Play all audios:
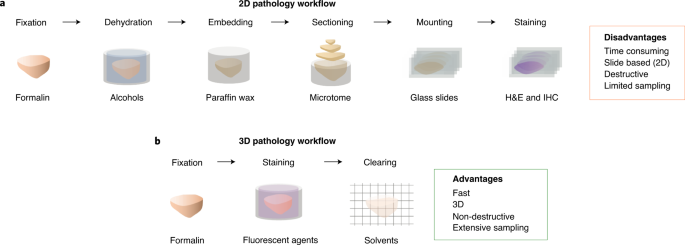
High-throughput methods for slide-free three-dimensional (3D) pathological analyses of whole biopsies and surgical specimens offer the promise of modernizing traditional histology workflows
and delivering improvements in diagnostic performance. Advanced optical methods now enable the interrogation of orders of magnitude more tissue than previously possible, where volumetric
imaging allows for enhanced quantitative analyses of cell distributions and tissue structures that are prognostic and predictive. Non-destructive imaging processes can simplify laboratory
workflows, potentially reducing costs, and can ensure that samples are available for subsequent molecular assays. However, the large size of the feature-rich datasets that they generate
poses challenges for data management and computer-aided analysis. In this Perspective, we provide an overview of the imaging technologies that enable 3D pathology, and the computational
tools—machine learning, in particular—for image processing and interpretation. We also discuss the integration of various other diagnostic modalities with 3D pathology, along with the
challenges and opportunities for clinical adoption and regulatory approval.
We acknowledge research grants from the Department of Defense (DoD) Prostate Cancer Research Program through W81XWH-18-10358 (J.T.C.L. and L.D.T.), W81XWH-19-1-0589 (N.P.R.),
W81XWH-20-1-0851(A.M. and J.T.C.L.) and W81XWH-15-1-0558 (A.M.); the DoD Breast Cancer Research Program W81XWH-19-1-0668 (A.M.); the DoD Lung Cancer Research Program W81XWH-18-1-0440 (A.M.);
the DoD Peer Reviewed Cancer Research Program W81XWH-16-1-0329 (A.M.); the Ohio Third Frontier Technology Validation Fund (A.M.); the Wallace H. Coulter Foundation Program in the Department
of Biomedical Engineering and The Clinical and Translational Science Award Program at Case Western Reserve University (A.M.); the Prostate Cancer Foundation (N.P.R.); and the National
Science Foundation 1934292 HDR: I-DIRSE-FW (J.T.C.L.). We also acknowledge grants from the National Institutes of Health (NIH) National Cancer Institute (NCI) through K99CA240681 (A.K.G.),
R01CA175391 (J.T.C.L.), R01CA244170 (J.T.C.L.), R01CA199996 (K.W.E.), U24CA199374 (A.M.), R01CA202752 (A.M.), R01CA208236 (A.M.), R01CA216579 (A.M.), R01CA220581 (A.M.), R01CA249992 (A.M.),
U01CA248226 (A.M.), U54CA254566 (A.M.) and U01CA239055 (A.M.); from the NIH National Institute of Biomedical Imaging and Bioengineering (NIBIB) through R43EB028736 (A.M.); from the NIH
National Heart, Lung and Blood Institute (NHLBI) through R01HL151277 (A.M.); from the NIH National Institute of General Medical Sciences (NIGMS) through P41GM135019 (K.W.E.); from the NIH
National Center for Research Resources through C06RR12463 (A.M.); from the US Department of Veterans Affairs IBX004121A (A.M.); from the Institute for Prostate Cancer Research at the
University of Washington (L.D.T.), and from the National Cancer Institute Breast Cancer SPORE/Safeway Foundation at the Fred Hutchinson Cancer Research Center (J.T.C.L). The content is
solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the US Department of Veterans Affairs, the Department of
Defense or the United States Government.
Department of Mechanical Engineering, University of Washington, Seattle, WA, USA
Department of Laboratory Medicine and Pathology, University of Washington, Seattle, WA, USA
Department of Bioengineering, University of Washington, Seattle, WA, USA
Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH, USA
Department of Medical Physics, University of Wisconsin, Madison, WI, USA
Department of Biomedical Engineering, University of Wisconsin, Madison, WI, USA
Louis Stokes Cleveland Veterans Administration Medical Center, Cleveland, OH, USA
All authors discussed and wrote the manuscript. J.T.C.L. and A.K.G. drew the figures. J.T.C.L. coordinated the effort and led the writing.
J.T.C.L., A.K.G., N.P.R. and L.D.T. are co-founders and shareholders of Lightspeed Microscopy, of which J.T.C.L. and N.P.R. are board members and N.P.R. is the chief executive officer.
Technology developed by J.T.C.L., A.K.G., N.P.R. and L.D.T. at the University of Washington has been licensed by Lightspeed Microscopy. K.W.E. is a co-founder and shareholder of OnLume, and
a scientific advisory consultant for Bruker Corporation and Elephas Corporation. A.M. is an equity holder in Elucid Bioimaging and Inspirata, has served as a scientific advisory board member
for Inspirata, AstraZeneca, Bristol Myers-Squibb, Aiforia and Merck, has had sponsored research agreements with Philips and Inspirata, has technology that is licensed to Elucid Bioimaging
and Inspirata, and is involved in a NIH U24 grant with PathCore Inc. and in three different R01 grants with Inspirata Inc. A.M. has sponsored research projects from AstraZeneca, Bristol
Myers-Squibb and Boehringer-Ingelheim.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Anyone you share the following link with will be able to read this content: