Play all audios:
ABSTRACT Super-concentrated water-in-salt electrolytes make high-voltage aqueous batteries possible, but at the expense of high cost and several adverse effects, including high viscosity,
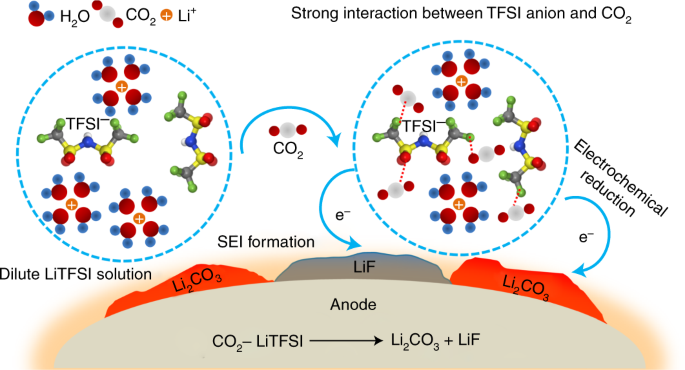
low conductivity and slow kinetics. Here, we observe a concentration-dependent association between CO2 and TFSI anions in water that reaches maximum strength at 5 mol kg−1 LiTFSI. This
TFSI–CO2 complex and its reduction chemistry allow us to decouple the interphasial responsibility of an aqueous electrolyte from its bulk properties, hence making high-voltage aqueous Li-ion
batteries practical in dilute salt-in-water electrolytes. The CO2/salt-in-water electrolyte not only inherits the wide electrochemical stability window and non-flammability from
water-in-salt electrolytes but also successfully circumvents the numerous disadvantages induced by excessive salt. This work represents a deviation from the water-in-salt pathway that not
only benefits the development of practical aqueous batteries, but also highlights how the complex interactions between electrolyte components can be used to manipulate interphasial
chemistry. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access
Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print
issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to
local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT
BEING VIEWED BY OTHERS WATER-IN-SALT ELECTROLYTES MADE SALTIER BY GEMINI IONIC LIQUIDS FOR HIGHLY EFFICIENT LI-ION BATTERIES Article Open access 07 February 2023 WATER-IN-POLYMER ELECTROLYTE
WITH A WIDE ELECTROCHEMICAL WINDOW AND RECYCLABILITY Article 18 April 2024 HYBRID ELECTROLYTE ENABLES SOLID-STATE SODIUM BATTERIES SUSTAINING 50,000 CYCLES Article 02 May 2025 DATA
AVAILABILITY All the data generated or analysed during this study are included in this article and its Supplementary Information. The details of the molecular dynamics simulation are
available in Supplementary Data 1. Source data are provided with this paper. REFERENCES * Li, W., Dahn, J. R. & Wainwright, D. S. Rechargeable lithium batteries with aqueous
electrolytes. _Science_ 264, 1115–1118 (1994). Article CAS PubMed Google Scholar * Suo, L. et al. “Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries.
_Science_ 350, 938–943 (2015). Article CAS PubMed Google Scholar * Luo, J.-Y., Cui, W.-J., He, P. & Xia, Y.-Y. Raising the cycling stability of aqueous lithium-ion batteries by
eliminating oxygen in the electrolyte. _Nat. Chem._ 2, 760–765 (2010). Article PubMed CAS Google Scholar * Yamada, Y. et al. Hydrate-melt electrolytes for high-energy-density aqueous
batteries. _Nat. Energy_ 1, 16129 (2016). Article CAS Google Scholar * Yang, C. et al. Aqueous Li-ion battery enabled by halogen conversion–intercalation chemistry in graphite. _Nature_
569, 245–250 (2019). Article CAS PubMed Google Scholar * Sun, W. et al. A rechargeable zinc-air battery based on zinc peroxide chemistry. _Science_ 371, 46–51 (2021). Article CAS
PubMed Google Scholar * Wu, X. et al. Reverse dual-ion battery via a ZnCl2 water-in-salt electrolyte. _J. Am. Chem. Soc._ 141, 6338–6344 (2019). Article CAS PubMed Google Scholar *
Abbas, Q. et al. Strategies to improve the performance of carbon/carbon capacitors in salt aqueous electrolytes. _J. Electrochem. Soc._ 162, A5148–A5157 (2015). Article CAS Google Scholar
* Liu, Q. et al. 2.2V high performance symmetrical fiber-shaped aqueous supercapacitors enabled by “water-in-salt” gel electrolyte and N-doped graphene fiber. _Energy Storage Mater._ 24,
495–503 (2020). Article Google Scholar * Yoshio, H., Katsuhei, K., Akira, M. & Shin, S. Production of methane and ethylene in electrochemical reduction of carbon dioxide at copper
electrode in aqueous hydrogencarbonate solution. _Chem. Lett._ 15, 897–898 (1986). Article Google Scholar * Tomita, Y., Teruya, S., Koga, O. & Hori, Y. Electrochemical reduction of
carbon dioxide at a platinum electrode in acetonitrile-water mixtures. _J. Electrochem. Soc._ 147, 4164–4167 (2000). Article CAS Google Scholar * Chen, Y.-T., Chen, H.-Y., Chien, Y.-S.,
Chuang, M.-C. & Chen, P.-Y. An excellent anode renders protic ionic liquids sustainable in metal electrodeposition. _Green Chem._ https://doi.org/10.1039/C9GC04169A 2020. * Huang, Q.
& Lyons, T. W. Electrodeposition of rhenium with suppressed hydrogen evolution from water-in-salt electrolyte. _Electrochem. Commun._ 93, 53–56 (2018). Article CAS Google Scholar *
Pasta, M., Wessells, C. D., Huggins, R. A. & Cui, Y. A high-rate and long cycle life aqueous electrolyte battery for grid-scale energy storage. _Nat. Commun._ 3, 1149 (2012). Article
PubMed CAS Google Scholar * Wu, X. et al. Diffusion-free Grotthuss topochemistry for high-rate and long-life proton batteries. _Nat. Energy_ 4, 123–130 (2019). Article CAS Google
Scholar * Wessells, C., Ruffο, R., Huggins, R. A. & Cui, Y. Investigations of the electrochemical stability of aqueous electrolytes for lithium battery applications. _Electrochem. Solid
State Lett._ 13, A59–A61 (2010). Article CAS Google Scholar * Winter, M. The solid electrolyte interphase – the most important and the least understood solid electrolyte in rechargeable
Li batteries. _Z. Phys. Chem._ 223, 1395–1406 (2009). Article CAS Google Scholar * Wood, S. M. et al. Predicting calendar aging in lithium metal secondary batteries: the impacts of solid
electrolyte interphase composition and stability. _Adv. Energy Mater._ 8, 1801427 (2018). Article CAS Google Scholar * Cheng, D. et al. Unveiling the stable nature of the solid
electrolyte interphase between lithium metal and LiPON via cryogenic electron microscopy. _Joule_ 4, 2484–2500 (2020). Article CAS Google Scholar * Li, W. & Dahn, J. R. Lithium-ion
cells with aqueous electrolytes. _J. Electrochem. Soc._ 142, 1742–1746 (1995). Article CAS Google Scholar * Suo, L. et al. How solid-electrolyte interphase forms in aqueous electrolytes.
_J. Am. Chem. Soc._ 139, 18670–18680 (2017). Article CAS PubMed Google Scholar * Zhao, M., Song, X., Wang, F., Dai, W. & Lu, X. Electrochemical performance of single crystalline
spinel LiMn2O4 nanowires in an aqueous LiNO3 solution. _Electrochim. Acta_ 56, 5673–5678 (2011). Article CAS Google Scholar * Wang, G. J. et al. Electrochemical behavior of LiCoO2 in a
saturated aqueous Li2SO4 solution. _Electrochim. Acta_ 54, 1199–1203 (2009). Article CAS Google Scholar * Liu, X.-H., Saito, T., Doi, T., Okada, S. & Yamaki, J.-I. Electrochemical
properties of rechargeable aqueous lithium ion batteries with an olivine-type cathode and a NASICON-type anode. _J. Power Sources_ 189, 706–710 (2009). Article CAS Google Scholar * Chen,
Y., Freunberger, S. A., Peng, Z., Bardé, F. & Bruce, P. G. Li–O2 battery with a dimethylformamide electrolyte. _J. Am. Chem. Soc._ 134, 7952–7957 (2012). Article CAS PubMed Google
Scholar * Khurram, A., He, M. & Gallant, B. M. Tailoring the discharge reaction in Li-CO2 batteries through incorporation of CO2 capture chemistry. _Joule_ 2, 2649–2666 (2018). Article
CAS Google Scholar * Yao, K. P. C. et al. Thermal stability of Li2O2 and Li2O for Li-air batteries: in situ XRD and XPS studies. _J. Electrochem. Soc._ 160, A824–A831 (2013). Article
CAS Google Scholar * Dong, Q. et al. Cathodically stable Li-O2 battery operations using water-in-salt electrolyte. _Chem_ 4, 1345–1358 (2018). Article CAS Google Scholar * Ding, M. S.
& Xu, K. Phase diagram, conductivity, and glass transition of LiTFSI–H2O binary electrolytes. _J. Phys. Chem. C_ 122, 16624–16629 (2018). Article CAS Google Scholar * Frost, R. L.
& Palmer, S. J. Infrared and infrared emission spectroscopy of nesquehonite Mg(OH)(HCO3)·2H2O–implications for the formula of nesquehonite. _Spectrochim. Acta A_ 78, 1255–1260 (2011).
Article CAS Google Scholar * Nunn, P. B. & O’Brien, P. The interaction of β-_N_-methylamino-L-alanine with bicarbonate: an 1H-NMR study. _FEBS Lett._ 251, 31–35 (1989). Article CAS
PubMed Google Scholar * Abbott, T. M., Buchanan, G. W., Kruus, P. & Lee, K. C. 13C nuclear magnetic resonance and Raman investigations of aqueous carbon dioxide systems. _Can. J.
Chem._ 60, 1000–1006 (1982). Article CAS Google Scholar * Jakobsen, J. P., Krane, J. & Svendsen, H. F. Liquid-phase composition determination in CO2−H2O−alkanolamine systems: an NMR
study. _Ind. Eng. Chem. Res._ 44, 9894–9903 (2005). Article CAS Google Scholar * Fears, T. M. et al. Evaluating the solid electrolyte interphase formed on silicon electrodes: a comparison
of _ex situ_ X-ray photoelectron spectroscopy and _in situ_ neutron reflectometry. _Phys. Chem. Chem. Phys._ 18, 13927–13940 (2016). Article CAS PubMed Google Scholar * Dubouis, N. et
al. The role of the hydrogen evolution reaction in the solid–electrolyte interphase formation mechanism for “_Water-in-Salt_” electrolytes. _Energy Environ. Sci._ 11, 3491–3499 (2018).
Article CAS Google Scholar * Jiang, L. et al. High-voltage aqueous Na-ion battery enabled by inert-cation-assisted water-in-salt electrolyte. _Adv. Mater._ 32, 1904427 (2020). Article
CAS Google Scholar * Huang, J. & Rüther, T. Why are ionic liquids attractive for CO2 absorption? An overview. _Aust. J. Chem._ 62, 298–308 (2009). Article CAS Google Scholar *
Cadena, C. et al. Why is CO2 so soluble in imidazolium-based ionic liquids? _J. Am. Chem. Soc._ 126, 5300–5308 (2004). Article CAS PubMed Google Scholar * Tan, P. et al. Solid-like
nano-anion cluster constructs a free lithium-ion-conducting superfluid framework in a water-in-salt electrolyte. _J. Phys. Chem. C_ 125, 11838–11847 (2021). Article CAS Google Scholar *
Jorgensen, W. L., Maxwell, D. S. & Tirado-Rives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. _J. Am. Chem.
Soc._ 118, 11225–11236 (1996). Article CAS Google Scholar * Canongia Lopes, J. N. & Pádua, A. A. H. Molecular force field for ionic liquids composed of triflate or bistriflylimide
anions. _J. Phys. Chem. B_ 108, 16893–16898 (2004). Article CAS Google Scholar * McEldrew, M., Goodwin, Z. A. H., Kornyshev, A. A. & Bazant, M. Z. Theory of the double layer in
water-in-salt electrolytes. _J. Phys. Chem. Lett._ 9, 5840–5846 (2018). Article CAS PubMed Google Scholar * Berendsen, H. J. C., Grigera, J. R. & Straatsma, T. P. The missing term in
effective pair potentials. _J. Phys. Chem._ 91, 6269–6271 (1987). Article CAS Google Scholar * Zhu, A., Zhang, X., Liu, Q. & Zhang, Q. A fully flexible potential model for carbon
dioxide. _Chin. J. Chem. Eng._ 17, 268–272 (2009). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS All authors except K.X. acknowledge the support of the National Natural
Science Foundation of China (51872322) and the Center for Clean Energy. J.Z., M.C. and G.F. thank the Hubei Provincial Natural Science Foundation of China (2020CFA093) and the Program for
Huazhong University of Science and Technology, Academic Frontier Youth Team. K.X. thanks the Joint Center of Energy Storage Research, an energy hub funded by the US Department of Energy,
Basic Energy Sciences, for support. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Beijing Advanced Innovation Center for Materials Genome Engineering, Key Laboratory for Renewable Energy,
Beijing Key Laboratory for New Energy Materials and Devices, Beijing National Laboratory for Condensed Matter Physics, Institute of Physics, Chinese Academy of Sciences, Beijing, China
Jinming Yue, Yuxin Tong, Lilu Liu, Liwei Jiang, Tianshi Lv, Yong-sheng Hu, Hong Li, Xuejie Huang, Lin Gu, Liumin Suo & Liquan Chen * Center of Materials Science and Optoelectronics
Engineering, University of Chinese Academy of Sciences, Beijing, China Jinming Yue, Yuxin Tong, Liwei Jiang, Tianshi Lv & Liumin Suo * State Key Laboratory of Coal Combustion, School of
Energy and Power Engineering, Huazhong University of Science and Technology, Wuhan, China Jinkai Zhang, Ming Chen & Guang Feng * Battery Science Branch, Sensor and Electron Devices
Directorate, US Army Research Laboratory, Adelphi, MD, USA Kang Xu * Yangtze River Delta Physics Research Center Co. Ltd, Liyang, China Liumin Suo Authors * Jinming Yue View author
publications You can also search for this author inPubMed Google Scholar * Jinkai Zhang View author publications You can also search for this author inPubMed Google Scholar * Yuxin Tong View
author publications You can also search for this author inPubMed Google Scholar * Ming Chen View author publications You can also search for this author inPubMed Google Scholar * Lilu Liu
View author publications You can also search for this author inPubMed Google Scholar * Liwei Jiang View author publications You can also search for this author inPubMed Google Scholar *
Tianshi Lv View author publications You can also search for this author inPubMed Google Scholar * Yong-sheng Hu View author publications You can also search for this author inPubMed Google
Scholar * Hong Li View author publications You can also search for this author inPubMed Google Scholar * Xuejie Huang View author publications You can also search for this author inPubMed
Google Scholar * Lin Gu View author publications You can also search for this author inPubMed Google Scholar * Guang Feng View author publications You can also search for this author
inPubMed Google Scholar * Kang Xu View author publications You can also search for this author inPubMed Google Scholar * Liumin Suo View author publications You can also search for this
author inPubMed Google Scholar * Liquan Chen View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS L.S. and K.X. conceived the idea. J.Y. and
L.S. designed the experiments. J.Y. performed the material preparation, electrochemical measurements and data analysis. L.J. performed the NMR measurements. Y.T. collected the TEM images,
and L.L. measured the XPS spectra. T.L. performed the cost analysis of the electrolyte. J.Z., M.C. and G.F. performed the molecular dynamics simulations and analysed the data. J.Y., G.F.,
K.X. and L.S. wrote the manuscript. All authors discussed the results and commented on the manuscript. CORRESPONDING AUTHORS Correspondence to Kang Xu or Liumin Suo. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Chemistry_ thanks Jin Han and the other, anonymous, reviewer(s) for
their contribution to the peer review of this work. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figs. 1–39, Tables 1–3 and experimental details. SUPPLEMENTARY DATA 1 Bulk files for molecular dynamics simulation. SOURCE
DATA SOURCE DATA FIG. 1 Source data for the graph in Fig. 1. SOURCE DATA FIG. 2 Source data for the graph in Fig. 2. SOURCE DATA FIG. 3 Source data for the graph in Fig. 3. SOURCE DATA FIG.
4 Source data for the graph in Fig. 4. SOURCE DATA FIG. 5 Source data for the graph in Fig. 5. SOURCE DATA FIG. 6 Source data for the graph in Fig. 6. RIGHTS AND PERMISSIONS Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yue, J., Zhang, J., Tong, Y. _et al._ Aqueous interphase formed by CO2 brings electrolytes back to salt-in-water regime. _Nat. Chem._ 13,
1061–1069 (2021). https://doi.org/10.1038/s41557-021-00787-y Download citation * Received: 08 February 2021 * Accepted: 13 August 2021 * Published: 11 October 2021 * Issue Date: November
2021 * DOI: https://doi.org/10.1038/s41557-021-00787-y SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative