Play all audios:
ABSTRACT Aqueous organic redox flow batteries offer a safe and potentially inexpensive solution to the problem of storing massive amounts of electricity produced from intermittent
renewables. However, molecular decomposition represents a major barrier to commercialization—and although structural modifications can improve stability, it comes at the expense of synthetic
cost and molecular weight. Now, utilizing 2,6-dihydroxy-anthraquinone (DHAQ) without further structural modification, we demonstrate that the regeneration of the original molecule after
decomposition represents a viable route to achieve low-cost, long-lifetime aqueous organic redox flow batteries. We used in situ (online) NMR and electron paramagnetic resonance, and
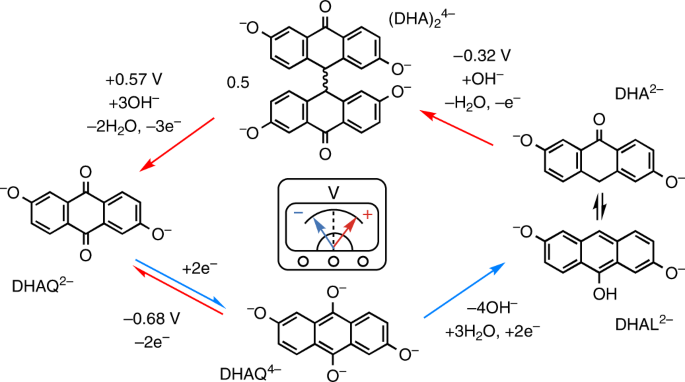
complementary electrochemical analyses to show that the decomposition compound 2,6-dihydroxy-anthrone (DHA) and its tautomer, 2,6-dihydroxy-anthranol (DHAL) can be recomposed to DHAQ
electrochemically through two steps: oxidation of DHA(L)2− to the dimer (DHA)24− by one-electron transfer followed by oxidation of (DHA)24− to DHAQ2− by three-electron transfer per DHAQ
molecule. This electrochemical regeneration process also rejuvenates the positive electrolyte—rebalancing the states of charge of both electrolytes without introducing extra ions. Access
through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other
Nature Portfolio journals Get Nature+, our best-value online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and online
access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which
are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS
AIR-STABLE NAPHTHALENE DERIVATIVE-BASED ELECTROLYTES FOR SUSTAINABLE AQUEOUS FLOW BATTERIES Article 28 August 2024 AN AMPHOTERIC AND HYDROGEN-BOND-RICH ARTIFICIAL Α-AMINO ACID FOR HIGHLY
DURABLE AQUEOUS REDOX FLOW BATTERIES Article Open access 21 May 2025 AN ACTIVE AND DURABLE MOLECULAR CATALYST FOR AQUEOUS POLYSULFIDE-BASED REDOX FLOW BATTERIES Article 02 October 2023 DATA
AVAILABILITY Data in the main paper and its Supplementary Information are available from https://doi.org/10.6084/m9.figshare.19612128. Source Data are provided with this paper. REFERENCES *
Huskinson, B. T. et al. A metal-free organic–inorganic aqueous flow battery. _Nature_ 505, 195–198 (2014). Article Google Scholar * Lin, K. et al. Alkaline quinone flow battery. _Science_
349, 1529–1532 (2015). Article Google Scholar * Kwabi, D. G., Ji, Y. & Aziz, M. J. Electrolyte lifetime in aqueous organic redox flow batteries: a critical review. _Chem. Rev._ 120,
6467 (2020). Article CAS PubMed Google Scholar * Beh, E. S. et al. A neutral pH aqueous organic–organometallic redox flow battery with extremely high capacity retention. _ACS Energy
Lett._ 2, 639–644 (2017). Article Google Scholar * Kwabi, D. G. et al. Alkaline quinone flow battery with long lifetime at pH 12. _Joule_ 2, 1894–1906 (2018). Article Google Scholar *
Ji, Y. et al. A phosphonate-functionalized quinone redox flow battery at near-neutral pH with record capacity retention rate. _Adv. Energy Mater._ 9, 1900039 (2019). Article Google Scholar
* Wu, M. et al. Extremely stable anthraquinone negolytes synthesized from common precursors. _Chem_ 6, 1432–1442 (2020). Article Google Scholar * Jin, S. et al. Near neutral pH redox
flow battery with low permeability and long-lifetime phosphonated viologen active species. _Adv. Energy Mater._ 10, 2000100 (2020). Article CAS Google Scholar * Pang, S., Wang, X., Wang,
P. & Ji, Y. Biomimetic amino acid functionalized phenazine flow batteries with long lifetime at near-neutral pH. _Angew. Chem. Int. Ed._ 60, 5289 (2021). Article CAS Google Scholar *
Xu, J., Pang, S., Wang, X., Wang, P. & Ji, Y. Ultrastable aqueous phenazine flow batteries with high capacity operated at elevated temperatures. _Joule_ 5, 2437–2449 (2021). Article CAS
Google Scholar * Gregory, T. D., Perry, M. L. & Albertus, P. Cost and price projections of synthetic active materials for redox flow batteries. _J. Power Sources_ 499, 229965 (2021).
Article CAS Google Scholar * Goulet, M.-A. et al. Extending the lifetime of organic flow batteries via redox state management. _J. Am. Chem. Soc._ 141, 8014–8019 (2019). Article CAS
PubMed Google Scholar * Zhao, E. W. et al. In situ NMR metrology reveals reaction mechanisms in redox flow batteries. _Nature_ 579, 224–228 (2020). Article CAS PubMed Google Scholar *
Zhao, E. W. et al. Coupled In situ NMR and EPR studies reveal the electron transfer rate and electrolyte decomposition in redox flow batteries. _J. Am. Chem. Soc._ 143, 1885–1895 (2021).
PubMed PubMed Central Google Scholar * Jing, Y. et al. In situ electrosynthesis of anthraquinone electrolytes in aqueous flow batteries. _Green Chem._ 22, 6084–6092 (2020). Article
Google Scholar * Goulet, M. A. et al. Correction to “Extending the lifetime of organic flow batteries via redox state management.”. _J. Am. Chem. Soc._ 143, 14019 (2021). Article CAS
PubMed Google Scholar * McCann, G. M., McDonnell, C. M., Magris, L. & O’Ferrall, R. A. M. Enol–keto tautomerism of 9-anthrol and hydrolysis of its methyl ether. _J. Chem. Soc._ 2,
784–795 (2002). Google Scholar * Zhao, E. W., Shellard, E. J. K., Klusener, P. A. A. & Grey, C. P. In situ bulk magnetization measurements reveal the state of charge of redox flow
batteries. _Chem. Commun._ 58, 1342–1345 (2022). Google Scholar * Mitra, A., Seaton, P. J., Assarpour, R. A. & Williamson, T. Unprecedented concentration dependent chemical shift
variation in 1H-NMR studies: a caveat in the investigations of molecular recognition and structure elucidation. _Tetrahedron_ 54, 15489 (1998). Article CAS Google Scholar * Yao, Y., Lei,
J., Shi, Y., Ai, F. & Lu, Y.-C. Assessment methods and performance metrics for redox flow batteries. _Nat. Energy_ 6, 582–588 (2021). Article Google Scholar * Fain, V. Y., Zaitsev, B.
E. & Ryabov, M. A. Anthraquinones tautomerism: VII. Hydroxy-substituted anthraquinones. _Russ. J. Org. Chem._ 43, 1460 (2007). Article CAS Google Scholar * Páez, T., Martínez-Cuezva,
A., Palma, J. & Ventosa, E. Revisiting the cycling stability of ferrocyanide in alkaline media for redox flow batteries. _J. Power Sources_ 471, 228453 (2020). Article Google Scholar *
Páez, T., Martínez-Cuezva, A., Marcilla, R., Palma, J. & Ventosa, E. Mitigating capacity fading in aqueous organic redox flow batteries through a simple electrochemical charge balancing
protocol. _J. Power Sources_ 512, 230516 (2021). Article Google Scholar * Dieterich, V. et al. Estimating the cost of organic battery active materials: a case study on anthraquinone
disulfonic acid. _Translational Mater. Res._ 5, 034001 (2018). Article Google Scholar * Goulet, M.-A. & Aziz, M. J. Flow battery molecular reactant stability determined by symmetric
cell cycling methods. _J. Electrochem. Soc._ 165, A1466 (2018). Article CAS Google Scholar * Wang, F. et al. Stable tetrasubstituted quinone redox reservoir for enhancing decoupled
hydrogen and oxygen evolution. _ACS Energy Lett._ 6, 1533–1539 (2021). Article CAS PubMed PubMed Central Google Scholar * Brushett, F. R., Aziz, M. J. & Rodby, K. E. On lifetime and
cost of redox-active organics for aqueous flow batteries. _ACS Energy Lett._ 5, 879–884 (2020). Article Google Scholar * Shaw, A. A. S., Christophe, Dauphin, Jean-Francois & Ancian,
Bernard Artifact-free PFG-enhanced double-quantum-filtered COSY experiments. _J. Magn. Reson. A_ 120, 110–115 (1996). Article Google Scholar Download references ACKNOWLEDGEMENTS Research
at Harvard was supported by the US National Science Foundation through grant no. CBET-1914543, by a US DOE award no. DE-AC05-76RL01830 through PNNL subcontract no. 535264, and by a grant
from the Massachusetts Clean Energy Center. Research at University of Cambridge was supported by Centre of Advanced Materials for Integrated Energy Systems (CAM-IES), via EPSRC grant no.
EP/P007767/1 and Shell. E.W.Z acknowledges the STFC Futures Early Career Award, grant no. ST/R006873/1. The funders had no role in study design, data collection and analysis, decision to
publish or preparation of the manuscript. We acknowledge P.A.A. Klusener from Shell for useful discussions. We thank D. A. Pollack, and K. Amini for useful discussions. AUTHOR INFORMATION
Author notes * Evan Wenbo Zhao Present address: Magnetic Resonance Research Center, Institute for Molecules and Materials, Radboud University Nijmegen, Nijmegen, the Netherlands *
Marc-Antoni Goulet Present address: Department of Chemical and Materials Engineering, Concordia University, Montreal, Quebec, Canada * Ali Davoodi Present address: Sichuan
University-Pittsburgh Institute, Sichuan University, Chengdu, China * These authors contributed equally: Yan Jing, Evan Wenbo Zhao, Marc-Antoni Goulet. AUTHORS AND AFFILIATIONS * Department
of Chemistry and Chemical Biology, Harvard University, Cambridge, MA, USA Yan Jing & Roy G. Gordon * Yusuf Hamied Department of Chemistry, University of Cambridge, Cambridge, UK Evan
Wenbo Zhao, Erlendur Jónsson & Clare P. Grey * John A. Paulson School of Engineering and Applied Sciences, Harvard University, Cambridge, MA, USA Marc-Antoni Goulet, Meisam Bahari, Eric
M. Fell, Shijian Jin, Ali Davoodi, Min Wu, Roy G. Gordon & Michael J. Aziz * Materials and Metallurgical Engineering Department, Faculty of Engineering, Ferdowsi University of Mashhad
(FUM), Mashhad, Iran Ali Davoodi Authors * Yan Jing View author publications You can also search for this author inPubMed Google Scholar * Evan Wenbo Zhao View author publications You can
also search for this author inPubMed Google Scholar * Marc-Antoni Goulet View author publications You can also search for this author inPubMed Google Scholar * Meisam Bahari View author
publications You can also search for this author inPubMed Google Scholar * Eric M. Fell View author publications You can also search for this author inPubMed Google Scholar * Shijian Jin
View author publications You can also search for this author inPubMed Google Scholar * Ali Davoodi View author publications You can also search for this author inPubMed Google Scholar *
Erlendur Jónsson View author publications You can also search for this author inPubMed Google Scholar * Min Wu View author publications You can also search for this author inPubMed Google
Scholar * Clare P. Grey View author publications You can also search for this author inPubMed Google Scholar * Roy G. Gordon View author publications You can also search for this author
inPubMed Google Scholar * Michael J. Aziz View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.J.A., R.G.G., C.P.G. supervised the project.
Y.J., E.W.Z., M.-A.G., M.J.A., R.G.G. and C.P.G. conceived the idea. Y.J., E.W.Z. and M.-A.G. designed the experiment. E.W.Z. performed the in situ NMR and EPR experiments and analysis. Y.J.
performed the ex situ NMR experiments and analysis. M.-A.G., M.B., E.M.F. and Y.J. performed DHA(L), ADS elctrochemical oxidation, and DHAQ and AQDS cell cycling. M.B. performed
three-electrode cell tests. M.-A.G., S.J. and Y.J. performed postmortem CV, NMR, LC-NMR experiments and a cell cycling. M.B. performed t-cell cycling with periodic aeration. E.J. made
intellectual contributions. M.W. and Y.J. synthesized DHA(L) and ADS, respectively. All authors contributed to the discussion of the project. Y.J., E.W.Z, M.-A.G., M.J.A., C.P.G. wrote the
manuscript with input from all co-authors. CORRESPONDING AUTHORS Correspondence to Clare P. Grey, Roy G. Gordon or Michael J. Aziz. ETHICS DECLARATIONS COMPETING INTERESTS The authors
declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Chemistry_ thanks Antoni Forner-Cuenca, Yi-Chun Lu and Edgar Ventosa for their contribution to the peer review of
this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. EXTENDED DATA
EXTENDED DATA FIG. 1 ELECTROCHEMICAL OXIDATION OF DHA(L)2−. A & B, Linear sweep voltammetry (LSV) and the 1st order differential of 10 mM DHA(L)2− in 1 M KOH supporting electrolyte
indicating a peak for dimerization reaction around −260 mV vs. SHE and a second oxidation reaction starting around +300 mV vs. SHE, before being overshadowed by oxygen evolution at around
+650 mV vs. SHE. We consider the potentials (−0.32 V, + 0.57 V) at the 1st order differential peak current as the approximations of oxidation potentials of [DHA(L)2− to (DHA)24−] and
[(DHA)24− to DHAQ2−]. The 1st order differential was from the LSV curve obtained at 20 mV/s. C & D, Cyclic voltammograms of 10 mM pure DHA in 1 M KOH before and after a + 110 mV vs. SHE
10-minute potential hold with a highly porous Zoltek PXFB working electrode. New peaks match those of dimer observed in a previous study[2]; cyclic voltammograms of 10 mM pure DHA in 1 M KOH
before and after +410 mV vs. SHE 10-minute potential hold with a highly porous working electrode. New peaks match those of DHAQ2−. E, Potential hold of 0.1 M DHA(L)2− in 1.2 M KOH negolyte
in full cell containing a 2X excess of 50 mM K4[Fe(CN)6] and 50 mM K3[Fe(CN)6] posolyte. The Y-axis represent the number of coulombs extracted from DHA(L)2− electrolyte over electrochemical
oxidation. F, 1H NMR spectra of DHA(L)2− negolyte before and after 30-min hold at different cell voltages. EXTENDED DATA FIG. 2 DHAQ REGENERATION HYSTERESIS. A, Repeated application of
electrochemical regeneration of negolyte in battery composed of 6 mL 100 mM DHAQ2− in the negolyte and a mixture of 35 mL 100 mM K4[Fe(CN)6] and 50 mM K3[Fe(CN)6] in the posolyte.
Galvanostatic cycling at ±50 mA/cm2 with potential holds at 1.0 and 1.5 V and current cutoff of ±1 mA/cm2 during normal cycles. Every 50 cycles an additional discharge step at −2 mA/cm2
until potential limit and a further hold until current decreases to −0.2 mA/cm2. B, Aggregate data for 3 cell replicates with varying potential limits for regeneration treatment indicating
partial dependence of recovery ratio on recovery from prior treatment. EXTENDED DATA FIG. 3 ENERGETIC COST OF ELECTROCHEMICAL REGENERATION IN FIG. 4A AND C. A, Integrated areas above and
below 0 V of the cell discharge voltage profile with electrochemical regeneration in Fig. 4c. B, Integrated areas above and below 0 V of the cell discharge voltage profiles with 13
regeneration processes in Fig. 4a. The area ratio (%) represents the percentage of discharged electrical energy that is subsequently used for regeneration during a single discharge process,
reflecting the energetic cost. It is, on average, 1.30%. Note that the electrochemical regeneration step is not performed in every cycle, but rather once every 51 cycles. Relative to the
total electricity discharged during 51 cycles, the energetic cost for regeneration is only 0.025%. EXTENDED DATA FIG. 4 DHAQ CONCENTRATION TRACKING. A, Cycling of battery composed of 5 mL
100 mM DHAQ2− in the negolyte and 20 mL of a mixture of 100 mM K4[Fe(CN)6] and 50 mM K3[Fe(CN)6] in the posolyte. Galvanostatic cycling at ±50 mA/cm2 with potential holds at 1.0 V and 1.5 V
and current cutoff of ±1 mA/cm2 during normal cycles. Three electrochemical oxidation treatments before an aliquot was removed from negolyte reservoir on day 10 for concentration
measurement. B, Cyclic voltammograms of 20 mM DHAQ2− in 1 M KOH on glassy carbon working electrode. Pristine DHAQ2− electrolyte measured before and after dilution by 10% to verify resolution
of measurement. Cycled DHAQ2− data refers to an aliquot removed from negolyte in part a, after ~25% capacity had been lost. No change in peak current suggests DHAQ2− concentration has not
changed during cycling after regeneration treatment. C & D, Comparison of DHAQ2− NMR peak integral with respect to DMSO internal standard. Similarity of the peak integral of cycled
electrolyte which had lost ~25% of capacity to that of pristine DHAQ2− does not indicate overall decrease in DHAQ2− concentration. An aliquot of the cycled DHAQ2− was taken for 1H NMR
measurement when ~25% capacity was lost on day 10.5. E, LC−MS result of the cycled DHAQ2−. The peak at 10.8–11.1 min is from DHAQ2−, which is the sole compound detected. Other peaks are from
background, which can be ignored. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figs. 1–15 and Discussion after some captions. SOURCE DATA SOURCE DATA FIG. 1 Unprocessed
excel data for DHAQ regeneration hysteresis. SOURCE DATA FIG. 2 Unprocessed in situ NMR data. SOURCE DATA FIG. 3 Unprocessed in situ NMR data. SOURCE DATA FIG. 4 Unprocessed excel and.csv
data for flow battery tests. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Jing, Y., Zhao, E.W., Goulet, MA. _et al._ In situ electrochemical
recomposition of decomposed redox-active species in aqueous organic flow batteries. _Nat. Chem._ 14, 1103–1109 (2022). https://doi.org/10.1038/s41557-022-00967-4 Download citation *
Received: 16 December 2021 * Accepted: 04 May 2022 * Published: 16 June 2022 * Issue Date: October 2022 * DOI: https://doi.org/10.1038/s41557-022-00967-4 SHARE THIS ARTICLE Anyone you share
the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative