Play all audios:
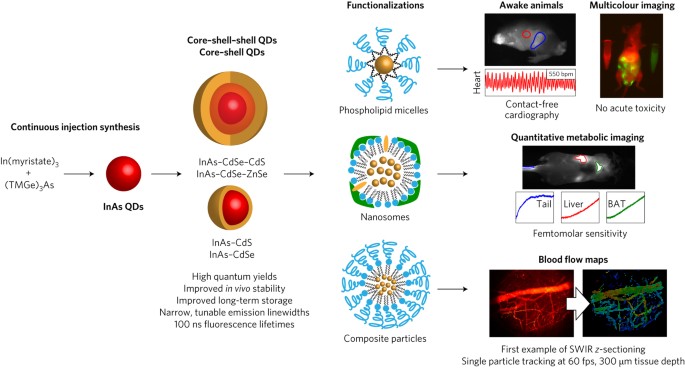
ABSTRACT For _in vivo_ imaging, the short-wavelength infrared region (SWIR; 1,000–2,000 nm) provides several advantages over the visible and near-infrared regions: general lack of
autofluorescence, low light absorption by blood and tissue, and reduced scattering. However, the lack of versatile and functional SWIR emitters has prevented the general adoption of SWIR
imaging by the biomedical research community. Here, we introduce a class of high-quality SWIR-emissive indium-arsenide-based quantum dots that are readily modifiable for various functional
imaging applications, and that exhibit narrow and size-tunable emission and a dramatically higher emission quantum yield than previously described SWIR probes. To demonstrate the
unprecedented combination of deep penetration, high spatial resolution, multicolour imaging and fast acquisition speed afforded by the SWIR quantum dots, we quantified, in mice, the
metabolic turnover rates of lipoproteins in several organs simultaneously and in real time as well as heartbeat and breathing rates in awake and unrestrained animals, and generated detailed
three-dimensional quantitative flow maps of the mouse brain vasculature. Access through your institution Buy or subscribe This is a preview of subscription content, access via your
institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel
any time Learn more Subscribe to this journal Receive 12 digital issues and online access to articles $119.00 per year only $9.92 per issue Learn more Buy this article * Purchase on
SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about
institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS IN VIVO NON-INVASIVE CONFOCAL FLUORESCENCE IMAGING BEYOND 1,700 NM USING
SUPERCONDUCTING NANOWIRE SINGLE-PHOTON DETECTORS Article 23 May 2022 FAST TIME-DOMAIN DIFFUSE CORRELATION SPECTROSCOPY WITH SUPERCONDUCTING NANOWIRE SINGLE-PHOTON DETECTOR: SYSTEM VALIDATION
AND IN VIVO RESULTS Article Open access 24 July 2023 SILICON-ROSINDOLIZINE FLUOROPHORES WITH SHORTWAVE INFRARED ABSORPTION AND EMISSION PROFILES ENABLE IN VIVO FLUORESCENCE IMAGING Article
25 March 2024 REFERENCES * De Jong, M., Essers, J. & van Weerden, W. M. Imaging preclinical tumour models: improving translational power. _Nat. Rev. Cancer_ 14, 481–493 (2014). Article
CAS Google Scholar * Welsher, K. et al. A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. _Nat. Nanotech_. 4, 773–780 (2009). Article CAS Google Scholar
* Yi, H. et al. M13 phage-functionalized single-walled carbon nanotubes as nanoprobes for second near-infrared window fluorescence imaging of targeted tumors. _Nano Lett._ 12, 1176–11 83
(2012). Article CAS Google Scholar * Hong, G. et al. Through-skull fluorescence imaging of the brain in a new near-infrared window. _Nat. Photon._ 8, 723–730 (2014). Article CAS Google
Scholar * Ghosh, D. et al. Deep, noninvasive imaging and surgical guidance of submillimeter tumors using targeted M13-stabilized single-walled carbon nanotubes. _Proc. Natl Acad. Sci. USA_
111, 13948–139 53 (2014). Article CAS Google Scholar * Bardhan, N. M., Ghosh, D. & Belcher, A. M. Carbon nanotubes as _in vivo_ bacterial probes. _Nat. Commun._ 5, 4918 (2014).
Article CAS Google Scholar * Welsher, K., Sherlock, S. P. & Dai, H. Deep-tissue anatomical imaging of mice using carbon nanotube fluorophores in the second near-infrared window.
_Proc. Natl Acad. Sci. USA_ 108, 8943–8948 (2011). Article CAS Google Scholar * Hong, G. et al. Multifunctional _in vivo_ vascular imaging using near-infrared II fluorescence. _Nat. Med_.
18, 1841–1846 (2012). Article CAS Google Scholar * Naczynski, D. J. et al. Rare-earth-doped biological composites as _in vivo_ shortwave infrared reporters. _Nat. Commun._ 4, 2199
(2013). Article CAS Google Scholar * Lim, Y. T. et al. Selection of quantum dot wavelengths for biomedical assays and imaging. _Mol. Imaging_ 2, 50–64 (2003). Article CAS Google Scholar
* Hong, G. et al. Ultrafast fluorescence imaging _in vivo_ with conjugated polymer fluorophores in the second near-infrared window. _Nat. Commun._ 5, 4206 (2014). Article CAS Google
Scholar * Tsukasaki, Y. et al. Short-wavelength infrared emitting mutimodal probe for non-invasive visualization of phagocyte cell migration in living mice. _Chem. Commun._ 50, 14356–1435 9
(2014). Article CAS Google Scholar * Tao, Z. et al. Biological imaging using nanoparticles of small organic molecules with fluorescence emission at wavelengths longer than 1000 nm.
_Angew. Chem. Int. Ed._ 52, 13002–1300 6 (2013). Article CAS Google Scholar * Dong, B. et al. Facile synthesis of highly photoluminescent Ag2Se quantum dots as a new fluorescent probe in
the second near-infrared window for _in vivo_ imaging. _Chem. Mater._ 25, 2503–2509 (2013). Article CAS Google Scholar * Zhu, C.-N. et al. Ag2Se quantum dots with tunable emission in the
second near-infrared window. _ACS Appl. Mater. Interfaces_ 5, 1186–1189 (2013). Google Scholar * Zhang, Y. Y. et al. Ag2S quantum dot: a bright and biocompatible fluorescent nanoprobe in
the second near-infrared window. _ACS Nano_ 6, 3695–3702 (2012). Article CAS Google Scholar * Tsukasaki, Y. et al. Synthesis and optical properties of emission-tunable PbS/CdS core–shell
quantum dots for _in vivo_ fluorescence imaging in the second near-infrared window. _RSC Adv_. 4, 41164–41171 (2014). Article CAS Google Scholar * Chen, O. et al. Compact high-quality
CdSe–CdS core–shell nanocrystals with narrow emission linewidths and suppressed blinking. _Nat. Mater._ 12, 445–451 (2013). Article CAS Google Scholar * Franke, D. et al. Continuous
injection synthesis of indium arsenide quantum dots emissive in the short-wavelength infrared. _Nat. Commun._ 7, 12749 (2016). Article CAS Google Scholar * Zhang, Y. et al.
Biodistribution, pharmacokinetics and toxicology of Ag2S near-infrared quantum dots in mice. _Biomaterials_ 34, 3639–3646 (2013). Article CAS Google Scholar * Dubertret, B. et al. _In
vivo_ imaging of quantum dots encapsulated in phospholipid micelles. _Science_ 298, 1759–1762 (2002). Article CAS Google Scholar * Stroh, M. et al. Quantum dots spectrally distinguish
multiple species within the tumor milieu _in vivo_. _Nat. Med_. 11, 678–682 (2005). Article CAS Google Scholar * Bruns, O. T. et al. Real-time magnetic resonance imaging and
quantification of lipoprotein metabolism _in vivo_ using nanocrystals. _Nat. Nanotech_. 4, 193–201 (2009). Article CAS Google Scholar * Bartelt, A. et al. Brown adipose tissue activity
controls triglyceride clearance. _Nat. Med_. 17, 200–205 (2011). Article CAS Google Scholar * Heeren, J. & Bruns, O. Nanocrystals, a new tool to study lipoprotein metabolism and
atherosclerosis. _Curr. Pharm. Biotechnol._ 13, 365–372 (2012). Article CAS Google Scholar * Fay, F. et al. Nanocrystal core lipoprotein biomimetics for imaging of lipoproteins and
associated diseases. _Curr. Cardiovasc. Imaging Rep_. 6, 45–54 (2013). Article Google Scholar * Jung, C. et al. Intraperitoneal injection improves the uptake of nanoparticle-labeled
high-density lipoprotein to atherosclerotic plaques compared with intravenous injection: a multimodal imaging study in ApoE knockout mice. _Circ. Cardiovasc. Imaging_ 7, 303–311 (2014).
Article Google Scholar * Jacobs, G. H., Neitz, J. & Deegan, J. F. Retinal receptors in rodents maximally sensitive to ultraviolet light. _Nature_ 353, 655–656 (1991). Article CAS
Google Scholar * Foster, H. L., Small, J. D. & Fox, J. G. _The Mouse in Biomedical Research: Normative Biology, Immunology, and Husbandry_ (Academic Press, 2006). Google Scholar *
Berndt, A., Leme, A. S., Paigen, B., Shapiro, S. D. & Svenson, K. L. Unrestrained plethysmograph and anesthetized forced oscillation methods of measuring lung function in 29 inbred
strains of mice. _Mouse Phenome Database_http://phenome.jax.org/db/q?rtn=projects/projdet&reqprojid=351 (2010). * Hillman, E. M. C. & Moore, A. All-optical anatomical co-registration
for molecular imaging of small animals using dynamic contrast. _Nat. Photon._ 1, 526–530 (2007). Article CAS Google Scholar * Kamoun, W. S. et al. Simultaneous measurement of RBC
velocity, flux, hematocrit and shear rate in vascular networks. _Nat. Methods_ 7, 655–660 (2010). Article CAS Google Scholar * Adrian, R. J. Twenty years of particle image velocimetry.
_Exp. Fluids_ 39, 159–169 (2005). Article Google Scholar * Adrian, R. J. Particle-imaging techniques for experimental fluid mechanics. _Annu. Rev. Fluid Mech._ 23, 261–304 (1991). Article
Google Scholar * Shi, Y., Cheng, J. C., Fox, R. O. & Olsen, M. G. Measurements of turbulence in a microscale multi-inlet vortex nanoprecipitation reactor. _J. Micromech. Microeng._
23, 75005 (2013). Article Google Scholar * Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. _Nat. Methods_ 9, 676–682 (2012). Article CAS Google Scholar
* Aharoni, A., Mokari, T., Popov, I. & Banin, U. Synthesis of InAs/CdSe/ZnSe core/shell1/shell2 structures with bright and stable near-infrared fluorescence. _J. Am. Chem. Soc._ 128,
257–264 (2006). Article CAS Google Scholar * Li, J. J. et al. Large-scale synthesis of nearly monodisperse CdSe/CdS core/shell nanocrystals using air-stable reagents via successive ion
layer adsorption and reaction. _J. Am. Chem. Soc._ 125, 12567–12575 (2003). Article CAS Google Scholar * Hines, M. A. & Scholes, G. D. Colloidal PbS nanocrystals with size-tunable
near-infrared emission: observation of post-synthesis self-narrowing of the particle size distribution. _Adv. Mater._ 15, 1844–1849 (2003). Article CAS Google Scholar * Pietryga, J. M. et
al. Utilizing the lability of lead selenide to produce heterostructured nanocrystals with bright, stable infrared emission. _J. Am. Chem. Soc._ 130, 4879–4885 (2008). Article CAS Google
Scholar * Supran, G. J. et al. High-performance shortwave-infrared light-emitting devices using core–shell (PbS–CdS) colloidal quantum dots. _Adv. Mater._ 27, 1437–1442 (2015). Article CAS
Google Scholar * Folch, J., Lees, M. & Sloane Stanley, G. H. A simple method for the isolation and purification of total lipides from animal tissues. _J. Biol. Chem._ 226, 497–509
(1957). CAS PubMed Google Scholar * Wurdinger, T. et al. A secreted luciferase for _ex vivo_ monitoring of _in vivo_ processes. _Nat. Methods_ 5, 171–173 (2008). Article CAS Google
Scholar * Kodack, D. P. et al. Combined targeting of HER2 and VEGFR2 for effective treatment of HER2-amplified breast cancer brain metastases. _Proc. Natl Acad. Sci. USA_ 109, E3119–E3127
(2012). Article CAS Google Scholar * Kloepper, J. et al. Ang-2/VEGF bispecific antibody reprograms macrophages and resident microglia to anti-tumor phenotype and prolongs glioblastoma
survival. _Proc. Natl Acad. Sci. USA_ 113, 4476–4481 (2016). Article CAS Google Scholar * Neher, R. A. et al. Blind source separation techniques for the decomposition of multiply labeled
fluorescence images. _Biophys. J._ 96, 3791–3800 (2009). Article CAS Google Scholar * Meinhart, C. D., Wereley, S. T. & Santiago, J. G. A PIV algorithm for estimating time-averaged
velocity fields. _J. Fluids Eng_. 122, 285–289 (2000). Article Google Scholar * Marxen, M., Sullivan, P. E., Loewen, M. R., Èhne, B. J. & Jähne, B. Comparison of Gaussian particle
center estimators and the achievable measurement density for particle tracking velocimetry. _Exp. Fluids_ 29, 145–153 (2000). Article Google Scholar Download references ACKNOWLEDGEMENTS
This work received support from the US National Institutes of Health (NIH) in part through 5-U54-CA151884 (M.G.B.), P01-CA080124 (R.K.J. and D.Fukumura), R35 CA197743, P50 CA165962 and
R01-CA126642 (R.K.J.), R01-CA096915 (D.Fukumura), the NIH funded Laser Biomedical Research Center through 4-P41-EB015871-30 (M.G.B.), and the US National Cancer Institute/Federal Share
Proton Beam Program Income (R.K.J.); the US National Foundation for Cancer Research (R.K.J.), and the Warshaw Institute for Pancreatic Cancer Research and Massachusetts General Hospital
Executive Committee on Research (D.Fukumura); the US Army Research Office through the Institute for Soldier Nanotechnologies (W911NF-13-D-0001; J.A.C., O.C., H.W., G.W.H. and M.G.B.); the US
Department of Defence through DoD W81XWH-10-1-0016 (R.K.J.); and the US National Science Foundation (NSF) through ECCS-1449291 (D.Franke and M.G.B.). This work was supported as part of the
Massachusetts Institute of Technology (MIT) Center for Excitonics, an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Office of Basic Energy
Sciences under Award Number DE-SC0001088 (T.S.B. and M.W.B.W.). O.T.B. is supported by an European Molecular Biology Organization long-term fellowship. A.B. is supported by a Deutsche
Forschungsgemeinschaft Research Fellowship (BA 4925/1-1). D.Franke is supported by a fellowship of the Evonik Stiftung and fellowship of the Boehringer Ingelheim Fonds. This research was
conducted with government support under and awarded by the US Department of Defence, Air Force Office of Scientific Research, National Defence Science and Engineering Graduate Fellowship 32
CFR 168a (J.A.C.). J.H. is supported by a grant from the Fondation Leducq—Triglyceride Metabolism in Obesity and Cardiovascular Disease. L.R. received a Mildred Scheel Fellowship (Deutsche
Krebshilfe). D.K.H., D.M.M., I.C. and O.B.A. were supported by NSF GRFP fellowships. J.K. was supported by fellowships from the Deutsche Forschungsgemeinschaft and the SolidarImmun
Foundation. C.J.R. and P.T.C.S. acknowledge support from NIH 4-P41-EB015871-30, DP3-DK101024 01, 1-U01-NS090438-01, 1-R01-EY017656 -0, 6A1, 1-R01-HL121386-01A1, the Biosym IRG of
Singapore–MIT Alliance Research and Technology Center, the Koch Institute for Integrative Cancer Research Bridge Initiative, Hamamatsu Inc., and the Samsung GRO program. We thank S. Roberge
and P. Huang for technical assistance. We also thank QDVision for providing an InAs–CdZnS QD sample (InAs-016) used in this study. We are grateful to Gökhan Hotamisligil for critical
discussion and continuing support. AUTHOR INFORMATION Author notes * Lars Riedemann, Mark W. B. Wilson, Ou Chen, Gyu Weon Hwang & Jonas Kloepper Present address: †Present addresses:
Neurology Clinic and National Center for Tumor Diseases, University Hospital Heidelberg, and Clinical Cooperation Unit Neuro-Oncology, German Cancer Consortium, German Cancer Research
Center, 69120 Heidelberg, Germany (L.R.); Department of Chemistry, University of Toronto, 80 Saint George Street, Toronto, Ontario M5S 3H6, Canada (M.W.B.W.); Department of Chemistry, Brown
University, Providence, Rhode Island 02912, USA (O.C.); Korea Institute of Science and Technology, Seoul 02792, Republic of Korea (G.W.H.); Centre Hospitalier Universitaire Vaudois,
Département de Médecine Interne, CHUV-UNIL, Rue du Bugnon 46, 1011 Lausanne, Switzerland (J.K.)., * Oliver T. Bruns and Thomas S. Bischof: These authors contributed equally to this work.
AUTHORS AND AFFILIATIONS * Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, 02139, Massachusetts, USA. Oliver T. Bruns, Thomas S. Bischof,
Daniel K. Harris, Daniel Franke, Jessica A. Carr, Mark W. B. Wilson, Ou Chen, He Wei, Gyu Weon Hwang, Daniel M. Montana, Igor Coropceanu, Odin B. Achorn & Moungi G. Bawendi * Department
of Materials Science and Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Massachusetts, Cambridge, 02139,, USA. Daniel K. Harris, Gyu Weon Hwang & Daniel M.
Montana * Department of Chemical Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Massachusetts, Cambridge, 02139,, USA. Yanxiang Shi & Klavs F. Jensen *
Edwin L. Steele Laboratories for Tumor Biology, Massachusetts General Hospital and Harvard Medical School, 100 Blossom Street, Cox-7, Massachusetts, Boston, 02114,, USA. Lars Riedemann,
Jonas Kloepper, Dai Fukumura & Rakesh K. Jain * Department of Genetics and Complex Diseases and Sabri Ülker Center, Harvard T.H. Chan School of Public Health, 665 Huntington Avenue,
Massachusetts, Boston, 02115,, USA. Alexander Bartelt * Raytheon Vision Systems, Goleta, 93117,, California, USA. Frank B. Jaworski * Department of Biological Engineering, Massachusetts
Institute of Technology, Cambridge, Massachusetts 02139, USA. Christopher J. Rowlands & Peter T. C. So * Department of Biochemistry and Molecular Cell Biology, University Medical Center
Hamburg-Eppendorf, Martinistrasse 52, Hamburg, 20246, Germany. Joerg Heeren * Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139,
USA. Peter T. C. So Authors * Oliver T. Bruns View author publications You can also search for this author inPubMed Google Scholar * Thomas S. Bischof View author publications You can also
search for this author inPubMed Google Scholar * Daniel K. Harris View author publications You can also search for this author inPubMed Google Scholar * Daniel Franke View author
publications You can also search for this author inPubMed Google Scholar * Yanxiang Shi View author publications You can also search for this author inPubMed Google Scholar * Lars Riedemann
View author publications You can also search for this author inPubMed Google Scholar * Alexander Bartelt View author publications You can also search for this author inPubMed Google Scholar
* Frank B. Jaworski View author publications You can also search for this author inPubMed Google Scholar * Jessica A. Carr View author publications You can also search for this author
inPubMed Google Scholar * Christopher J. Rowlands View author publications You can also search for this author inPubMed Google Scholar * Mark W. B. Wilson View author publications You can
also search for this author inPubMed Google Scholar * Ou Chen View author publications You can also search for this author inPubMed Google Scholar * He Wei View author publications You can
also search for this author inPubMed Google Scholar * Gyu Weon Hwang View author publications You can also search for this author inPubMed Google Scholar * Daniel M. Montana View author
publications You can also search for this author inPubMed Google Scholar * Igor Coropceanu View author publications You can also search for this author inPubMed Google Scholar * Odin B.
Achorn View author publications You can also search for this author inPubMed Google Scholar * Jonas Kloepper View author publications You can also search for this author inPubMed Google
Scholar * Joerg Heeren View author publications You can also search for this author inPubMed Google Scholar * Peter T. C. So View author publications You can also search for this author
inPubMed Google Scholar * Dai Fukumura View author publications You can also search for this author inPubMed Google Scholar * Klavs F. Jensen View author publications You can also search for
this author inPubMed Google Scholar * Rakesh K. Jain View author publications You can also search for this author inPubMed Google Scholar * Moungi G. Bawendi View author publications You
can also search for this author inPubMed Google Scholar CONTRIBUTIONS O.T.B., T.S.B., D.K.H., D.Franke, L.R., A.B., F.B.J., J.A.C., M.W.B.W., O.C., H.W., G.W.H., D.M.M., I.C., O.B.A. and
J.K. performed the experiments. O.T.B, T.S.B., Y.S. and C.J.R. analysed the data, O.T.B., T.S.B. and M.G.B. wrote the paper and were assisted by D.Franke, A.B. and R.K.J. J.H., P.T.C.S,
D.Fukumura, K.F.J. and R.K.J. provided guidance on the study design. J.H. provided lipid samples. All authors reviewed and edited the manuscript. CORRESPONDING AUTHORS Correspondence to
Oliver T. Bruns, Thomas S. Bischof or Moungi G. Bawendi. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY
INFORMATION Supplementary figures and video captions. (PDF 6792 kb) SUPPLEMENTARY VIDEO 1 Five InAs core-shell quantum dot samples with distinct emission spectra, dissolved in hexanes.
Please refer to the Supplementary Information file for the full description. (MOV 2521 kb) SUPPLEMENTARY VIDEO 2 SWIR emission from a mouse with activated brown adipose tissue. Please refer
to the Supplementary Information file for the full description. (MOV 1309 kb) SUPPLEMENTARY VIDEO 3 Biodistribution of PEGylated SWIR QDs (1,200 nm emission) in a mouse. Please refer to the
Supplementary Information file for the full description. (MOV 5674 kb) SUPPLEMENTARY VIDEO 4 Same as Supplementary Video 3, after reaching equilibrium. Please refer to the Supplementary
Information file for the full description. (MOV 4812 kb) SUPPLEMENTARY VIDEO 5 Awake mouse injected with PEGylated QDs emitting at 1,080 nm. Please refer to the Supplementary Information
file for the full description. (MOV 4035 kb) SUPPLEMENTARY VIDEO 6 Imaging of the brain of a mouse with glioblastoma multiforme through a cranial window, during injection of PEGylated QDs.
Please refer to the Supplementary Information file for the full description. (MOV 29665 kb) SUPPLEMENTARY VIDEO 7 High-speed intravital SWIR microscopy in the healthy hemisphere of a
mouse's brain. Please refer to the Supplementary Information file for the full description. (MOV 8493 kb) SUPPLEMENTARY VIDEO 8 High-speed intravital SWIR microscopy of the tumour
margin of the mouse shown in Supplementary Video 7. Please refer to the Supplementary Information file for the full description. (MOV 8305 kb) SUPPLEMENTARY VIDEO 9 Z-stack images of blood
flow in the brain of a mouse. Please refer to the Supplementary Information file for the full description. (AVI 2350 kb) SUPPLEMENTARY VIDEO 10 Z-resolved imaging of blood flow in the brain
of a mouse. Please refer to the Supplementary Information file for the full description. (MOV 10131 kb) SUPPLEMENTARY VIDEO 11 Z-resolved imaging of blood flow in the brain of a mouse.
Please refer to the Supplementary Information file for the full description. (MOV 7114 kb) SUPPLEMENTARY VIDEO 12 Z-resolved imaging of blood flow in the brain of a mouse. Please refer to
the Supplementary Information file for the full description. (AVI 1801 kb) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Bruns, O., Bischof, T.,
Harris, D. _et al._ Next-generation _in vivo_ optical imaging with short-wave infrared quantum dots. _Nat Biomed Eng_ 1, 0056 (2017). https://doi.org/10.1038/s41551-017-0056 Download
citation * Received: 26 October 2016 * Accepted: 02 March 2017 * Published: 10 April 2017 * DOI: https://doi.org/10.1038/s41551-017-0056 SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative