Play all audios:
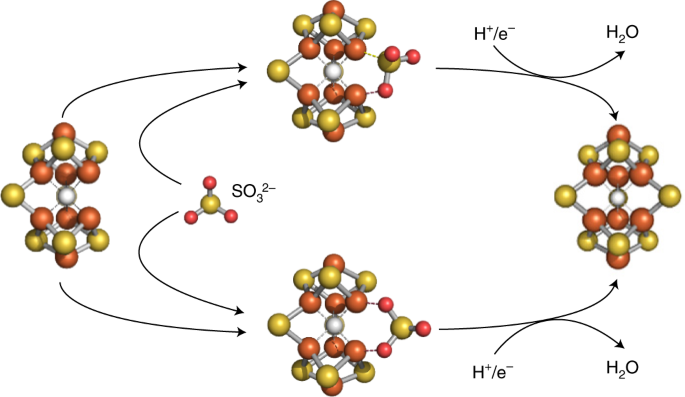
ABSTRACT Molybdenum nitrogenase catalyses the reduction of N2 to NH3 at its cofactor, an [(_R_-homocitrate)MoFe7S9C] cluster synthesized via the formation of a [Fe8S9C] L-cluster prior to
the insertion of molybdenum and homocitrate. We have previously identified a [Fe8S8C] L*-cluster, which is homologous to the core structure of the L-cluster but lacks the ‘ninth sulfur’ in
the belt region. However, direct evidence and mechanistic details of the L*- to L-cluster conversion upon ‘ninth sulfur’ insertion remain elusive. Here we trace the ‘ninth sulfur’ insertion
using SeO32− and TeO32− as ‘labelled’ SO32−. Biochemical, electron paramagnetic resonance and X-ray absorption spectroscopy/extended X-ray absorption fine structure studies suggest a role of
the ‘ninth sulfur’ in cluster transfer during cofactor biosynthesis while revealing the incorporation of Se2−- and Te2−-like species into the L-cluster. Density functional theory
calculations further point to a plausible mechanism involving in situ reduction of SO32− to S2−, thereby suggesting the utility of this reaction to label the catalytically important belt
region for mechanistic investigations of nitrogenase. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS
Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more
Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full
article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs *
Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS EVIDENCE OF SUBSTRATE BINDING AND PRODUCT RELEASE VIA BELT-SULFUR MOBILIZATION OF THE NITROGENASE COFACTOR Article 16 May 2022
CONNECTING THE GEOMETRIC AND ELECTRONIC STRUCTURES OF THE NITROGENASE IRON–MOLYBDENUM COFACTOR THROUGH SITE-SELECTIVE 57FE LABELLING Article 13 March 2023 BIOASSEMBLY OF COMPLEX IRON–SULFUR
ENZYMES: HYDROGENASES AND NITROGENASES Article 22 July 2020 DATA AVAILABILITY The authors declare that all data supporting the findings of this study are available within the article, the
Supplementary Information and the source files published alongside the article. Source data are provided with this paper. REFERENCES * Burgess, B. K. & Lowe, D. J. Mechanism of
molybdenum nitrogenase. _Chem. Rev._ 96, 2983–3012 (1996). Article CAS Google Scholar * Buscagan, T. M. & Rees, D. C. Rethinking the nitrogenase mechanism: activating the active site.
_Joule_ 3, 2662–2678 (2019). Article CAS Google Scholar * Rutledge, H. L. & Tezcan, F. A. Electron transfer in nitrogenase. _Chem. Rev._ 120, 5158–5193 (2020). Article CAS Google
Scholar * Jasniewski, A. J., Lee, C. C., Ribbe, M. W. & Hu, Y. Reactivity, mechanism, and assembly of the alternative nitrogenases. _Chem. Rev._ 120, 5107–5157 (2020). Article CAS
Google Scholar * Spatzal, T. et al. Evidence for interstitial carbon in nitrogenase FeMo cofactor. _Science_ 334, 940 (2011). Article CAS Google Scholar * Wiig, J. A., Hu, Y., Lee, C. C.
& Ribbe, M. W. Radical SAM-dependent carbon insertion into the nitrogenase M-cluster. _Science_ 337, 1672–1675 (2012). Article CAS Google Scholar * Lee, S. C., Lo, W. & Holm, R.
H. Developments in the biomimetic chemistry of cubane-type and higher nuclearity iron–sulfur clusters. _Chem. Rev._ 114, 3579–3600 (2014). Article CAS Google Scholar * Ohki, Y. &
Tatsumi, K. New synthetic routes to metal–sulfur clusters relevant to the nitrogenase metallo-clusters. _Z. Anorg. Allg. Chem._ 639, 1340–1349 (2013). Article CAS Google Scholar * Ribbe,
M. W., Hu, Y., Hodgson, K. O. & Hedman, B. Biosynthesis of nitrogenase metalloclusters. _Chem. Rev._ 114, 4063–4080 (2014). Article CAS Google Scholar * Hu, Y. & Ribbe, M. W.
Biosynthesis of the metalloclusters of nitrogenases. _Annu. Rev. Biochem._ 85, 455–483 (2016). Article CAS Google Scholar * Tanifuji, K. et al. Tracing the ‘ninth sulfur’ of the
nitrogenase cofactor via a semi-synthetic approach. _Nat. Chem._ 10, 568–572 (2018). Article CAS Google Scholar * Jasniewski, A. J. et al. Spectroscopic characterization of an eight-iron
nitrogenase cofactor precursor that lacks the “9th sulfur”. _Angew. Chem. Int. Ed._ 58, 14703–14707 (2019). Article CAS Google Scholar * Hu, Y. et al. FeMo cofactor maturation on NifEN.
_Proc. Natl Acad. Sci. USA_ 103, 17119–17124 (2006). Article CAS Google Scholar * Hu, Y. et al. Nitrogenase Fe protein: a molybdate/homocitrate insertase. _Proc. Natl Acad. Sci. USA_ 103,
17125–17130 (2006). Article CAS Google Scholar * Hu, Y. & Ribbe, M. W. Maturation of nitrogenase cofactor—the role of a class E radical SAM methyltransferase NifB. _Curr. Opin. Chem.
Biol._ 31, 188–194 (2016). Article CAS Google Scholar * Kang, W. et al. Crystallographic analysis of NifB with a full complement of clusters: structural insights into the radical
SAM-dependent carbide insertion during nitrogenase cofactor assembly. _Angew. Chem. Int. Ed._ 60, 2364–2370 (2020). Article Google Scholar * Rettberg, L. A. et al. Identity and function of
an essential nitrogen ligand of the nitrogenase cofactor biosynthesis protein NifB. _Nat. Commun._ 11, 1757 (2020). Article CAS Google Scholar * Rettberg, L. A. et al. Probing the
coordination and function of Fe4S4 modules in nitrogenase assembly protein NifB. _Nat. Commun._ 9, 2824 (2018). Article Google Scholar * Musgrave, K. B., Angove, H. C., Burgess, B. K.,
Hedman, B. & Hodgson, K. O. All-ferrous titanium(iii) citrate reduced Fe protein of nitrogenase: an XAS study of electronic and metrical structure. _J. Am. Chem. Soc._ 120, 5325–5326
(1998). Article CAS Google Scholar * Einsle, O. et al. Nitrogenase MoFe-protein at 1.16 Å resolution: a central ligand in the FeMo-cofactor. _Science_ 297, 1696–1700 (2002). Article CAS
Google Scholar * Ciurli, S., Yu, S. B., Holm, R. H., Srivastava, K. K. P. & Munck, E. Synthetic nickel–iron NiFe3Q4 cubane-type clusters (_S_ = 3/2) by reductive rearrangement of
linear [Fe3Q4(SEt)4]3− (Q = sulfur, selenium). _J. Am. Chem. Soc._ 112, 8169–8171 (1990). Article CAS Google Scholar * Bobrik, M. A. et al. Selenium substitution in [Fe4S4(SR)4]2−:
synthesis and comparative properties of [Fe4X4(YC6H5)4]2− (X, Y = sulfur, selenium) and the structure of [(CH3)4N]2[Fe4Se4(SC6H5)4]. _Inorg. Chem._ 17, 1402–1410 (1978). Article CAS Google
Scholar * Barbaro, P., Bencini, A., Bertini, I., Briganti, F. & Midollini, S. The tetranuclear trianion [Fe4Te4(SC6H5)4]3−: crystal and molecular structure and magnetic properties. _J.
Am. Chem. Soc._ 112, 7238–7246 (1990). Article CAS Google Scholar * Simon, W., Wilk, A., Krebs, B. & Henkel, G. [Fe4Te4(TePh)4]3−, the first telluride–tellurolate complex. _Angew.
Chem. Int. Ed._ 26, 1009–1010 (1987). Article Google Scholar * Zimmermann, M. D. & Tossell, J. A. Acidities of arsenic(iii) and arsenic(v) thio- and oxyacids in aqueous solution using
the CBS-QB3/CPCM method. _J. Phys. Chem. A_ 113, 5105–5111 (2009). Article CAS Google Scholar * Kang, W., Lee, C. C., Jasniewski, A. J., Ribbe, M. W. & Hu, Y. Structural evidence for
a dynamic metallocofactor during N2 reduction by Mo-nitrogenase. _Science_ 368, 1381–1385 (2020). Article CAS Google Scholar * Spatzal, T., Perez, K. A., Howard, J. B. & Rees, D. C.
Catalysis-dependent selenium incorporation and migration in the nitrogenase active site iron–molybdenum cofactor. _Elife_ 4, e11620 (2015). Article Google Scholar * Spatzal, T., Perez, K.
A., Einsle, O., Howard, J. B. & Rees, D. C. Ligand binding to the FeMo-cofactor: structures of CO-bound and reactivated nitrogenase. _Science_ 345, 1620–1623 (2014). Article CAS Google
Scholar Download references ACKNOWLEDGEMENTS This work was supported by NIH-NIGMS grants GM67626 (to M.W.R. and Y.H.), GM141046 (to Y.H. and M.W.R.), R35 GM126961 (to R.D.B.), GM110501 (to
J.Y.) and GM126289 (to J.K.). Y.O. was supported by Grant-in-Aids for Scientific Research (MEXT Japan) (nos. 19H02733 and 20K21207), International Collaborative Research Program of ICR,
Kyoto University, Takeda Science Foundation and Tatematsu Foundation. K.T. recieved support from the Kyoto University Research Fund for Young Scientist (Start-Up). XAS data were collected at
Beamlines 7-3 and 9-3 at the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory. SLAC is supported by the US Department of Energy, Office of Science, Office of
Basic Energy Sciences under contract no. DE-AC02-76SF00515. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research, and by the
National Institutes of Health, National Institute of General Medical Sciences (including P30GM133894). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Molecular Biology and
Biochemistry, University of California, Irvine, Irvine, CA, USA Kazuki Tanifuji, Andrew J. Jasniewski, Martin T. Stiebritz, Chi Chung Lee, Yilin Hu & Markus W. Ribbe * International
Research Center for Elements Science, Institute for Chemical Research, Kyoto University, Kyoto, Japan Kazuki Tanifuji & Yasuhiro Okhi * Department of Chemistry, University of California,
Davis, Davis, CA, USA David Villarreal & R. David Britt * Department of Chemistry and Biochemistry, University of Wisconsin, Milwaukee, WI, USA Jarett Wilcoxen * Molecular Biophysics
and Integrated Bioimaging Division, Lawrence Berkeley National Laboratory, Berkeley, CA, USA Ruchira Chatterjee, Isabel Bogacz, Junko Yano & Jan Kern * Stanford Synchrotron Radiation
Lightsource, SLAC National Accelerator Laboratory, Stanford University, Menlo Park, CA, USA Britt Hedman & Keith O. Hodgson * Department of Chemistry, Stanford University, Stanford, CA,
USA Keith O. Hodgson * Department of Chemistry, University of California, Irvine, Irvine, CA, USA Markus W. Ribbe Authors * Kazuki Tanifuji View author publications You can also search for
this author inPubMed Google Scholar * Andrew J. Jasniewski View author publications You can also search for this author inPubMed Google Scholar * David Villarreal View author publications
You can also search for this author inPubMed Google Scholar * Martin T. Stiebritz View author publications You can also search for this author inPubMed Google Scholar * Chi Chung Lee View
author publications You can also search for this author inPubMed Google Scholar * Jarett Wilcoxen View author publications You can also search for this author inPubMed Google Scholar *
Yasuhiro Okhi View author publications You can also search for this author inPubMed Google Scholar * Ruchira Chatterjee View author publications You can also search for this author inPubMed
Google Scholar * Isabel Bogacz View author publications You can also search for this author inPubMed Google Scholar * Junko Yano View author publications You can also search for this author
inPubMed Google Scholar * Jan Kern View author publications You can also search for this author inPubMed Google Scholar * Britt Hedman View author publications You can also search for this
author inPubMed Google Scholar * Keith O. Hodgson View author publications You can also search for this author inPubMed Google Scholar * R. David Britt View author publications You can also
search for this author inPubMed Google Scholar * Yilin Hu View author publications You can also search for this author inPubMed Google Scholar * Markus W. Ribbe View author publications You
can also search for this author inPubMed Google Scholar CONTRIBUTIONS K.T., A.J.J., M.T.S., C.C.L, Y.H. and M.W.R. designed experiments. K.T., A.J.J., M.T.S., C.C.L, D.V., J.W., R.C., I.B.,
J.Y., J.K., R.D.B., Y.H. and M.W.R. analysed data. K.T., A.J.J., M.T.S., C.C.L., D.V., J.W., R.C., I.B., J.Y and J.K. performed experiments. Y.O., B.H. and K.O.H provided material and/or
technical resources. Y.H. and M.W.R. wrote the manuscript with input from all authors. CORRESPONDING AUTHORS Correspondence to R. David Britt, Yilin Hu or Markus W. Ribbe. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Chemistry_ thanks Benoit D’Autréaux and the other,
anonymous, reviewer(s) for their contribution to the peer review of this work. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary methods, Figs. 1–3, Tables 1–7 and references. REPORTING SUMMARY SOURCE DATA SOURCE DATA FIG. 1
Source Data Fig. 1a–c SOURCE DATA FIG. 2 Source Data Fig. 2a,b. SOURCE DATA FIG. 3 Source Data Fig. 3a–c. SOURCE DATA FIG. 4 Source Data Fig. 4a–c. SOURCE DATA FIG. 5 Source Data Fig. 5c
SOURCE DATA FIG. 6 Source Data Fig. 6a,b. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Tanifuji, K., Jasniewski, A.J., Villarreal, D. _et al._ Tracing
the incorporation of the “ninth sulfur” into the nitrogenase cofactor precursor with selenite and tellurite. _Nat. Chem._ 13, 1228–1234 (2021). https://doi.org/10.1038/s41557-021-00799-8
Download citation * Received: 11 December 2020 * Accepted: 25 August 2021 * Published: 11 October 2021 * Issue Date: December 2021 * DOI: https://doi.org/10.1038/s41557-021-00799-8 SHARE
THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative