Play all audios:
ABSTRACT The three-spined stickleback (_Gasterosteus aculeatus_) is an important model system for the study of parallel evolution in the wild, having repeatedly colonized and adapted to
freshwater from the sea throughout the northern hemisphere. Previous studies identified numerous genomic regions showing consistent genetic differentiation between freshwater and marine
ecotypes but these had typically limited geographic sampling and mostly focused on the Eastern Pacific region. We analysed population genomic data from global samples of the three-spined
stickleback marine and freshwater ecotypes to detect loci involved in parallel evolution at different geographic scales. Most signatures of parallel evolution were unique to the Eastern
Pacific and trans-oceanic marine–freshwater differentiation was restricted to a limited number of shared genomic regions, including three chromosomal inversions. On the basis of simulations
and empirical data, we demonstrate that this could result from the stochastic loss of freshwater-adapted alleles during the invasion of the Atlantic basin and selection against
freshwater-adapted variants in the sea, both of which can reduce standing genetic variation available for freshwater adaptation outside the Eastern Pacific region. Moreover, the elevated
linkage disequilibrium associated with marine–freshwater differentiation in the Eastern Pacific is consistent with secondary contact between marine and freshwater populations that evolved in
isolation from each other during past glacial periods. Thus, contrary to what earlier studies from the Eastern Pacific region have led us to believe, parallel marine–freshwater
differentiation in sticklebacks is far less prevalent and pronounced in all other parts of the species global distribution range. Access through your institution Buy or subscribe This is a
preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value
online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 digital issues and online access to articles $119.00 per year only $9.92 per issue
Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL
ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS INTERCONTINENTAL GENOMIC PARALLELISM IN
MULTIPLE THREE-SPINED STICKLEBACK ADAPTIVE RADIATIONS Article 30 November 2020 GENOMIC DATA AND MULTI-SPECIES DEMOGRAPHIC MODELLING UNCOVER PAST HYBRIDIZATION BETWEEN CURRENTLY ALLOPATRIC
FRESHWATER SPECIES Article 30 August 2021 FINE-SCALE CONTEMPORARY RECOMBINATION VARIATION AND ITS FITNESS CONSEQUENCES IN ADAPTIVELY DIVERGING STICKLEBACK FISH Article Open access 05 June
2024 DATA AVAILABILITY The RAD-seq data have been uploaded to the GenBank under accession numbers SAMN14078677 to SAMN14078738 (https://www.ncbi.nlm.nih.gov/Traces/study/?acc=PRJNA605695).
Previously published sequencing data are retrieved from studies specified in Supplementary Table 1. CODE AVAILABILITY The scripts used for analysing empirical data (genotype likelihood
estimation, filtering, LDna) and simulated data are available in DRYAD repository: https://doi.org/10.5061/dryad.b2rbnzsb1. CHANGE HISTORY * _ 22 APRIL 2021 A Correction to this paper has
been published: https://doi.org/10.1038/s41559-021-01447-7 _ REFERENCES * Schluter, D. & Conte, G. L. Genetics and ecological speciation. _Proc. Natl Acad. Sci. USA_ 106, 9955–9962
(2009). Article CAS PubMed PubMed Central Google Scholar * Arendt, J. & Reznick, D. Convergence and parallelism reconsidered: what have we learned about the genetics of adaptation?
_Trends Ecol. Evol._ 23, 26–32 (2008). Article PubMed Google Scholar * DeFaveri, J., Shikano, T., Shimada, Y., Goto, A. & Merila, J. Global analysis of genes involved in freshwater
adaptation in threespine sticklebacks (_Gasterosteus aculeatus_). _Evolution_ 65, 1800–1807 (2011). Article PubMed Google Scholar * Stern, D. L. The genetic causes of convergent
evolution. _Nat. Rev. Genet._ 14, 751–764 (2013). Article CAS PubMed Google Scholar * Bell, M. A. & Foster, S. A. _The Evolutionary Biology of the Threespine Stickleback_ (Oxford
Univ. Press, 1994). * Gibson, G. The synthesis and evolution of a supermodel. _Science_ 307, 1890–1891 (2005). Article CAS PubMed Google Scholar * Hendry, A. P., Peichel, C. L.,
Matthews, B., Boughman, J. W. & Nosil, P. Stickleback research: the now and the next. _Evol. Ecol. Res._ 15, 111–141 (2013). Google Scholar * Lescak, E. A. et al. Evolution of
stickleback in 50 years on earthquake-uplifted islands. _Proc. Natl Acad. Sci. USA_ 112, E7204–E7212 (2015). Article CAS PubMed PubMed Central Google Scholar * Östlund-Nilsson, S.,
Mayer, I. & Huntingford, F. A. _Biology of the Three-spined Stickleback_ (CRC Press, 2006). * McKinnon, J. S. & Rundle, H. D. Speciation in nature: the threespine stickleback model
systems. _Trends Ecol. Evol._ 17, 480–488 (2002). Article Google Scholar * Jones, F. C. et al. The genomic basis of adaptive evolution in threespine sticklebacks. _Nature_ 484, 55–61
(2012). Article CAS PubMed PubMed Central Google Scholar * Ferchaud, A. L. & Hansen, M. M. The impact of selection, gene flow and demographic history on heterogeneous genomic
divergence: three-spine sticklebacks in divergent environments. _Mol. Ecol._ 25, 238–259 (2016). Article CAS PubMed Google Scholar * Hohenlohe, P. A. et al. Population genomics of
parallel adaptation in threespine stickleback using sequenced RAD tags. _PLoS Genet._ 6, e1000862 (2010). Article PubMed PubMed Central CAS Google Scholar * Hohenlohe, P. A. &
Magalhaes, I. S. in _Population Genomics_ (eds Oleksiak, M. F. & Rajora, O.P.) 249–276 (Springer, 2020). * Liu, S., Ferchaud, A. L., Gronkjaer, P., Nygaard, R. & Hansen, M. M.
Genomic parallelism and lack thereof in contrasting systems of three-spined sticklebacks. _Mol. Ecol._ 27, 4725–4743 (2018). Article PubMed Google Scholar * Pujolar, J. M., Ferchaud, A.
L., Bekkevold, D. & Hansen, M. M. Non-parallel divergence across freshwater and marine three-spined stickleback _Gasterosteus aculeatus_ populations. _J. Fish Biol._ 91, 175–194 (2017).
Article CAS PubMed Google Scholar * Terekhanova, N. V., Barmintseva, A. E., Kondrashov, A. S., Bazykin, G. A. & Mugue, N. S. Architecture of parallel adaptation in ten lacustrine
threespine stickleback populations from the White Sea area. _Genome Biol. Evol._ 11, 2605–2618 (2019). Article PubMed PubMed Central Google Scholar * Terekhanova, N. V. et al. Fast
evolution from precast bricks: genomics of young freshwater populations of threespine stickleback _Gasterosteus aculeatus_. _PLoS Genet._ 10, e1004696 (2014). Article PubMed PubMed Central
CAS Google Scholar * Chan, Y. F. et al. Adaptive evolution of pelvic reduction in sticklebacks by recurrent deletion of a Pitx1 enhancer. _Science_ 327, 302–305 (2010). Article CAS
PubMed Google Scholar * Colosimo, P. F. et al. Widespread parallel evolution in sticklebacks by repeated fixation of ectodysplasin alleles. _Science_ 307, 1928–1933 (2005). Article CAS
PubMed Google Scholar * Nelson, T. C. & Cresko, W. A. Ancient genomic variation underlies repeated ecological adaptation in young stickleback populations. _Evol. Lett._ 2, 9–21 (2018).
Article PubMed PubMed Central Google Scholar * Kemppainen, P. et al. Linkage disequilibrium network analysis (LDna) gives a global view of chromosomal inversions, local adaptation and
geographic structure. _Mol. Ecol. Resour._ 15, 1031–1045 (2015). Article CAS PubMed PubMed Central Google Scholar * Betancur, R. R., Orti, G. & Pyron, R. A. Fossil-based comparative
analyses reveal ancient marine ancestry erased by extinction in ray-finned fishes. _Ecol. Lett._ 18, 441–450 (2015). Article Google Scholar * Matschiner, M., Hanel, R. & Salzburger,
W. On the origin and trigger of the notothenioid adaptive radiation. _PLoS ONE_ 6, e18911 (2011). Article CAS PubMed PubMed Central Google Scholar * Meynard, C. N., Mouillot, D.,
Mouquet, N. & Douzery, E. J. A phylogenetic perspective on the evolution of Mediterranean teleost fishes. _PLoS ONE_ 7, e36443 (2012). Article CAS PubMed PubMed Central Google
Scholar * Sanciangco, M. D., Carpenter, K. E. & Betancur, R. R. Phylogenetic placement of enigmatic percomorph families (Teleostei: Percomorphaceae). _Mol. Phylogenet. Evol._ 94,
565–576 (2016). Article PubMed Google Scholar * Fang, B., Merila, J., Matschiner, M. & Momigliano, P. Estimating uncertainty in divergence times among three-spined stickleback clades
using the multispecies coalescent. _Mol. Phylogenet. Evol._ 142, 106646 (2020). Article PubMed Google Scholar * Fang, B., Merila, J., Ribeiro, F., Alexandre, C. M. & Momigliano, P.
Worldwide phylogeny of three-spined sticklebacks. _Mol. Phylogenet. Evol._ 127, 613–625 (2018). Article PubMed Google Scholar * Orti, G., Bell, M. A., Reimchen, T. E. & Meyer, A.
Global survey of mitochondrial DNA sequences in the threespine stickleback: evidence for recent migrations. _Evolution_ 48, 608–622 (1994). Article PubMed Google Scholar * Halliburton, R.
& Halliburton, R. _Introduction to Population Genetics_ (Pearson/Prentice Hall, 2004). * Hyten, D. L. et al. Impacts of genetic bottlenecks on soybean genome diversity. _Proc. Natl
Acad. Sci. USA_ 103, 16666–16671 (2006). Article CAS PubMed PubMed Central Google Scholar * Johannesson, K. et al. Repeated evolution of reproductive isolation in a marine snail:
unveiling mechanisms of speciation. _Philos. Trans. R. Soc. Lond. B_ 365, 1735–1747 (2010). Article Google Scholar * Kemppainen, P., Lindskog, T., Butlin, R. & Johannesson, K. Intron
sequences of arginine kinase in an intertidal snail suggest an ecotype-specific selective sweep and a gene duplication. _Heredity_ 106, 808–816 (2011). Article CAS PubMed Google Scholar
* Roesti, M., Gavrilets, S., Hendry, A. P., Salzburger, W. & Berner, D. The genomic signature of parallel adaptation from shared genetic variation. _Mol. Ecol._ 23, 3944–3956 (2014).
Article PubMed PubMed Central Google Scholar * Varadharajan, S. et al. A high-quality assembly of the nine-spined stickleback (_Pungitius pungitius_) genome. _Genome Biol. Evol_. 11,
3291–3308 (2019). * Feder, J. L. & Nosil, P. The efficacy of divergence hitchhiking in generating genomic islands during ecological speciation. _Evolution_ 64, 1729–1747 (2010). Article
PubMed Google Scholar * Ramachandran, S. et al. Support from the relationship of genetic and geographic distance in human populations for a serial founder effect originating in Africa.
_Proc. Natl Acad. Sci. USA_ 102, 15942–15947 (2005). Article CAS PubMed PubMed Central Google Scholar * Bierne, N., Gagnaire, P. A. & David, P. The geography of introgression in a
patchy environment and the thorn in the side of ecological speciation. _Curr. Zool._ 59, 72–86 (2013). Article Google Scholar * Baker, V. R. & Bunker, R. C. Cataclysmic Late
Pleistocene flooding from glacial Lake Missoula—a review. _Quat. Sci. Rev._ 4, 1–41 (1985). Article Google Scholar * Bretz, J. H. The Lake Missoula floods and the channeled scabland. _J.
Geol._ 77, 505–543 (1969). Article Google Scholar * Oviatt, C. G. Chronology of Lake Bonneville, 30,000 to 10,000 yr BP. _Quat. Sci. Rev._ 110, 166–171 (2015). Article Google Scholar *
Upham, W. _The Glacial Lake Agassiz_ Vol. 25 (US Government Printing Office, 1896). * Hohenlohe, P. A., Bassham, S., Currey, M. & Cresko, W. A. Extensive linkage disequilibrium and
parallel adaptive divergence across threespine stickleback genomes. _Philos. Trans. R. Soc. Lond. B_ 367, 395–408 (2012). Article CAS Google Scholar * Bolnick, D. I., Barrett, R. D. H.,
Oke, K. B., Rennison, D. J. & Stuart, Y. E. (Non)parallel evolution. _Annu. Rev. Ecol. Evol. Syst._ 49, 303–330 (2018). Article Google Scholar * Roda, F., Walter, G. M., Nipper, R.
& Ortiz-Barrientos, D. Genomic clustering of adaptive loci during parallel evolution of an Australian wildflower. _Mol. Ecol._ 26, 3687–3699 (2017). Article CAS PubMed Google Scholar
* Barghi, N. et al. Genetic redundancy fuels polygenic adaptation in _Drosophila_. _PLoS Biol._ 17, e3000128 (2019). Article CAS PubMed PubMed Central Google Scholar * Kautt, A. F.,
Elmer, K. R. & Meyer, A. Genomic signatures of divergent selection and speciation patterns in a ‘natural experiment’, the young parallel radiations of Nicaraguan crater lake cichlid
fishes. _Mol. Ecol._ 21, 4770–4786 (2012). Article PubMed Google Scholar * Le Moan, A., Gagnaire, P. A. & Bonhomme, F. Parallel genetic divergence among coastal–marine ecotype pairs
of European anchovy explained by differential introgression after secondary contact. _Mol. Ecol._ 25, 3187–3202 (2016). Article PubMed CAS Google Scholar * Westram, A. et al. Do the same
genes underlie parallel phenotypic divergence in different _Littorina saxatilis_ populations? _Mol. Ecol._ 23, 4603–4616 (2014). Article CAS PubMed PubMed Central Google Scholar *
Morales, H. E. et al. Genomic architecture of parallel ecological divergence: beyond a single environmental contrast. _Sci. Adv._ 5, eaav9963 (2019). Article PubMed PubMed Central Google
Scholar * Roesti, M., Kueng, B., Moser, D. & Berner, D. The genomics of ecological vicariance in threespine stickleback fish. _Nat. Commun._ 6, 8767 (2015). Article CAS PubMed Google
Scholar * Twyford, A. D. & Friedman, J. Adaptive divergence in the monkey flower _Mimulus guttatus_ is maintained by a chromosomal inversion. _Evolution_ 69, 1476–1486 (2015). Article
PubMed PubMed Central Google Scholar * Faria, R. et al. Multiple chromosomal rearrangements in a hybrid zone between _Littorina saxatilis_ ecotypes. _Mol. Ecol._ 28, 1375–1393 (2018).
Article CAS Google Scholar * Westram, A. M. et al. Clines on the seashore: the genomic architecture underlying rapid divergence in the face of gene flow. _Evol. Lett._ 2, 297–309 (2018).
Article PubMed PubMed Central Google Scholar * Paccard, A. et al. Repeatability of adaptive radiation depends on spatial scale: regional versus global replicates of stickleback in lake
versus stream habitats. _J. Hered._ 111, 43–56 (2020). CAS PubMed Google Scholar * Conte, G. L. et al. Extent of QTL reuse during repeated phenotypic divergence of sympatric threespine
stickleback. _Genetics_ 201, 1189–1200 (2015). Article PubMed PubMed Central CAS Google Scholar * Conte, G. L., Arnegard, M. E., Peichel, C. L. & Schluter, D. The probability of
genetic parallelism and convergence in natural populations. _Proc. Biol. Sci._ 279, 5039–5047 (2012). PubMed PubMed Central Google Scholar * Hubbard, T. et al. Ensembl 2005. _Nucleic
Acids Res._ 33, D447–D453 (2005). Article CAS PubMed Google Scholar * Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. _Bioinformatics_ 25,
1754–1760 (2009). CAS PubMed PubMed Central Google Scholar * Catchen, J., Hohenlohe, P. A., Bassham, S., Amores, A. & Cresko, W. A. Stacks: an analysis tool set for population
genomics. _Mol. Ecol._ 22, 3124–3140 (2013). Article PubMed PubMed Central Google Scholar * Li, H. A statistical framework for SNP calling, mutation discovery, association mapping and
population genetical parameter estimation from sequencing data. _Bioinformatics_ 27, 2987–2993 (2011). Article CAS PubMed PubMed Central Google Scholar * Korneliussen, T. S.,
Albrechtsen, A. & Nielsen, R. ANGSD: analysis of next generation sequencing data. _BMC Bioinform._ 15, 356 (2014). Article Google Scholar * Kitano, J. et al. A role for a neo-sex
chromosome in stickleback speciation. _Nature_ 461, 1079–1083 (2009). Article CAS PubMed PubMed Central Google Scholar * Natri, H. M., Shikano, T. & Merilä, J. Progressive
recombination suppression and differentiation in recently evolved neo-sex chromosomes. _Mol. Biol. Evol._ 30, 1131–1144 (2013). Article CAS PubMed PubMed Central Google Scholar *
Hedrick, P. W. Sex: differences in mutation, recombination, selection, gene flow, and genetic drift. _Evolution_ 61, 2750–2771 (2007). Article PubMed Google Scholar * Schaffner, S. F. The
X chromosome in population genetics. _Nat. Rev. Genet._ 5, 43–51 (2004). Article CAS PubMed Google Scholar * Li, Z., Kemppainen, P., Rastas, P. & Merila, J. Linkage disequilibrium
clustering-based approach for association mapping with tightly linked genomewide data. _Mol. Ecol. Resour._ 18, 809–824 (2018). Article CAS PubMed Google Scholar * Fox, E. A., Wright, A.
E., Fumagalli, M. & Vieira, F. G. ngsLD: evaluating linkage disequilibrium using genotype likelihoods. _Bioinformatics_ 35, 3855–3856 (2019). Article CAS PubMed Google Scholar *
Roesti, M., Moser, D. & Berner, D. Recombination in the threespine stickleback genome—patterns and consequences. _Mol. Ecol._ 22, 3014–3027 (2013). Article CAS PubMed Google Scholar
* Matthey‐Doret, R. & Whitlock, M. C. Background selection and FST: consequences for detecting local adaptation. _Mol. Ecol._ 28, 3902–3914 (2019). Article PubMed CAS Google Scholar
* Stankowski, S. et al. Widespread selection and gene flow shape the genomic landscape during a radiation of monkeyflowers. _PLoS Biol._ 17, e3000391 (2019). Article PubMed PubMed Central
CAS Google Scholar * Neuenschwander, S., Hospital, F., Guillaume, F. & Goudet, J. quantiNemo: an individual-based program to simulate quantitative traits with explicit genetic
architecture in a dynamic metapopulation. _Bioinformatics_ 24, 1552–1553 (2008). Article CAS PubMed Google Scholar * Hu, A. et al. Influence of Bering Strait flow and North Atlantic
circulation on glacial sea-level changes. _Nat. Geosci._ 3, 118–121 (2010). Article CAS Google Scholar * Meiri, M. et al. Faunal record identifies Bering isthmus conditions as constraint
to end-Pleistocene migration to the New World. _Proc. Biol. Sci._ 281, 20132167 (2014). PubMed PubMed Central Google Scholar * Zheng, X. et al. A high-performance computing toolset for
relatedness and principal component analysis of SNP data. _Bioinformatics_ 28, 3326–3328 (2012). Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We
are grateful to the following people who helped in obtaining the samples used in this study: J. DeFaveri, A. Adill, W. Aguirre, T. Bakker, A. Bell, M. Bell, B. Borg, F. Franzén, A. Goto, A.
Hendry, G. Herczeg, F. von Hippel, A. Hirvonen, J. Hämäläinen, M. Kaukoranta, A. Kijewska, D. Kingsley, Y. Kosaka, L. Kvarnemo, D. Lajus, T. Leinonen, A. Levsen, S. McCairns, A. Millet, J.
Morozinska, C. Munk, H. Mäkinen, A. Nolte, K. Østbye, W. Pekkola, J. Pokela, M. Ravinet, K. Räsänen, D. Schluter, M. Seymor, T. Shikano, P. Sjöstrand, G. Staines, B. Stelbrink, I. Syvänperä,
A. Vasemägi, M. Webster, J. Willacker, H. Winkler and L. Zaveik. Our research was supported by Academy of Finland grant nos. 250435, 263722, 265211 and 1307943 to J.M. and grant no. 316294
to P.M., the Finnish Cultural Foundation grant no. 00190489 to P.K. and the Chinese Scholarship Council grant no. 201606270188 to B.F. We thank J. DeFaveri for feedback and linguistic
corrections. AUTHOR INFORMATION Author notes * These authors contributed equally: Bohao Fang, Petri Kemppainen. AUTHORS AND AFFILIATIONS * Ecological Genetics Research Unit, Organismal and
Evolutionary Biology Research Programme, Faculty of Biological and Environmental Sciences, University of Helsinki, Helsinki, Finland Bohao Fang, Petri Kemppainen, Paolo Momigliano, Xueyun
Feng & Juha Merilä Authors * Bohao Fang View author publications You can also search for this author inPubMed Google Scholar * Petri Kemppainen View author publications You can also
search for this author inPubMed Google Scholar * Paolo Momigliano View author publications You can also search for this author inPubMed Google Scholar * Xueyun Feng View author publications
You can also search for this author inPubMed Google Scholar * Juha Merilä View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS P.K. and J.M.
conceived the concept of the study, with contributions from P.M. and B.F. B.F. and P.K. carried out analyses with significant contributions from P.M. P.K. and B.F. led the writing, with
significant contributions from P.M. and J.M. X.F. contributed to LiftOver analysis. B.F. visualized the data. All authors accepted the final version of this manuscript. CORRESPONDING AUTHORS
Correspondence to Bohao Fang or Petri Kemppainen. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature
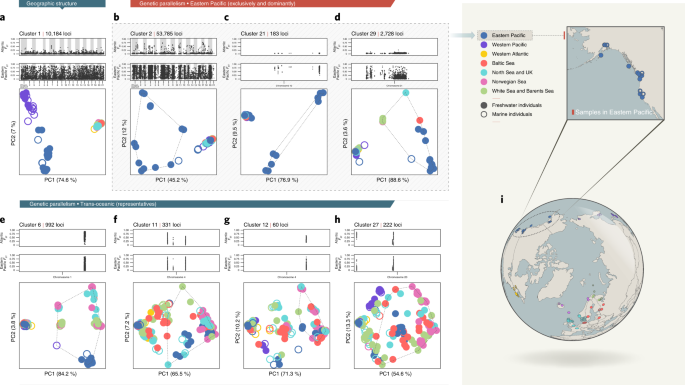
remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. EXTENDED DATA EXTENDED DATA FIG. 1 VISUALIZATION OF ALL LD-CLUSTERS IDENTIFIED BY
LDNA. In each panel, I) the top and II) middle plots represent the marine–freshwater differentiation (_F_ST) of the clustered loci of the individuals in the Atlantic and Eastern Pacific,
respectively. III) The bottom left plot shows population differentiation based on loci in each LD-cluster (principal component analysis; PCA). Only one chromosome is presented on the x axis
when the clustered loci were located on a single chromosome. IV) The bottom right plot depicts the number of in-group samples (as positive value) and the remaining samples (as negative
value). Global samples from various regions are shown in different colours; freshwater ecotypes are indicated by light-colour and marine ecotype by dark-colour. The same colour scheme was
used in the PCA. The p-values were obtained from permutation tests of cluster separation (Supplementary Information 1). EXTENDED DATA FIG. 2 ABILITY OF LDNA TO RECOVER MARINE–FRESHWATER
DIFFERENTIATED REGIONS FROM JONES ET AL.11. Jones et al.11 identified 812 regions showing parallel marine–freshwater differentiation in the Eastern Pacific (“_i-_regions”) and 81 regions
showing global parallelism (“_m-f_ regions”). (A) The proportions of _m-f_ and _i-_regions that were correctly recovered by LDna (red; at least one SNP from 29 LD-clusters mapped to these
regions), the proportion or regions for which we had data but LDna analyses failed to recover (cyan), and regions for which we had no genetic data (blue). (B) Number of high LD-SNPs
(produced by the first LDna-filtering step) and raw SNPs (bottom row) in regions that were and were not recovered by LDna and (C) size of the regions that were and were not recovered by LDna
(on _log__10_ scale). (D) _F_ST from raw SNPs located within regions that were and were not recovered by LDna. Overall, _m-f_ regions and _i-_ regions that were not recovered by LDna were
generally smaller, contained fewer SNPs (that is had lower sequencing coverage) and exhibited lower _F_ST than the regions correctly recovered by LDna. EXTENDED DATA FIG. 3 GENOME-WIDE
MARINE–FRESHWATER DIFFERENTIATION (_F_ST) IN THE ATLANTIC, EASTERN PACIFIC AND WESTERN PACIFIC OCEANS. (A–C) SNP-based _F_ST of the individuals in the Atlantic (ATL), Eastern Pacific (EP)
and Western Pacific (WP), respectively. Ecotype pairs follow the main analyses (Extended Data Table 2). (D) Window-based _F_ST (win-size=100 kb) between EP freshwater samples (n = 13) and EP
marine samples (n = 4). (E) Window-based _F_ST between EP freshwater samples (n = 13) and all Pacific marine samples (n = 13). (D, E) are significantly correlated (_r_ = 0.904, p <
0.0001). (F, G) SNP-based EP genetic parallelism (LD-clusters 2, 21, 29) for the same ecotype comparison as (D, E), respectively. Loci from LD-clusters involved in genetic parallelism are
colour-coded for all panels (refer to main Fig. 2). EXTENDED DATA FIG. 4 PCA PLOT OF LDNA CLUSTERS WITH POPULATION IDENTIFICATION. See Supplementary Table 1 for population identifiers.
EXTENDED DATA FIG. 5 POPULATION DIVERSITY AND ISOLATION-BY-DISTANCE (IBD) IN MARINE THREE-SPINED STICKLEBACK POPULATIONS. (A) Boxplots of individual heterozygosity (proportion heterozygous
positions per individual) of marine individuals in different geographical regions (EP = Eastern Pacific, WP = Western Pacific and ATL = Atlantic; GLM, _F_2,64 = 43.05, _P_ < 0.001). (B)
Boxplots of individual heterozygosity of LD-cluster 2 in different geographical regions (GLM, _F_2,64 = 91.9, _P_ < 0.001). (C) IBD between marine populations. Note that the different
scales of empirical and simulated heterozygosity in (A, B) are not relevant. This is because in the simulations of all allele frequencies started from 0.5 and while a burn in of 10k
generations was appropriate for loci linked to QTL, neutral loci would have required four times more generations to reach equilibrium (see Supplementary Information 3). However, the trends
in terms of loss of heterozygosity away from the ancestral Eastern Pacific marine populations is still informative and consistent with the empirical data. EXTENDED DATA FIG. 6 MERCATOR
PROJECTION OF GLOBAL THREE-SPINED STICKLEBACK POPULATIONS USED IN THE STUDY. 166 three-spined stickleback individuals from 63 localities were used, including 119 freshwater individuals and
47 marine individuals. For a complete list of samples, see Supplementary Table 1. EXTENDED DATA FIG. 7 SUMMARY OF ALL LD-CLUSTERS. Shaded rows (LD-clusters) contribute to genetic parallelism
of regional or trans-oceanic freshwater populations. EXTENDED DATA FIG. 8 SAMPLING SCHEMES FOR _F_ST ANALYSES. The table specifies sampling schemes used for _F_ST analyses and figures.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary notes 1–5, references, Figs. 1–3 and Table 1. REPORTING SUMMARY RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Fang, B., Kemppainen, P., Momigliano, P. _et al._ On the causes of geographically heterogeneous parallel evolution in sticklebacks. _Nat Ecol Evol_ 4, 1105–1115
(2020). https://doi.org/10.1038/s41559-020-1222-6 Download citation * Received: 01 April 2019 * Accepted: 14 May 2020 * Published: 22 June 2020 * Issue Date: 01 August 2020 * DOI:
https://doi.org/10.1038/s41559-020-1222-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative