Play all audios:
ABSTRACT Transcranial direct current stimulation (tDCS) is a promising noninvasive intervention for Parkinson’s disease (PD). However, studies of its motor and cognitive effect have produced
mixed results. We conducted a systematic review including 38 studies and meta-analysis of 12 randomized sham-controlled trials with 263 PD patients. No significant differences were found
between active and sham tDCS in motor function (UPDRS-III: SMD = –0.14, p = 0.74), gait (SMD = 0.10, p = 0.513), attention and working memory (SMD = 0.24, p = 0.13), executive function (SMD
= 0.03, p = 0.854), and memory and learning (SMD: −0.07, p = 0.758). The prediction intervals indicated substantial heterogeneity among studies. Meta-regression showed small positive effects
in younger PD patients with milder symptoms. These findings are preliminary but suggest tDCS may benefit some PD patients while being neutral or harmful to others. SIMILAR CONTENT BEING
VIEWED BY OTHERS COMPARISON OF STIMULATION SITES ENHANCING DUAL-TASK PERFORMANCE USING TRANSCRANIAL DIRECT CURRENT STIMULATION IN PARKINSON’S DISEASE Article Open access 19 January 2025 A
SYSTEMATIC REVIEW AND META-ANALYSIS OF TRANSCRANIAL DIRECT-CURRENT STIMULATION EFFECTS ON COGNITIVE FUNCTION IN PATIENTS WITH ALZHEIMER’S DISEASE Article 03 February 2022 ASSESSMENT OF
NONINVASIVE BRAIN STIMULATION INTERVENTIONS IN PARKINSON’S DISEASE: A SYSTEMATIC REVIEW AND NETWORK META-ANALYSIS Article Open access 20 June 2024 INTRODUCTION Parkinson’s disease (PD) is a
progressive brain disease characterized by motor and non-motor symptoms1,2,3. In addition to motor symptoms such as tremor, bradykinesia, and rigidity, non-motor symptoms such as autonomic
dysfunction and cognitive deficits significantly affect a patient’s quality of life and even prognosis, in particular the cases of dementia4,5. Similar to motor symptoms, cognitive non-motor
symptoms vary individually, ranging from mild cognitive impairment to dementia, and may potentially be predicted by genetic or clinical features6. Although dopaminergic medications are
available that can help to alleviate motor symptoms in the initial stages of PD, these medications become less effective with illness progression and carry risk for serious adverse effects,
such as motor complications and hallucinations7. Furthermore, no interventions are available that can improve the cognitive symptoms of PD or halt further cognitive decline. As a result,
extensive research is underway to explore new or more advanced therapeutic interventions for PD8. One promising intervention is noninvasive brain stimulation, which includes transcranial
magnetic stimulation (TMS) and transcranial electrical stimulation (tES; encompassing both direct current, tDCS, and alternating current, tACS)9. TMS, first introduced by Barker et al. in
1985, employs pulsed magnetic fields to stimulate specific cortical regions, thereby modulating neural activity and influencing brain function10. The evolution of this technology led to the
development of repetitive TMS (rTMS), which allows for extended treatment sessions and has significantly enhanced the therapeutic potential of TMS in modulating brain activity11. With tES,
patients receive weak (1–2 mA) electric currents aimed at alleviating clinical symptoms by directly modulating brain activity. Although its precise mechanism of action remains elusive, pre
and clinical improvements following tES have been attributed to effects on cell membrane potentials, neurotrophic signaling, and long-term potentiation/depression-type neuroplasticity12. At
a macroscopic level, the clinical benefits of tDCS have been linked to its effects on cortical excitability13, while tACS has been associated with modulating brain oscillatory dynamics14,
and both improving large-scale motor and cognitive network functioning via corticostriatal circuit15. Compared to TMS, tES is relatively inexpensive, painless, easy to administer, and
portable9. Over the past 15 years, several randomized clinical trials (RCTs) and meta-analytic reviews have been published on motor and cognitive outcomes in patients with PD following tDCS
delivery. However, the results of different meta-analytic reviews have been mixed, probably in part due to use of different study inclusion criteria and data analytic approach. For example,
some meta-analytic reviews16,17 report that tDCS given to PD patients produced a significant improvement in overall motor function, as indexed by a reduced score on the Unified Parkinson’s
Disease Ranking Scale (UPDRS)-III, but other meta-analyzes report no significant differences18,19. On the other hand, meta-analytical reviews of cognitive outcomes of tDCS seem to have
yielded somewhat more consistent results, including a small but significant improvement on tests of working memory and attention, which was evident in not only PD patients but also patients
with other neurological or psychiatric disorders20. It appears, thus, that tDCS may exert beneficial effects on cognitive functioning in patients with PD while having no or only minor
effects on motor symptoms19. However, such a conclusion would be based primarily on point effect size estimates and corresponding confidence intervals (CIs) and p-values, which do not
provide the range of _true_ tDCS effects expected to be observed in similar studies and patients, such as in a next study or in a comparable real-life setting21,22. In contrast, the
prediction interval (PI) conveys this information. Unfortunately, the PI has not yet been utilized to capture variation in the true effect size across different populations of studies and
patients included in the meta-analysis. Thus, the PI could disclose large between-study heterogeneity of tDCS intervention effects, which may range from beneficial effects to zero effect in
some populations and, perhaps, even effects in the opposite direction of the summary point effect size estimate (i.e., detrimental effects) in other populations22. In this context, we
conducted a systematic review and meta-analysis of studies that utilized tDCS intervention for symptom relief in patients with PD. Our aim was to provide a better understanding of the
strength and heterogeneity of the effects of tDCS on patients’ motor and cognitive functioning, as measured by established clinical symptom rating scales or standardized motor and cognitive
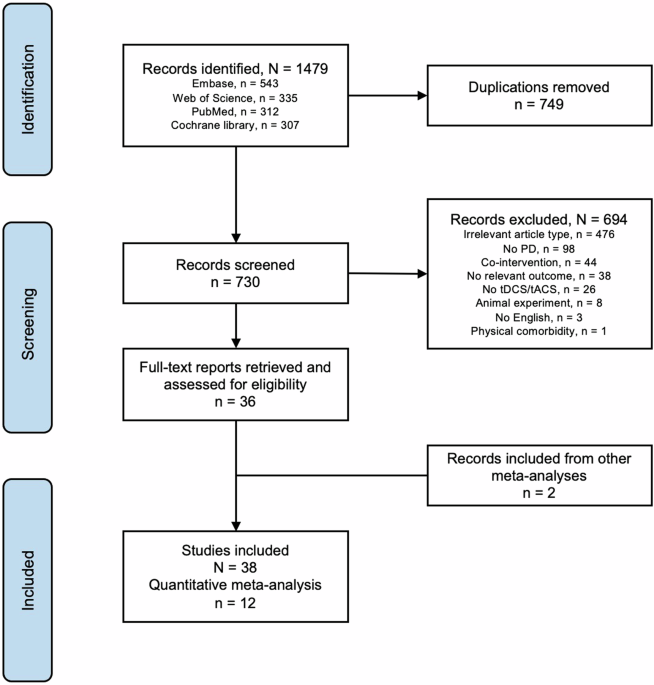
tests. RESULTS From the databases, we identified 1479 records (operators in Supplementary). After removing 749 duplicates, we retained 730 records for screening and selection (Fig. 1).
Thirty-eight studies examined tDCS effects in a total of 754 patients with PD (Table 1). Thirty-four of these studies were RCTs and 4 were non-RCTs, including observational studies. Twelve
of the 38 studies were included in the meta-analysis (7 using a parallel group and 5 a cross-over design), encompassing a total of 263 patients with PD [mean age: 65 years, range: 47–74;
mean illness duration: 8.3 years, range: 0.5 to 10 years; mean illness severity/disease stage measured by the Hoehn and Yahr (H-Y) scale: mean 2.2, range: 1.5–2.5]. One study measured
working memory in the “off” medication status, two studies regarding motor function examined both “on” and “off” medication status, and the rest were performed with patients “on” medication
(Table 1). Motor outcome measures in the 12 RCTs mainly included overall motor function as assessed by the UPDRS-III (_n_ = 5), and gait and balance (_n_ = 5). Cognitive outcome measures
primarily fell into the domains of attention and working memory (_n_ = 6), executive function (_n_ = 4), and memory and learning (_n_ = 2). Outcomes were measured immediately after one or
more sessions of tDCS, with some studies also including follow-up assessments a few weeks or months later (Table 1). QUALITY OF INCLUDED STUDIES Most studies showed a low risk of bias in the
randomization process (D1) and deviations from intended interventions (D2). There was more variability in the risk of bias due to missing outcome data (D3), with several studies showing
some concerns or high risk. The measurement of outcomes (D4) was mostly at low risk, while the selection of reported results (D5) presented some concerns across multiple studies. The highest
levels of bias were observed in the domains of missing outcome data and measurement of outcomes, with several studies showing high or some concerns in these areas. The details of the risk
of bias of all the included studies were available in Fig. 2a and summarized in Fig. 2b. MOTOR OUTCOMES Figure 3 presents forest plots illustrating the individual and weighted average effect
size estimates for the outcome measures of overall motor function (UPDRS-III score) (panel a) and gait and balance (panel b). UPDRS-III SCORE For the UPDRS-III score, Hedge’s g pooled
across 5 studies [77 patients after active tDCS, 76 patients after sham tDCS; target: premotor-M1 area + DLPFC (_k_ = 1), M1 + DLPFC (_k_ = 1), M1 + SMA (_k_ = 1), M1 alone and cerebellum
alone (_k_ = 1), M1 alone (_k_ = 1)] reached a value of −0.14 (95% CI: −1.23 to 0.95) (Fig. 3a). This result indicates that the mean UPDRS-III score tended to be slightly higher (toward
motor impairment) after active tDCS compared to sham tDCS, but the mean difference was not statistically significant (_t_ = −0.36, df = 4, _p_ = 0.737). However, considerable effect size
heterogeneity was evident (tau-squared = 0.64, I-squared = 0.84), along with a significant Q-test statistic (chi-square = 25.93, df = 4, _p_ < 0.001), rejecting the null hypothesis of
homogeneous true effect sizes across studies. Similarly, the 95% PI (−2.97 to 2.69) was substantially wider than the 95% CI. The I-squared index signified that up to 84% of the total
variance in observed tDCS effects on the UPDRS-III score reflected variance in true effects across studies rather than random sampling error. A sensitivity analysis indicated that the mean
effect estimate reached values of −0.48 (SE = 0.28, _k_ = 4) and 0.10 (SE = 0.42, _k_ = 4) when excluding the study displaying the largest positive effect (_g_ = 1.24, weight = 19%;
Simonette et al.) and the study with the largest negative effect (_g_ = −0.99, weight = 22%; Manor et al.), respectively. However, heterogeneity was still marked after excluding either of
these studies (tau-squared = 0.20 and 0.58, respectively). Consequently, random-effects meta-regression was used to identify sources of effect size heterogeneity. Five potential moderators
were considered: age, illness duration, illness stage, motor symptom severity at baseline, and the number of tDCS sessions. Two variables emerged as effect-modifiers: patients’ severity of
motor symptoms and age at baseline (Fig. 4). Higher UPDRS-III scores at baseline were associated with progressively less positive (i.e., more negative) effect sizes (Fig. 4a). Specifically,
as patients’ baseline motor symptom severity scores increased by 27 points (from score 13 to 40), the predicted effect size decreased by up to 156 points (1.56 SD units, from 0.53 to −1.03).
The meta-regression model with symptom severity at baseline as the single covariate explained more than half of the total between-study effect size heterogeneity (R-squared analogous =
59%), although significant residual heterogeneity remained (tau-squared = 0.26, I-squared = 0.64; chi-squared = 8.22, df = 3, _p_ = 0.042). However, we could not formally reject the null
hypothesis that the true value of the slope of the meta-regression prediction line was zero (baseline UPDRS-III score: beta = −0.06, _t_ = −2.20, df = 3, _p_ = 0.116, 95% CI: −0.14 to 0.03).
Similarly, increasing age at baseline was associated with progressively less positive (i.e., more negative) effect sizes (beta = −0.07, _t_ = −1.46, df = 3, _p_ = 0.240, 95% CI: −0.22 to
0.08, R-squared analogous = 25%). The predicted effect size decreased by a total of 149 points (1.49 SD units, from 0.78 to −0.71) across the study-level age range of 52 to 74 years (Fig.
4b). By contrast, meta-regression models including illness duration (range: 5.5 to 10.8 years, _k_ = 5), illness stage (range: 1.5 to 2.5 on H-Y scale, _k_ = 5), or number of stimulation
sessions (values: 1, 5, or 10 sessions, _k_ = 5) did not account for any heterogeneity variance (all three R-squared analogous values = 0%). GAIT AND BALANCE For gait and balance, effect
size estimates were obtained from 5 studies [79 patients 1after active tDCS, 77 patients after sham tDCS; target: premotor-M1 area + DLPFC (_k_ = 1), M1 + DLPFC (_k_ = 1), DLPFC (_k_ = 2),
M1 alone, DLPFC alone, and cerebellum alone (_k_ = 1)]. The mean intervention effect reached a value of 0.10 (_t_ = 0.72, df = 4, _p_ = 0.513), indicating a slight, but not significant,
improvement in patients’ timed TUG performance after active tDCS compared to sham tDCS (Fig. 3b). The 95% CI (−0.28 to 0.48) was wide, reflecting low precision of the mean effect estimate,
and heterogeneity appeared trivial (tau- and I-squared = 0). A sensitivity analysis indicated that the observed mean effect (_g_ = 0.10, SE = 0.14, _k_ = 5) showed modest changes when
excluding the study with the largest positive effect (_g_ = 0.35, weight = 9%; Wong et al., _g_ = 0.07, SE = 0.14, _k_ = 4) and the study with the largest negative effect (_g_ = −0.02,
weight = 22%; Bueno et al., _g_ = 0.13, SE = 0.16, _k_ = 4). The effect size estimate from Wong et al. was also characterized by poor precision, suggesting possible reporting bias.
Inspection of a funnel plot under the fixed-effect model suggested that reporting bias might have inflated the observed mean intervention effect, as effect estimates from studies with
similar precision were not symmetrically distributed around the mean effect size (Fig. 5a). Assuming neither heterogeneity nor chance caused the apparent funnel plot asymmetry, some studies
with relatively low precision seemed to be missing above the point of zero effect and in areas of small negative effects. A trim-and-fill analysis estimated the number of missing studies to
be 2, producing an adjusted fixed-effect point estimate of 0.05 (_k_ = 7, 5 observed and 2 imputed studies, _z_ = 0.38, _p_ = 0.706, 95% CI: −0.20 to 0.29). Thus, the small positive mean
effect size initially observed for gait measures (_g_ = 0.10, _k_ = 5) likely overestimates the real effect of active tDCS intervention. OTHER STUDIES OF MOTOR OUTCOMES Eight studies of tDCS
for PD not included in the meta-analysis also utilized the UPDRS-III score as an outcome measure (Table 1). Four out of the 8 studies (50%) found significant reductions in the UPDRS-III
after tDCS, mainly using M1 as the target of stimulation23,24,25,26. The other four studies (50%) did not find significant effects after tDCS applied to DLPFC27 or cerebellum28,29,30. Seven
studies examined gait and balance after tDCS (Table 1). Two out of these 7 studies (29%) found significant improvements in patients’ gait and balance scores after stimulating M124,31. Three
studies (43%) observed improvement only with specific settings or durations targeting M126, cerebellum32, or DLPFC33. Two studies (29%) did not report significant effects after stimulating
M123 or DLPFC27. Finally, three studies examined upper limb function in PD patients after tDCS to various targets: M1 (_n_ = 1), SMA (_n_ = 1), sensorimotor cortex (_n_ = 1), and DLPFC (_n_
= 1) (Table 1). One study (33%) found significant improvements after stimulating the sensorimotor cortex34, whereas the other two studies (66%) detected no significant differences following
tDCS after M1 or SMA27,35. COGNITIVE OUTCOMES Figure 6 presents forest plots illustrating the individual and weighted average effect size estimates for measures of attention and working
memory (panel a), executive function (panel b). ATTENTION AND WORKING MEMORY Hedges’ g for measures of attention and working memory, pooled across 7 studies [69 patients after active tDCS,
67 patients after sham tDCS; target: DLPFC (_k_ = 3), premotor-M1 area + DLPFC (_k_ = 1), M1 alone, DLPFC alone (_k_ = 2), cerebellum (_k_ = 1)], reached a value of 0.24 (_t_ = 1.77, _p_ =
0.128, 95% CI: −0.09 to 0.58) (Fig. 6a). This finding indicates that the patients’ level of attention/working memory performance after active tDCS was higher, though not significantly, than
the performance level after sham tDCS. No evidence was found that the true effect size varied across studies (tau- and I-squared = 0). A sensitivity analysis showed that, relative to the
initially observed mean effect size (_g_ = 0.24, _k_ = 7), the mean effect estimate reached a moderately lower value (_g_ = 0.18, 95% CI: −0.21 to 0.57, _k_ = 6) and a higher value (_g_ =
0.30, 95% CI: −0.08 to 0.67, _k_ = 6) when excluding the studies with the highest positive effect size (Hedges’ _g_ = 0.48, weight = 15%; Boggio et al.) and the largest negative effect size
(Hedges’ _g_ = −0.19, weight = 20%; Lau et al.), respectively. Despite the wide range of observed effect sizes, a funnel plot under the fixed-effect model demonstrated that all studies were
located within the expected triangular region, suggesting no heterogeneity or reporting bias (Fig. 5b). The individual effect size estimates were symmetrically scattered around the mean
fixed-effect size (_g_ = 0.24), further indicating the absence of heterogeneity and reporting bias. EXECUTIVE FUNCTION For measures of executive function, Hedges’ g pooled across 4 studies
[43 patients active tDCS, 43 patients sham tDCS; target: DLPFC (_k_ = 4)] reached a nonsignificant value of 0.03 (_t_ = 0.20, df = 3, _p_ = 0.854) (Fig. 6b). The margin of error for the
estimated mean effect size was large, with the 95% CI (−0.50 to 0.57) encompassing the null effect as well as small-to-medium negative and positive effects. No evidence of between-study
effect size heterogeneity (tau- and I-squared = 0) or reporting bias was found, as the effect estimates visualized in a funnel plot were symmetrically distributed around the mean effect
size. MEMORY AND LEARNING There were only 2 studies available for estimating the intervention effect on measures of memory and learning [22 patients active tDCS, 22 patients sham tDCS;
target: DLPFC (_k_ = 1), M1 alone, cerebellum alone (_k_ = 1)]. The weighted average effect size estimate was −0.07 (_t_ = −0.40, df = 1, _p_ = 0.758, 95% CI: −2.27 to 2.13), reflecting a
nonsignificant trend toward impaired memory/learning performance following active tDCS. The wide 95% CI indicated poor precision of the point estimate, suggesting that the intervention
effect in the population might actually be zero. OTHER STUDIES OF COGNITIVE OUTCOMES Four studies not included in the meta-analysis examined attention and working memory in PD patients
following tDCS to DLPFC (_n_ = 2) and M1 (_n_ = 2) (Table 1). Two studies (50%) observed improvements by targeting M1 with certain stimulation conditions or at one of the measuring time
points but not with others23,36, while the remaining two studies (50%) did not detect significant differences27,37. Seven studies examined executive functioning in PD patients following tDCS
to DLPFC (_n_ = 6), M1 (_n_ = 1), TPC (_n_ = 1), and MFC (_n_ = 1) (Table 1). One study (14%) reported significant improvements in tests of executive functioning after tDCS on MFC38. Four
studies (57%) observed improvements with certain stimulation conditions, measuring times, or in one of the examination tests but not others after stimulating DLPFC27,39,40,41 and TPC39. The
remaining two studies (29%) did not detect significant differences after stimulating DLPFC37,42 or M142. One study targeted DLPFC and measured visuospatial function but detected no
significant improvement after the stimulation27. DISCUSSION This meta-analytical review found that patients with PD who received tDCS intervention did not differ significantly from those who
received sham tDCS. The meta-regression model disclosed three possible types of tDCS effects: small positive effects (effect size of 0.32) in patients with mild motor symptoms (UPDRS-III
score at 13); null effects in patients with moderate symptoms (UPDRS-III scores 22–25); and small-to-medium negative effects (effect size of −0.48) in patients with severe symptoms
(UPDRS-III score of 40). A similar tendency was observed with age, showing a near null effect at 64 years, while illness duration, illness stage, or number of stimulation sessions did not
account for any heterogeneity variance. The findings suggest tDCS could potentially benefit younger patients with less severe symptoms. Additionally, results from other qualitative studies,
including observational studies, highlighted that tDCS on M1 might improve UPDRS score23,24,25,26 as well as gait and balance24,31. However, the evidence is not compelling due to large
variability and inconsistent effects within and across studies. The inconsistent findings were also reported in 3 studies provided long-term follow up including Benninger et al. reported
significant improvement in gait on the first day but no improvement after one month, while Valentino et al. observed consistent improvement up to four weeks. Failure to improve executive
function was noted in two studies from immediate post-stimulation to three-month follow-up27,43. Assuming that tDCS effects are specific to individual patients, focusing on inter- and
intra-individual differences such as genetic background or comorbid conditions could offer a personalized and potentially more fruitful approach than relying solely on group-averaged data
and summary effect size estimates. In particular, genetic polymorphisms (e.g. _LRRK2, PARK2, SNCA, DJ-1, COMT_ and _ALDH2_) or socio-cultural factors that vary across individuals or ethnic
groups may not only contribute to differences in symptom manifestation and drug efficacy but could also influence the therapeutic outcomes of neuromodulation44,45. To examine this
possibility in more detail, it would be useful if future studies routinely analyze and present individual patient data. Moreover, extensive research, particularly large-scale multicenter
trials with patient characteristic-based subgroup analyzes, is needed to identify and understand sources of between-study variability in tDCS effects, including clinical and methodological
differences such as patient sample characteristics, tDCS parameters and protocols, duration of follow-up, and outcome measurement. It seems plausible to assume that the large variability in
tDCS effects across studies and patients originates partly from the manner in which the electric current stimulation has been delivered to patients, posing significant technological and
methodological challenges. In this context, it may be informative to contrast tDCS with deep brain stimulation (DBS) targeting the globus pallidus interna or subthalamic nucleus, which has
been successfully applied to the management of severely disabled PD patients with medication-refractory motor symptoms and complications46. First, DBS is a neurosurgical implant that usually
delivers continuous and sustained electric stimulation over years, whereas tDCS is most commonly given only in a number of sessions occurring over days or weeks. Second, DBS stimulation
parameters, such as intensity, are carefully adjusted for individual patients during and after surgery to improve clinical benefits while minimizing side effects, whereas tDCS parameters are
typically fixed across the period of intervention and not personalized. Third, DBS is surgically implanted into a precisely targeted brain region using detailed anatomical information
obtained from individual MR images. In contrast, the spatial resolution of tDCS is severely limited by volume-conduction effects, which shunt, weaken, and distort scalp-applied electric
currents flowing from the stimulation electrode into the skin, subcutaneous tissue, skull, cerebrospinal fluid, and brain47,48. Additionally, tDCS is typically delivered via relatively large
stimulation electrodes, which may not always be positioned exactly across participants and studies49, inducing cortical activation patterns likely to extend well beyond the focal area of
interest50. Finally, the possibility should be considered that tDCS in its current form does not evoke any direct brain changes in human participants, which is not an issue of significant
concern in DBS. On this view, the transient motor and cognitive effects seen in human participants following tDCS could originate from indirect and nonspecific mechanisms, including placebo
effects51, differences in task strategy (e.g., speed-accuracy tradeoff), effects on comorbid mood symptoms, peripheral effects52, and heightened arousal and alertness53. Even with DBS
showing promise, improving cognitive function remains challenging and sometimes even has negative impacts54. Cognitive functions are difficult to modulate with unspecific stimulation
focusing on a single cortical target. In Parkinson’s disease, degeneration of multiple neurotransmitter systems, including dopaminergic, cholinergic, and noradrenergic systems, plays a
critical role in cognitive impairment55. Loss of the lateral dopaminergic system in frontal, parietal and temporal cortical regions were found in PD patient with cognitive decline56. The
extended interplay of these neurotransmitter systems as well as their networking effects make the cognitive function difficult to handle. Considering this, more targeted and precise brain
stimulation techniques are needed to effectively address cognitive decline. Yet, the assessment and reporting of adverse events following tDCS have not always received adequate consideration
in tDCS studies57, and unknown risks remain. However, to date, only transient and reversible side effects of tDCS have been reported, and not significant or enduring negative effects58.
Intervention in patients with PD remains unclear since the initial promising findings reported more than 15 years ago23,43. In fact, based on the present meta-analysis, as well as on prior
meta-analyzes, the null hypothesis that tDCS intervention has no genuine effect on patients’ gait and cognitive function can still not be rejected. The present results, however, should be
viewed as preliminary due to the limited number of studies and patients included. We also found some tentative evidence for funnel plot asymmetry, especially for cognitive outcomes where
null and negative findings appeared to be underrepresented, indicating the presence of publication bias. This may have inflated effect sizes, although other sources of funnel plot asymmetry,
such as chance, cannot be excluded, given the small number of studies included. Furthermore, statistical power of most studies is typically low due to small patient samples, making it
possible to detect only large tDCS effects, which may not be realistic to emerge, while the number of tests and intervention concealment and placebo effects remain issues of concern. In this
meta-analytic review, we found insufficient evidence for clinically significant effects of tDCS on motor and cognitive functions in patients with PD. Given the technological and
methodological challenges involved in noninvasive electric stimulation therapy, along with an incomplete understanding of volume-conduction effects, further research is required to identify
and understand sources of between-study variability in tDCS effects. Novel non-invasive methods like transcranial temporal interference stimulation could improve the accuracy of transcranial
electrical stimulation, deserving more focus for developing customized and potent therapies for Parkinson's disease59. METHODS SEARCH STRATEGY We conducted a comprehensive literature
search using the key terms “transcranial alternating current stimulation”, “tACS”, “transcranial direct current stimulation”, “tDCS”, “Parkinson*”, and “PD”. These terms were submitted to
the PubMed, Web of Science, Cochrane Library, and EMBASE databases up to April 29, 2024. This approach allowed us to capture diverse geographical and disciplinary perspectives, ensuring the
inclusion of high-quality, peer-reviewed content, therefore, enhancing the reliability and validity of our study. Additionally, we reviewed studies from relevant previous meta-analyzes to
expand our pool of potential records. SELECTION CRITERIA Two Authors independently screened the titles and abstracts of retrieved records to determine eligibility. Both RCTs and non-RCTs
were included if they met the following criteria: (1) involved patients with idiopathic PD in inpatient or outpatient settings; (2) used tDCS intervention; (3) for RCTs, compared active and
sham tDCS interventions using either a between-group or cross-over design; and (4) assessed clinical outcomes related to motor or cognitive function using established clinical rating scales
(e.g., UPDRS-III) or tests (e.g., digit span). Exclusion criteria were: studies that did not involve PD patients or included PD patients with physical or psychiatric comorbidities, or
received co-interventions such as TMS, physiotherapy, cognitive training, or antipsychotic medication (excluding antiparkinson medications). Studies not published in English, animal
research, and irrelevant article types (reviews, meta-analyzes, abstracts, and study protocols) were also excluded. Additionally, studies using physiological measures of motor or cognitive
outcomes following tDCS were excluded to maintain a focus on behavioral and clinical outcomes, which are directly relevant to the therapeutic efficacy of tDCS in PD. The exclusion of studies
employing physiological measures enhances the homogeneity of the included studies, facilitating a more accurate synthesis of the clinical impact of tDCS. tDCS studies with insufficient
quantitative data for effect size calculation underwent qualitative analysis. Finally, tACS studies were excluded due to the low number of studies (_n_ = 1) . DATA EXTRACTION Data extracted
from each clinical study comprised: (a) study authors, publication date, study design (between- or within-subjects), patient sample size, and drop-out rate; (b) demographic (age, gender) and
clinical characteristics (e.g., symptom severity, medication status, illness duration); (c) tDCS parameters and protocol (e.g., scalp region of interest, anodal/cathodal stimulation,
stimulation intensity, number and duration of sessions); (d) instruments used to measure motor or cognitive outcomes (e.g., clinical rating scale, motor or cognitive test); and (e)
quantitative outcome data (e.g., means, standard deviations) from active and sham tDCS, and qualitative data expressed as improvement “+”, no statistically significant change “±”, and
negative impact “−”. Including parameters such as symptom severity and medication status is crucial as they significantly influence the response to tDCS and help understand baseline
characteristics and potential confounders in clinical outcomes. Symptom severity provides insight into the initial condition of patients, important for evaluating the magnitude of
improvement. Medication status is critical since concurrent treatments can modulate the effects of tDCS, impacting the study’s findings. Similarly, specific tDCS parameters (e.g., intensity,
duration, and stimulation site) are key to replicating and understanding the heterogeneity in study outcomes. We aimed to classify each cognitive outcome into one of five cognitive domains
relevant to PD: attention and working memory, executive function, language, memory and learning, and visuospatial function60. META-ANALYSIS Standardized mean difference (SMD) effect size
estimates (Hedge’s _g_ with standard error and 95% CI) between active tDCS and sham tDCS groups were computed for each outcome using Comprehensive Meta-Analysis (CMA). We accounted for study
design (between- or within-subjects) when calculating Hedge’s _g_ to ensure comparability of effects size estimates from studies using different designs61. Individual Hedge’s _g_ values
were averaged across studies using an inverse variance method and analyzed with a random-effects model in SPSS (version 28.0). The 95% CI indicated the precision of the estimated mean effect
size, while the 95% PI (from CMI Prediction Intervals software) measured between-study heterogeneity21,22. In the absence of between-study heterogeneity, the PI coincides with the
respective CI. In the presence of heterogeneity, however, the prediction covers a wider range than the CI21,22. Cochran’s Q and Higgins’s I2 tests assessed statistical heterogeneity21. If
heterogeneity was evident, we conducted meta-regression analyzes to identify sources of variability, considering age, symptom severity, illness severity, illness duration, and tDCS
parameters (intensity, duration, and sessions) as potential moderators. Sensitivity analysis evaluated each study’s influence by excluding one study at a time. Publication bias was assessed
via funnel plots and Egger’s test for funnel plot asymmetry. STUDY QUALITY ASSESSMENT The methodological quality of each study was evaluated using the Cochrane risk of bias tool for
individually-randomized clinical trials (RoB-2)62 covering 5 domains: (1) risk of bias from the randomization process, (2) risk of bias due to deviations from intended interventions, (3)
risk of bias from missing outcome data, (4) risk of bias in outcome measurement, and (5) risk of bias in selecting the reported result. Each domain was rated as ‘low risk’, ‘some concerns’,
or ‘high risk’ of bias. Judgments were based on answers to signaling questions addressing systematic errors, independently assessed by two reviewers. Discrepancies were resolved through
consensus. All reviewers adhered to the Methodological Expectations for Cochrane Intervention Reviews (MECIR) standards for assessing risk of bias DATA AVAILABILITY All data generated or
analyzed during this study are included in this published article. REFERENCES * Armstrong, M. J. & Okun, M. S. Diagnosis and treatment of Parkinson disease: a review. _JAMA_ 323, 548–560
(2020). Article PubMed Google Scholar * Jankovic, J. Parkinson’s disease: clinical features and diagnosis. _J. Neurol., Neurosurg., psychiatry_ 79, 368–376 (2008). Article CAS PubMed
Google Scholar * Chaudhuri, K. R. & Schapira, A. H. V. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. _Lancet Neurol._ 8, 464–474 (2009). Article
CAS PubMed Google Scholar * Yu R. L., Wu R. M. Mild cognitive impairment in patients with Parkinson’s disease: An updated mini-review and future outlook. _Frontiers in Aging
Neuroscience_. 2022;14. https://www.frontiersin.org/journals/aging-neuroscience/articles/10.3389/fnagi.2022.943438 * Fan, Y. et al. Determinants of quality of life according to cognitive
status in Parkinson’s disease. _Front. Aging Neurosci._ 12, 269 (2020). Article PubMed PubMed Central Google Scholar * Guo, Y. et al. Predictors of cognitive impairment in Parkinson’s
disease: a systematic review and meta-analysis of prospective cohort studies. _J. Neurol._ 268, 2713–2722 (2021). Article CAS PubMed Google Scholar * Jankovic, J. & Stacy, M. Medical
management of levodopa-associated motor complications in patients with Parkinson’s disease. _CNS drugs_ 21, 677–692 (2007). Article CAS PubMed Google Scholar * Serva, S. N., Bernstein,
J., Thompson, J. A., Kern, D. S. & Ojemann, S. G. An update on advanced therapies for Parkinson’s disease: From gene therapy to neuromodulation. _Front. Surg._ 9, 863921 (2022). Article
PubMed PubMed Central Google Scholar * Thair, H., Holloway, A. L., Newport, R. & Smith, A. D. Transcranial direct current stimulation (tDCS): a beginner’s guide for design and
implementation. _Front. Neurosci._ 11, 641 (2017). Article PubMed PubMed Central Google Scholar * Barker, A. T., Jalinous, R. & Freeston, I. L. Non-invasive magnetic stimulation of
human motor cortex. _Lancet_ 325, 1106–1107 (1985). Article Google Scholar * Huerta, P. T. & Volpe, B. T. Transcranial magnetic stimulation, synaptic plasticity and network
oscillations. _J. Neuroeng. rehabilitation_ 6, 1–10 (2009). Article Google Scholar * Pelletier, S. J. & Cicchetti, F. Cellular and molecular mechanisms of action of transcranial direct
current stimulation: evidence from in vitro and in vivo models. _Int J. Neuropsychopharmacol._ 18, pyu047 (2014). Article PubMed Google Scholar * Reed, T. & Cohen Kadosh, R.
Transcranial electrical stimulation (tES) mechanisms and its effects on cortical excitability and connectivity. _J. Inherit. Metab. Dis._ 41, 1123–1130 (2018). Article PubMed PubMed
Central Google Scholar * Lee, H. J., Jung, D. H., Jung, Y. J., Shin, H. K. & Choi, B. T. Transcranial alternating current stimulation rescues motor deficits in a mouse model of
Parkinson’s disease via the production of glial cell line-derived neurotrophic factor. _Brain stimulation_ 15, 645–653 (2022). Article PubMed Google Scholar * Chase, H. W., Boudewyn, M.
A., Carter, C. S. & Phillips, M. L. Transcranial direct current stimulation: a roadmap for research, from mechanism of action to clinical implementation. _Mol. psychiatry_ 25, 397–407
(2020). Article PubMed Google Scholar * Elsner, B., Kugler, J., Pohl, M. & Mehrholz, J. Transcranial direct current stimulation (tDCS) for idiopathic Parkinson’s disease. _Cochrane
Database Syst. Rev._ 7, CD010916 (2016). PubMed Google Scholar * Zhang, X. et al. Effects of non-invasive brain stimulation on walking and balance ability in Parkinson’s patients: a
systematic review and meta-analysis. _Front. aging Neurosci._ 14, 1065126–1065126 (2022). Article CAS PubMed Google Scholar * Goodwill, A. M. et al. Using non-invasive transcranial
stimulation to improve motor and cognitive function in Parkinson’s disease: a systematic review and meta-analysis. _Sci. Rep._ 7, 14840 (2017). Article PubMed PubMed Central Google
Scholar * Liu, X. et al. Transcranial direct current stimulation for Parkinson’s disease: a systematic review and meta-analysis. _Front. aging Neurosci._ 13, 746797 (2021). Article PubMed
PubMed Central Google Scholar * Begemann, M. J., Brand, B. A., Ćurčić-Blake, B., Aleman, A. & Sommer, I. E. Efficacy of non-invasive brain stimulation on cognitive functioning in
brain disorders: a meta-analysis. _Psychological Med._ 50, 2465–2486 (2020). Article Google Scholar * Borenstein, M. In a meta-analysis, the I-squared statistic does not tell us how much
the effect size varies. _J. Clin. Epidemiol._ 152, 281–284 (2022). Article PubMed Google Scholar * IntHout, J., Ioannidis, J. P. A., Rovers, M. M. & Goeman, J. J. Plea for routinely
presenting prediction intervals in meta-analysis. _BMJ Open_ 6, e010247 (2016). Article PubMed PubMed Central Google Scholar * Fregni, F. et al. Noninvasive cortical stimulation with
transcranial direct current stimulation in Parkinson’s disease. _Mov. Disord._ 21, 1693–1702 (2006). Article PubMed Google Scholar * Valentino, F. et al. Transcranial direct current
stimulation for treatment of freezing of gait: a cross-over study. _Mov. Disord.: Off. J. Mov. Disord. Soc._ 29, 1064–1069 (2014). Article Google Scholar * Salimpour, Y., Mari, Z. K. &
Shadmehr, R. Altering effort costs in Parkinson’s disease with noninvasive cortical stimulation. _J. Neurosci.: Off. J. Soc. Neurosci._ 35, 12287–12302 (2015). Article CAS Google Scholar
* Zhang B., Huang F., Liu J., Zhang D. Bilateral transcranial direct current stimulation may be a feasible treatment of Parkinsonian tremor. _Frontiers in Neuroscience_. 2023;17.
https://www.frontiersin.org/journals/neuroscience/articles/10.3389/fnins.2023.1101751 * Doruk, D., Gray, Z., Bravo, G. L., Pascual-Leone, A. & Fregni, F. Effects of tDCS on executive
function in Parkinson’s disease. _Neurosci. Lett._ 582, 27–31 (2014). Article CAS PubMed Google Scholar * Lima de Albuquerque L., et al. An Acute Application of Cerebellar Transcranial
Direct Current Stimulation Does Not Improve Motor Performance in Parkinson’s Disease. _Brain sciences_. 2020;10. https://doi.org/10.3390/brainsci10100735 * de Albuquerque, L. L. et al.
Long-term application of cerebellar transcranial direct current stimulation does not improve motor learning in Parkinson’s disease. _Cerebellum (Lond., Engl.)_ 21, 333–349 (2022). Article
Google Scholar * de Albuquerque L. L., et al. A Single Application of Cerebellar Transcranial Direct Current Stimulation Fails to Enhance Motor Skill Acquisition in Parkinson’s Disease: A
Pilot Study. _Biomedicines_. 2023;11. https://doi.org/10.3390/biomedicines11082219 * Beretta, V. S. et al. tDCS application for postural control in Parkinson’s disease: Effects are
associated with baseline characteristics. _Parkinsonism Relat. Disord._ 93, 62–65 (2021). Article CAS PubMed Google Scholar * Workman C. D., Fietsam A. C., Uc E. Y., Rudroff T.
Cerebellar Transcranial Direct Current Stimulation in People with Parkinson’s Disease: A Pilot Study. _Brain sciences_. 2020;10. https://doi.org/10.3390/brainsci10020096 * Manenti, R. et al.
Time up and go task performance improves after transcranial direct current stimulation in patient affected by Parkinson’s disease. _Neurosci. Lett._ 580, 74–77 (2014). Article CAS PubMed
Google Scholar * Schoellmann, A. et al. Anodal tDCS modulates cortical activity and synchronization in Parkinson’s disease depending on motor processing. _NeuroImage Clin._ 22,
101689–101689 (2019). Article PubMed PubMed Central Google Scholar * Sadler, C. M., Kami, A. T., Nantel, J., Lommen, J. & Carlsen, A. N. Transcranial direct current stimulation over
motor areas improves reaction time in Parkinson’s disease. _Front. Neurol._ 13, 913517 (2022). Article PubMed PubMed Central Google Scholar * Firouzi, M. et al. Transcranial
direct-current stimulation enhances implicit motor sequence learning in persons with Parkinson’s disease with mild cognitive impairment. _J. Neuropsychol._ 15, 363–378 (2021). Article
PubMed Google Scholar * Lau, C. I. et al. Effect of single-session transcranial direct current stimulation on cognition in Parkinson’s disease. _CNS Neurosci. therapeutics_ 25, 1237–1243
(2019). Article Google Scholar * Adenzato, M. et al. Transcranial direct current stimulation enhances theory of mind in Parkinson’s disease patients with mild cognitive impairment: a
randomized, double-blind, sham-controlled study. _Transl. Neurodegeneration_ 8, 1 (2019). Article Google Scholar * Pereira, J. B. et al. Modulation of verbal fluency networks by
transcranial direct current stimulation (tDCS) in Parkinson’s disease. _Brain stimulation_ 6, 16–24 (2013). Article PubMed Google Scholar * Mishra, R. K. & Thrasher, A. T.
Transcranial direct current stimulation of dorsolateral prefrontal cortex improves dual-task gait performance in patients with Parkinson’s disease: A double blind, sham-controlled study.
_Gait posture_ 84, 11–16 (2021). Article PubMed Google Scholar * Mishra, R. K. & Thrasher, A. T. Effect of concurrent transcranial direct current stimulation on instrumented timed up
and go task performance in people with Parkinson’s disease: A double-blind and cross-over study. _J. Clin. Neurosci.: Off. J. Neurosurgical Soc. Australas._ 100, 184–191 (2022). Article
Google Scholar * Terenzi, D. et al. Effects of tDCS on reward responsiveness and valuation in Parkinson’s patients with impulse control disorders. _J. Neurol._ 269, 1557–1565 (2022).
Article PubMed Google Scholar * Benninger, D. H. et al. Transcranial direct current stimulation for the treatment of Parkinson’s disease. _J. Neurol., Neurosurg. Psychiatry_ 81, 1105
LP–1101111 (2010). Article Google Scholar * Yu R. L., Tu S. C., Wu R. M., Lu P. A., Tan C. H. Interactions of COMT and ALDH2 Genetic Polymorphisms on Symptoms of Parkinson’s Disease.
_Brain Sciences_. 2021;11. https://doi.org/10.3390/brainsci11030361 * Ben-Joseph, A., Marshall, C. R., Lees, A. J. & Noyce, A. J. Ethnic variation in the manifestation of parkinson’s
disease: a narrative review. _J. Parkinson’s. Dis._ 10, 31–45 (2020). Article Google Scholar * Ramirez-Zamora, A. & Ostrem, J. L. Globus Pallidus Interna or subthalamic nucleus deep
brain stimulation for parkinson disease: a review. _JAMA Neurol._ 75, 367–372 (2018). Article PubMed Google Scholar * Jwa A. S., Goodman J. S., Glover G. H. Inconsistencies in mapping
current distribution in transcranial direct current stimulation. _Frontiers in Neuroimaging_. 2023;1. https://www.frontiersin.org/journals/neuroimaging/articles/10.3389/fnimg.2022.1069500 *
Vöröslakos, M. et al. Direct effects of transcranial electric stimulation on brain circuits in rats and humans. _Nat. Commun._ 9, 483 (2018). Article PubMed PubMed Central Google Scholar
* Horvath J. C., Carter O., Forte J. D. Transcranial direct current stimulation: five important issues we aren’t discussing (but probably should be). _Frontiers in Systems Neuroscience_.
2014;8. https://www.frontiersin.org/journals/systems-neuroscience/articles/10.3389/fnsys.2014.00002 * Sadleir, R. J., Vannorsdall, T. D., Schretlen, D. J. & Gordon, B. Transcranial
direct current stimulation (tDCS) in a realistic head model. _NeuroImage_ 51, 1310–1318 (2010). Article PubMed Google Scholar * Workman C. D., Fietsam A. C., Rudroff T. Tolerability and
Blinding of Transcranial Direct Current Stimulation in People with Parkinson’s Disease: A Critical Review. _Brain Sci_. 2020;10. https://doi.org/10.3390/brainsci10070467 * van Boekholdt, L.,
Kerstens, S., Khatoun, A., Asamoah, B. & Mc Laughlin, M. tDCS peripheral nerve stimulation: a neglected mode of action? _Mol. Psychiatry_ 26, 456–461 (2021). Article PubMed Google
Scholar * Esposito, M., Ferrari, C., Fracassi, C., Miniussi, C. & Brignani, D. Responsiveness to left-prefrontal tDCS varies according to arousal levels. _Eur. J. Neurosci._ 55, 762–777
(2022). Article PubMed PubMed Central Google Scholar * Rački V., et al. Cognitive Impact of Deep Brain Stimulation in Parkinson’s Disease Patients: A Systematic Review. _Frontiers in
Human Neuroscience_. 2022;16. https://www.frontiersin.org/journals/human-neuroscience/articles/10.3389/fnhum.2022.867055 * Aarsland, D. et al. Parkinson disease-associated cognitive
impairment. _Nat. Rev. Dis. Prim._ 7, 47 (2021). Article PubMed Google Scholar * Sasikumar, S. & Strafella, A. P. Imaging mild cognitive impairment and dementia in Parkinson’s
disease. _Front. Neurol._ 11, 47 (2020). Article PubMed PubMed Central Google Scholar * Brunoni, A. R. et al. A systematic review on reporting and assessment of adverse effects
associated with transcranial direct current stimulation. _Int. J. Neuropsychopharmacol._ 14, 1133–1145 (2011). Article PubMed Google Scholar * Matsumoto, H. & Ugawa, Y. Adverse events
of tDCS and tACS: a review. _Clin. Neurophysiol. Pract._ 2, 19–25 (2017). Article PubMed Google Scholar * Yang, C. et al. Transcranial temporal interference stimulation of the right
globus pallidus in parkinson’s disease. _Mov. Disord._ https://doi.org/10.1002/mds.29967 (2024). * Litvan, I. et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease:
movement disorder society task force guidelines. _Mov. Disord.: Off. J. Mov. Disord. Soc._ 27, 349–356 (2012). Article Google Scholar * Lakens, D. Calculating and reporting effect sizes
to facilitate cumulative science: a practical primer for t-tests and ANOVAs. _Front. Psychol._ 4, 863 (2013). Article PubMed PubMed Central Google Scholar * Lundh, A. & Gøtzsche, P.
C. Recommendations by Cochrane review groups for assessment of the risk of bias in studies. _BMC Med Res Methodol._ 8, 22 (2008). Article PubMed PubMed Central Google Scholar Download
references ACKNOWLEDGEMENTS We would like to express our gratitude to Prof. Odin van der Stelt for his invaluable consulting as well as Chenhao Yang and Yichao Du for supporting the study
quality assessment. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Clinical Neuroscience Center, Ruijin Hospital Luwan Branch, Shanghai Jiaotong University School of Medicine, Shanghai, China
Zhuo Duan & Chencheng Zhang * Clinical Neuroscience Center, Department of Psychiatry & Mental Health, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai,
China Zhuo Duan & Chencheng Zhang * Laboratory of Stereotaxy and Interventional Neurosciences, Department of Stereotactic and Functional Neurosurgery, University Hospital Freiburg,
Freiburg, Germany Zhuo Duan * Department of Stereotactic and Functional Neurosurgery, University Hospital Freiburg, Freiburg, Germany Zhuo Duan Authors * Zhuo Duan View author publications
You can also search for this author inPubMed Google Scholar * Chencheng Zhang View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Z.D. and C.Z.
contributed to conceptualization, synthesize, review and agreed with the manuscript. CORRESPONDING AUTHORS Correspondence to Zhuo Duan or Chencheng Zhang. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission
under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons
licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Duan, Z., Zhang, C. Transcranial direct current stimulation for Parkinson’s
disease: systematic review and meta-analysis of motor and cognitive effects. _npj Parkinsons Dis._ 10, 214 (2024). https://doi.org/10.1038/s41531-024-00821-z Download citation * Received: 11
July 2023 * Accepted: 19 October 2024 * Published: 06 November 2024 * DOI: https://doi.org/10.1038/s41531-024-00821-z SHARE THIS ARTICLE Anyone you share the following link with will be
able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative