reaction of the titanium alloy almost stops, resulting in formation of a porous layer almost without TiO2. At this moment, the hydrolysis reaction mainly occurs with AlO2− and a
small amount of SiO32−. According to the potential pH diagram, AlO2− can completely transform into Al(OH)3 when the pH is below 9 and is further sintered at high temperature by plasma
discharge to form Al2O3. Thus, the XRD pattern shows a highly crystalline α and γ-Al2O3 phase due to the molten Al(OH)3 and a trace SiO2 phase because of the hydrolysis of SiO32−. For the
compact layer, it undergoes the oxidation reaction and hydrolysis reaction during the MAO process, where Ti dissolves and combines with O to form TiO2, accompanied by the formation of Al2O3
and SiO2. It should be mentioned that the properties of the oxide coating formed on the LPBF TiNi samples are quite different from those of forged samples using the same processing
parameters and electrolytes. For examples, for the MAO 450 V 40 min samples, the oxide coating on LPBF samples exhibit better bonding than that of forged sample54. These may be due to the
much fine equiaxed grains and rougher surface in LPBF samples. It was reported that the superfine crystallization of TiNi samples may result in a significant increase in crystal defects,
free energy, and diffusion capacity, which provides additional energy and diffusion channels for the nucleation and growth of the surface MAO coating55. Thus, the much fine grains in LPBF
may accelerate the MAO process and improve the performance of the surface coating modified layer and interfacial bonding. In addition, the surface roughness of the AM component is much
rougher than those of forged samples because of the partial melting of powder particles and sintering to the surface56, which may also contribute the better bonding strength of MAO
coatings57. The detailed comparison on the formation and properties of MAO coating between LPBF and forged samples needs to be further studied for their practical application. INFLUENCE OF
MICRO-ARC OXIDATION LAYER ON THE ION PRECIPITATION OF TINI ALLOYS Titanium and titanium alloys, in contact with acidic media containing fluoride ions, will precipitate metal ions and
accordingly result in allergic reactions58,59,60. As an implant material, the corrosion resistance of TiNi depends on the passivation film formed on the surface. The passivation film will
react in an acidic solution containing fluoride ions as follows61,62,63: $${{\rm{TiO}}}_{2}+6{{\rm{F}}}^{-}+4{{\rm{H}}}^{+}\to {[{{\rm{TiF}}}_{6}]}^{2-}+2{{\rm{H}}}_{2}{\rm{O}}$$ (7)
$${{\rm{TiO}}}_{2}+4{{\rm{F}}}^{-}+4{{\rm{H}}}^{+}\to {\rm{TiF}}4+2{{\rm{H}}}_{2}{\rm{O}}$$ (8) The titanium-fluorine complex ([TiF6]2−) in the reaction formula is a water-soluble molecule.
Its formation indicates that the TiNi matrix is corroded and a dissolution reaction occurs. At this time, Ti and Ni ions are precipitated into the medium. Ni is an essential trace element
for the human body64, and the intake of nickel in the human body is 200–300 ug d−1. Ni is involved in carbohydrate, amino acid and lipid metabolism65. TiNi undergoes electrochemical
corrosion in the oral environment and releases excessive Ni, which may cause toxicity, allergy, and potential carcinogenic effects7. In addition, Ni can be used as a cofactor for enzymatic
reactions to affect cellular morphology and proliferation64. However, the tolerance limit of Ni content in vivo remains elusive. Widely varying concentrations (1–100 mg L−1) and durations
(hours to months) of Ni ion release have also been reported in physiological or saline environments66. A range of 3–30 mg L−1 reported by Messer et al. could alter cell metabolic
functions49. Similarly, Bour et al. found a threshold value of 30 mg L−1 could trigger a cytotoxic response in vitro67. In the presence of fluoride with low pH, the release of Ti ions in the
titanium samples is increased, which further accelerates the dissolution effect68. The increasing release of Ti ions indicates the deterioration of the TiNi alloy surface film layer, which
leads to a simultaneous increase in Ni ion release. The heavy metal ions released from the alloy are likely the largest portion of cytotoxicity due to contact with metal alloy. To prevent
the release of metal ions, the surface modifications are widely discussed. The present study tested the difference in the release of ions between alloys with or without MAO coating. Even in
a more serious corrosive solution, the Ni ion released from the MAO-coated LPBF TiNi alloy is 0.93 ± 0.03 mg L−1 in 24 h, significantly lower than those from the uncoated samples, which is
also much lower than the reported that could cause cell toxicity67. After the micro-arc oxidation treatment is completed, the ion precipitation amount of TiNi is reduced, and the ion
precipitation rate is also slowed down. The above analysis demonstrates that the amount of Ni ions released from uncoated TiNi reaches the peak, and the natural oxide layer on the surface
cannot serve as a long-term barrier for Ni release; while the Al2O3 oxide layer formed on the surface of TiNi alloys by micro-arc oxidation technology can block the leachable Ni ions from
the substrate, and significantly improve the corrosion resistance of the alloys in the fluorine-containing acidic artificial saliva. It is noted that in order to shorten the corrosion test
time, the immersion environment used in this study is more corrosive (pH set to 4, added fluoride ion concentration of 0.5%) than those of human body fluid (HBF). In the less corrosive HBF
solution, the MAO oxidation layer is anticipated to exhibit much superior protection for TiNi samples69. In addition to the role of Al2O3 as a coating that blocks Ni release, it shows good
corrosion resistance as there were no Al ion release detected in this study. Al2O3 has been widely applied as dental and orthopedic biomaterials due to its biocompatibility. Feighan et al.
showed that Al2O3 blasted implants presented woven and lamellar bone in direct apposition to the implant surface, which was an evidence of active bone formation toward the implants70. In
addition, osteoblasts were able to proliferate and differentiate on the Al2O3 substrates evaluated in the long-term culture, suggesting that Al2O3 could not affect osteoblast behavior71. It
should be pointed that although the Al2O3 coating can significantly improve the corrosion resistance of the LPBF TiNi alloy, the pores on the surface of the coating may have detrimental
effect on the fatigue properties of the studied alloy72. Further studies need to be conducted to clarify this issue for the long-term safe application of MAO coated LPBF TiNi alloy. In this
work, Al2O3 ceramic oxide layers were fabricated on the surface of LPBF produced TiNi samples using the micro-arc oxidation technique, and their microstructure and corrosion behavior were
investigated. The results are summarized as follows: (1) The studied oxide films are composed of two layers: i.e., a thin, compact and uniform inner layer and a porous outer layer. The
thickness of oxide film increases with increasing the working voltage and the bonding between the coating and substrate become better. The optimum parameters are at a voltage of 450 V and a
time of 40 min. The oxide films mainly consist of Al2O3. (2) By the protection of the oxide layer, the attack of fluorine ions on the substrate is effectively hindered. The release of Ti and
Ni ions and the release rates of the LPBF samples are retarded. The corrosion resistance of the TiNi sample is significantly improved. The presence of the corrosion-resistant phase α-Al2O3
and the small porosity of the dense structure ensure the stability of the coating, thereby effectively preventing aggressive ion corrosion from contacting the substrate through the pores.
The results presented in this work prove the possibility of forming oxide layer on AM TiNi alloys by micro-arc oxidation treatment, which provides an avenue to improve the surface properties
(e.g., corrosion, wear and biocompatibility) for their special application. METHODS MATERIALS AND SAMPLE PREPARATION The TiNi parts with the dimensions of 10 × 10 × 10 mm3 were fabricated
in a Realizer LPBF 250 machine using TiNi pre-alloyed powder with particle size ranging from 15 to 60 μm. The chemical compositions obtained by wet chemical and gas analyses of TiNi powder
are: 55.5 wt% Ni, 0.0071 wt% Fe, 0.013 wt% C, 0.065 wt% O and balanced Ti. The laser power was 200 W, the diameter of focused spot was 40 μm, and the thickness of fixed powder layer and
scanning interval were 50 μm and 100 μm, respectively. The process of preparing Al2O3 coatings by micro-arc oxidation was performed with a 50 kW lab-made pulsed direct-current power source.
A plastic-vessel container was used as the electrolytic cell, and graphite and TiNi discs were used as the cathode and anode, respectively. Before micro-arc oxidation, the LPBF samples were
ground using 150-grit SiC paper. The electrolyte was prepared by mixing 1–3 g L−1 sodium silicate, 5–8 g L−1 sodium phosphate, 3 g L−1 sodium acetate, and 1 g L−1 sodium aluminate. A
stirring and cooling system was installed to maintain uniform concentration and temperature of electrolyte at about 35 °C and to deposit a uniform layer on the discs. Since the phase
transformation temperature from austenite (B2) to martensite (B19) in the LPBF TiNi is about 60 °C, the deposition process can not affect the shape memory effects of the TiNi alloy73. The
process was carried out at a voltage between 350 V and 450 V, and the oxidation time was set at 30~40 min. After the oxidation process, samples were rinsed with deionized water and dried in
cool air. The samples were divided into four groups with the following parameters, i.e., (i) voltage of 350 V and time of 40 min, (ii) voltage of 400 V and time of 40 min, (iii) voltage of
450 V and time of 40 min, and (iv) voltage of 450 V and time of 30 min. MORPHOLOGY AND ELECTROCHEMICAL TESTS The surface morphology and chemical composition of micro-arc oxidized (MAO)
samples were analyzed using a scanning electron microscope (SEM, JSM-6510A) equipped with energy dispersive spectrometry (EDS). Porosity and pore size of the MAO coatings were analyzed using
Image J and Image-Pro Plus software, respectively. A total of 150 micro-pore sizes were statistically counted for the pore size analysis. Some MAO samples were cut along the cross-section
by electric spark methods. The samples were ground with successive SiC papers from 57 to 10 μm and polished mechanically using colloidal silica suspension for SEM observations. All samples
were cleaned with anhydrous ethanol and distilled water then dried before characterization. The phase constitution was examined by X-ray diffraction (XRD, Buker D8 DISCOVER) with a Cu-Kα
radiation source. The XRD patterns were quantificationally analyzed using a TOPAS software. All electrochemical tests were carried out using a computer-controlled constant potential
instrument (PARSTAT 2273) for data acquisition. Saturated calomel (SCE) was used as the reference electrode using a conventional three-electrode system. A large platinum sheet with size of
15 × 20 × 0.5 mm3 and the sample served as the auxiliary electrode and the working electrode, respectively. Experiments were carried out in a 37 ± 1 °C water bath. The electrolytic for
electrochemical measurements was artificial saliva solution composed of (g L−1) 0.4 NaCl, 0.4 KCl, 0.69 NaH2PO4 ∙ 2H2O, 0.906 CaCl2 ∙ 2H2O, 0.005 Na2S ∙ 9H2O, and 1 CH4N2O (carbamide). The
pH was adjusted to 4 using 2% lactic acid, and NaF was added to the artificial saliva solution to bring its fluoride ion concentration to 0.5%. All potential values were reported with
reference to SCE. The open circuit potential (OCP) tests were recorded for 4500 s after the work electrode was put into the electrolyte. Subsequent electrochemical impedance spectra (EIS)
were measured at open circuit potential in a frequency range of 105 ~ 10−2 Hz. The spectra were interpreted utilizing ZsimpWin 3.10 software (EG & G, USA) as well as fitted to obtain a
suitable equivalent circuit. Dynamic potential polarization measurements were performed after each EIS test. Each sample was swept from a potential of −500 mV concerning OCP to 1600 mV at a
scan rate of 0.33 mV S−1. Five samples were used for each group of corrosion test. IMMERSION EXPERIMENTS In addition, coated and uncoated LPBF samples were immersed in 15 ml of the
artificial saliva solution at pH 4 and containing 0.5% F− at 37 ± 1 °C. The samples were hung vertically in the centrifuge tubes, ensuring that the sample surface was not in contact with the
wall or bottom of centrifuge tubes. The immersion tests were performed for 1, 7, 14, 21, and 28 days with fresh solution changed every 7 days. The concentrations of Ti and Ni elements
precipitated in the solution during the immersion process were measured using inductively-coupled plasma optical emission spectrometer (ICP-OES, iCAP7400). After immersion, the samples were
washed by deionized water and the corrosion morphology was examined using SEM. DATA AVAILABILITY Available from the corresponding author upon reasonable request. REFERENCES * Chern Lin, J.
H., Lo, S. J. & Ju, C. P. Biocorrosion study of titanium-nickel alloys. _J. Oral. Rehabil._ 23, 129–134 (1996). Article CAS Google Scholar * Sharma, N., Jangra, K. K. & Raj, T.
Fabrication of NiTi alloy: a review, proceedings of the institution of mechanical engineers. _Proc. Inst. Mech. Eng._ 232, 250–269 (2018). CAS Google Scholar * Miura, F., Mogi, M., Ohura,
Y. & Hamanaka, H. The super-elastic property of the Japanese NiTi alloy wire for use in orthodontics. _Am. J. Orthod._ 90, 1–10 (1986). Article CAS Google Scholar * Jayasathyakawin,
S. et al. Mechanical properties and applications of Magnesium alloy–Review. _Mater. Today. Proc._ 27, 909–913 (2020). Article CAS Google Scholar * Chen, J. et al. Mechanical properties of
magnesium alloys for medical application: a review. _J. Mech. Behav. Biomed. Mater._ 87, 68–79b (2018). Article CAS Google Scholar * Patel, S. K. et al. A review on NiTi alloys for
biomedical applications and their biocompatibility. _Mater. Today. Proc._ 33, 5548–5551 (2020). Article CAS Google Scholar * Veverkova, J. et al. Effect of Ni ion release on the cells in
contact with NiTi alloys. _Environ. Sci. Pollut. Res._ 27, 7934–7942 (2020). Article CAS Google Scholar * Aksoy, C. B., Canadinc, D. & Yagci, M. B. Assessment of Ni ion release from
TiTaHfNbZr high entropy alloy coated NiTi shape memory substrates in artificial saliva and gastric fluid. _Mater. Chem. Phys._ 236, 121802 (2019). Article CAS Google Scholar * Nasakina,
E. O., Sudarchikova, M. A., Sergienko, K. V., Konushkin, S. V. & Sevost’yanov, M. A. Ion release and surface characterization of nanostructured nitinol during long-term testing.
_Nanomaterials_ 9, 1569 (2019). Article CAS Google Scholar * Li, C., Zheng, Y. F. & Zhao, L. C. Electrochemical corrosion behavior of Ti44Ni47Nb9 alloy in simulated body fluids.
_Mater. Sci. Eng. A_ 438, 504–508 (2006). Article Google Scholar * Zhang, T. C. & Li, D. Y. An experimental study on the erosion behavior of pseudoelastic TiNi alloy in dry sand and in
aggressive media. _Mater. Sci. Eng. A_ 293, 208–214 (2000). Article Google Scholar * Kayumova, E. M., Churakova, A. & Latypov, O. R. Comparison of the corrosion behavior of the TiNi
alloy in the coarse-grained and ultrafine-grained state. _JPCS_ 2124, 012026 (2021). Google Scholar * Zhang, L. C., Chen, L. Y. & Wang, L. Surface modification of titanium and titanium
alloys: technologies, developments, and future interests. _Adv. Eng. Mater._ 22, 1901258 (2020). Article CAS Google Scholar * Ohtsu, N., Hirano, Y. & Takiguchi, K. Comparison of NiTi
alloy surfaces formed by anodization in nitric, phosphoric, and sulfuric acid electrolytes. _Surf. Coat. Technol._ 335, 306–313 (2018). Article CAS Google Scholar * Meisner, L. L. et al.
Effect of silicon, titanium, and zirconium ion implantation on NiTi biocompatibility. _Adv. Mater. Sci. Eng._ 2012, 706094.1–706094.16 (2012). Article Google Scholar * Xu, J. L., Liu, F.
& Luo, J. M. Microstructure and corrosion resistance of microarc oxidation coating on surface of biomedical nickel-titanium alloy. _Electroplat. Finish._ 30, 41–44 (2011). Google Scholar
* Wang, J. H., Wang, J., Lu, Y., Du, M. H. & Han, F. Z. Effects of single pulse energy on the properties of ceramic coating prepared by micro-arc oxidation on Ti alloy. _Appl. Surf.
Sci._ 324, 405–413 (2015). Article CAS Google Scholar * Wang, F., Hou, B., Yuan, K. & Wang, Y. Compactness of coatings treated by MAO and LSM on Ti alloy. _Emerg. Mater. Res._ 4,
265–272 (2015). Google Scholar * Lim, Y. W. et al. Enhanced cell integration to titanium alloy by surface treatment with microarc oxidation: a pilot study. _Clin. Orthop. Relat. Res._ 467,
2251–2258 (2009). Article Google Scholar * Cui, Y. & Khan, A. W. A case study: reliability-based product development and improvement model. _Qual. Reliab. Eng. Int._ 24, 361–373
(2008). Article Google Scholar * Liu, F., Xu, J., Wang, F., Zhao, L. & Shimizu, T. Biomimetic deposition of apatite coatings on micro-arc oxidation treated biomedical NiTi alloy.
_Surf. Coat. Technol._ 204, 3294–3299 (2010). Article CAS Google Scholar * Sukuroglu, E. E. et al. The effect of TiO2 coating on biological NiTi alloys after micro-arc oxidation treatment
for corrosion resistance. _Proc. Inst. Mech. Eng. H_ 231, 699–704 (2017). Article Google Scholar * Sukuroglu, S., Sukuroglu, E. E. D., Totik, Y. & Küükosman, R. Investigation of
in-vitro properties of NiTi alloy after micro arc oxidation. _J. Polytech._ 24, 373–382 (2021). Google Scholar * Chen, L. Y., Liang, S. X., Liu, Y. & Zhang, L. C. Additive manufacturing
of metallic lattice structures: unconstrained design, accurate fabrication, fascinated performances, and challenges. _Mater. Sci. Eng. R Rep._ 146, 100648 (2021). Article Google Scholar *
Li, S. J. et al. Compression fatigue behavior of Ti–6Al–4V mesh arrays fabricated by electron beam melting. _Acta Mater._ 60, 793–802 (2012). Article CAS Google Scholar * Shahrubudin,
N., Lee, T. C. & Ramlan, R. An overview on 3D printing technology: technological. _Mater. Appl. Procedia Manuf._ 35, 1286–1296 (2019). Google Scholar * Lin, X., Yang, H., Chen, J.,
Huang, W. & Yue, T. M. Microstructural evolution in laser rapid forming of a graded titanium-nickel alloy. in _26th International Congress on Applications of Lasers and Electro-Optics,
ICALEO 2007 - Congress Proceedings_ (Laser Institute of America, 2007). * Kondoh, K., Umeda, J., Soba, R. & Tanabe Y. _Titanium in Medical and Dental Applications_ 583–590 (Elsevier,
2018) * Jia, C. F. et al. Corrosion behavior of TiNi alloy fabricated by selective laser melting in simulated saliva. _Coatings_ 12, 840 (2022). Article CAS Google Scholar * Wang, H. Y.
et al. Effect of sandblasting intensity on microstructures and properties of pure titanium micro-arc oxidation coatings in an optimized composite technique. _Appl. Surf. Sci._ 292, 204–212
(2014). Article CAS Google Scholar * Dai, W. B. et al. The effect of surface roughness of the substrate on fatigue life of coated aluminum alloy by micro-arc oxidation. _J. Alloy. Compd._
765, 1018–1025 (2018). Article CAS Google Scholar * Fazel, M., Salimijazi, H. R., Golozar, M. A. & Garsivaz, M. R. A comparison of corrosion, tribocorrosion and electrochemical
impedance properties of pure Ti and Ti6Al4V alloy treated by micro-arc oxidation process. _Appl. Surf. Sci._ 324, 751–756 (2015). Article CAS Google Scholar * Maj, L. et al. Titania
coating formation on hydrostatically extruded pure titanium by micro-arc oxidation method. _J. Mater. Sci. Technol._ 111, 224–235 (2022). Article CAS Google Scholar * Yao, Z. Q. et al.
Synthesis and properties of hydroxyapatite-containing porous titania coating on ultrafine-grained titanium by micro-arc oxidation. _Acta Biomater._ 6, 2816–2825 (2010). Article CAS Google
Scholar * Samadianfard,, S. R., Seifzadeh, D. & Dikici, B. Application of g-C3N4/sol-gel nanocomposite on AM60B magnesium alloy and investigation of its properties. _Int. J. Miner.
Metall. Mater._ 30, 1113–1127 (2023). Article CAS Google Scholar * Marti, A. Inert bioceramics (Al2O3, ZrO2) for medical application. _Injury_ 31, 33–36 (2000). Article Google Scholar *
Rahmati, M. & Mozafari, M. Biocompatibility of alumina-based biomaterials—a review. _J. Cell Physiol._ 234, 3321–3335 (2019). Article CAS Google Scholar * Sekine, I., Sakaguchi, K.
& Yuasa, M. Estimation and prediction of degradation of coating films by frequency at maximum phase angle. _J. Coat. Tech._ 64, 45–49 (1992). CAS Google Scholar * Zuo, Y., Pang, R.,
Li, W., Xiong, J. P. & Tang, Y. M. The evaluation of coating performance by the variations of phase angles in middle and high frequency domains of EIS. _Corros. Sci._ 50, 3322–3328
(2008). Article CAS Google Scholar * Kaseem, M. & Choe, H. C. Synchronized improvements in the protective and bioactive properties of plasma-electrolyzed layers via cellulose
microcrystalline. _Acs. Biomater. Sci. Eng._ 9, 197–210 (2022). Article Google Scholar * Kaseem, M. et al. Guar gum-driven high-energy plasma electrolytic oxidation for concurrent
improvements in the electrochemical and catalytic properties of Ti-15 Zr alloy. _Surf. Interfaces_ 3, 102403 (2022). Article Google Scholar * Kaseem, M. & Choe, H. C. The effect of
in-situ reactive incorporation of MoOx on the corrosion behavior of Ti-6Al-4 V alloy coated via micro-arc oxidation coating. _Corros. Sci._ 192, 109764 (2021). Article CAS Google Scholar
* Kaseem, M. & Choe, H. C. Simultaneous improvement of corrosion resistance and bioactivity of a titanium alloy via wet and dry plasma treatments. _J. Alloy. Compd._ 851, 156840 (2021).
Article CAS Google Scholar * Touzain, S. Some comments on the use of the EIS phase angle to evaluate organic coating degradation. _Electrochim. Acta_ 55, 6190–6194 (2010). Article CAS
Google Scholar * Yao, Z. P., Jiang, Z. H., Xin, S. G., Sun, X. T. & W, X. H. Electrochemical impedance spectroscopy of ceramic coatings on Ti–6Al–4V by micro-plasma oxidation.
_Electrochim. Acta_ 50, 3273–3279 (2005). Article CAS Google Scholar * Bai, Y. et al. Improved corrosion behavior of electron beam melted Ti-6Al-4V alloy in phosphate buffered saline.
_Corros. Sci._ 123, 289–296 (2017). Article CAS Google Scholar * Huang, H. H. et al. Ion release from NiTi orthodontic wires in artificial saliva with various acidities. _Biomaterials_
24, 3585–3592 (2003). Article CAS Google Scholar * Katic, V. et al. Effect of pH, fluoride and hydrofluoric acid concentration on ion release from NiTi wires with various coatings. _Dent.
Mater._ 36, 149–156 (2017). Article CAS Google Scholar * Messer, R. L. & Lucas, L. C. Evaluations of metabolic activities as biocompatibility tools: a study of individual ions’
effects on fibroblasts. _Dent. Mater._ 15, 1–6 (1999). Article CAS Google Scholar * Lin, Z., Wang, T., Yu, X., Sun, X. & Yang, H. Functionalization treatment of micro-arc oxidation
coatings on magnesium alloys: a review. _J. Alloy. Compd._ 879, 160453 (2021). Article CAS Google Scholar * Velten, D. et al. Preparation of TiO2 layers on cp-Ti and Ti6Al4V by thermal
and anodic oxidation and by sol-gel coating techniques and their characterization. _J. Biomed. Mater. Res._ 59, 18–28 (2002). Article CAS Google Scholar * Liu, F. & Shimizu, T.
Effects of NaAlO2 concentrations on structure and characterization of micro-arc oxidation coatings formed on biomedical NiTi alloy. _J. Ceram. Soc. Jpn._ 118, 113–117 (2010). Article CAS
Google Scholar * Xu, J. L., Zhong, Z. C., Yu, D. Z., Liu, F. & Luo, J. M. Effect of micro-arc oxidation surface modification on the properties of the NiTi shape memory alloy. _J. Mater.
Sci. Mater. Med._ 23, 2839–2846 (2012). Article CAS Google Scholar * Jia, C. F. _Study on Microstructure and Corrosion Characteristics of TiNi Alloy Prepared by Selective Laser Melting_
(Jiamusi University, 2022). * Zhu, L. H. et al. Effects of micro-arc oxidation on surface properties of conventional and ultrafine-grained TiNi alloy. _Chin. J. Nonferr. Metal._ 24,
1014–1019 (2014). CAS Google Scholar * Zhao, X. L. et al. Comparison of the microstructures and mechanical properties of Ti–6Al–4V fabricated by selective laser melting and electron beam
melting. _Mater. Des._ 95, 21–31 (2016). Article CAS Google Scholar * Kajanek, D. et al. Impact of shot peening on corrosion performance of AZ31 magnesium alloy coated by PEO: comparison
with conventional surface pre-treatments. _Surf. Coat. Technol._ 446, 128773 (2022). Article CAS Google Scholar * Zigante, M. et al. Symptoms of titanium and nickel allergic sensitization
in orthodontic treatment. _Prog. Orthod._ 21, 17 (2020). Article Google Scholar * Koike, M. & Fujii, H. In vitro assessment of corrosive properties of titanium as a biomaterial. _J.
Oral. Rehabil._ 28, 540–548 (2001). Article CAS Google Scholar * Matomo, Y., Nakagawa, M., Mausuya, S., Ishikawa, K. & Terada, Y. Corrosion behavior of pure titanium and titanium
alloys in various concentrations of acidulated phosphate fluoride (APF) Solutions. _Dent. Mater._ 25, 104–112 (2006). Article Google Scholar * Mirjalili, M., Momeni, M., Ebrahimi, N. &
Moayed, M. H. Comparative study on corrosion behaviour of Nitinol and stainless steel orthodontic wires in simulated saliva solution in presence of fluoride ions. _Mater. Sci. Eng. C_ 33,
2084–2093 (2013). Article CAS Google Scholar * Kwon, Y. H., Cheon, Y. D., Seol, H. J., Lee, J. H. & Kim, H. I. Changes on NiTi orthodontic wired due to acidic fluoride solution.
_Dent. Mater._ 23, 557–565 (2004). Article CAS Google Scholar * Guo, J. & Mi, C. In vitro experimental study on the precipitation of titanium ions from four nickel-titanium
orthodontic arch wires. _Oral. Med. Res._ 31, 328–331 (2015). CAS Google Scholar * Aǧaoǧlu, G., Arun, T., Izgü, B. & Yarat, A. Nickel and chromium levels in the saliva and serum of
patients with fixed orthodontic appliances. _Angle Orthod._ 71, 375–379 (2001). Google Scholar * Denkhaus, E. & Salnikow, K. Nickel essentiality, toxicity, and carcinogenicity. _Crit.
Rev. Oncol. Hematol._ 42, 35–56 (2002). Article CAS Google Scholar * Shabalovskaya, S. A. et al. The influence of surface oxides on the distribution and release of nickel from nitinol
wires. _Biomaterials_ 30, 468–477 (2009). Article CAS Google Scholar * Bour, H., Nicolas, J. F., Garrigue, J. L., Demiden, A. & Schmitt, D. Establishment of nickel-specific T cell
lines from patients with allergic contact dermatitis: comparison of dierent protocols. _Clin. Immunol. Immunopathol._ 73, 142–145 (1994). Article CAS Google Scholar * Al-Mayouf, A. M.,
Al-Swayih, A. A., Al-Mobarak, N. A. & Al-Jabab, A. S. Corrosion behavior of a new titanium alloy for dental implant applications in fluoride media. _Mater. Chem. Phys._ 86, 320–329
(2004). Article CAS Google Scholar * Tao, X. J. et al. Synthesis of a porous oxide layer on a multifunctional biomedical titanium by micro-arc oxidation. _Mater. Sci. Eng. C_ 29,
1923–1930 (2009). Article CAS Google Scholar * Feighan, J. E., Goldberg, V. M., Davy, D., Parr, J. A. & Stevenson, S. The influence of surface-blasting on the incorporation of
titanium alloy implants in a rabbit intramedullary model. _J. Bone Jt. Surg._ 77-A, 1380–1395 (1995). Article Google Scholar * Sader, M. S., Balduino, A., Soares, G. A. & Borojevic, R.
Effect of three distinct treatments of titanium surface on osteoblast attachment, proliferation, and differentiation. _Clin. Oral. Implants Res._ 16, 667–675 (2005). Article Google Scholar
* Wen, L. et al. Influence of coating microstructure on fatigue performance of microarc oxidation coated LY12 alloy specimen. _Rare Met. Mater. Eng._ 38, 747–750 (2009). CAS Google
Scholar * Ren, D. C. et al. Microstructure and properties of equiatomic Ti-Ni alloy fabricated by selective laser melting. _Mater. Sci. Eng. A_ 771, 138286 (2020). Article Google Scholar
Download references ACKNOWLEDGEMENTS This work was supported by National Natural Science Foundation of China (82372379, 81902191, U2241245), Aeronautical Science Foundation of China
(2022Z053092001), CAS Interdisciplinary Innovation Team Project (JCTD-2020-10) and the Opening Project of National Key Laboratory of Shock Wave and Detonation Physics (2022JCJQLB05702).
AUTHOR INFORMATION Author notes * These authors contributed equally: Xin Zhao, Yicheng Liu. AUTHORS AND AFFILIATIONS * Department of Operation Room, The First Hospital of China Medical
University, Shenyang, 110001, China Xin Zhao * Department of Orthopedics, The First Hospital of China Medical University, Shenyang, 110001, China Yicheng Liu & Wei Yuan * Orthopaedic
Biomaterials Engineering Research Center of Liaoning Province, Shenyang, 110001, China Yicheng Liu & Wei Yuan * Beijing Chunlizhengda Medical Instruments Co., Ltd., No.10 Xinmi West
Second Road, Tongzhou District, Beijing, 101112, China Chenfan Jia * Shi-Changxu Innovation Center for Advanced Materials, Institute of Metal Research, Chinese Academy of Sciences, 62 Wencui
Road, Shenyang, 110016, China Hao Chang, Wei Zhang, Yun Bai & Shujun Li * School of Materials Science and Engineering, University of Science and Technology of China, No. 62 Wenhua Road,
Shenyang, 110016, China Hao Chang * School of Engineering, Edith Cowan University, 270 Joondalup Drive, Joondalup, Perth, WA, 6027, Australia Lai-Chang Zhang Authors * Xin Zhao View author
publications You can also search for this author inPubMed Google Scholar * Yicheng Liu View author publications You can also search for this author inPubMed Google Scholar * Chenfan Jia View
author publications You can also search for this author inPubMed Google Scholar * Hao Chang View author publications You can also search for this author inPubMed Google Scholar * Wei Zhang
View author publications You can also search for this author inPubMed Google Scholar * Yun Bai View author publications You can also search for this author inPubMed Google Scholar * Shujun
Li View author publications You can also search for this author inPubMed Google Scholar * Lai-Chang Zhang View author publications You can also search for this author inPubMed Google Scholar
* Wei Yuan View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS X.X.W.: Methodology, Data curation, Writing - original draft preparation.
Y.C.L.: Data curation, Writing - original draft preparation. X.Z. and Y.C.L. are co-first authors. C.F.J.: Methodology, Investigation. HC: Software, Data curation. WZ: Methodology, Software,
Supervision, Writing-review & editing. Y.B.: Methodology, Software, Investigation. SL: Conceptualization, Resources, Methodology, Data curation, Funding acquisition. L.C.Z.:
Visualization, Formal analysis, Writing-review & editing. W.Y.: Conceptualization, Funding acquisition, Writing-review & editing. CORRESPONDING AUTHORS Correspondence to Wei Zhang or
Wei Yuan. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons
license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zhao, X., Liu, Y., Jia, C. _et al._ Corrosion behavior of laser powder bed fusion
additive manufacturing produced TiNi alloy by micro-arc oxidation. _npj Mater Degrad_ 8, 13 (2024). https://doi.org/10.1038/s41529-024-00430-4 Download citation * Received: 13 October 2023 *
Accepted: 11 January 2024 * Published: 25 January 2024 * DOI: https://doi.org/10.1038/s41529-024-00430-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative