Play all audios:
ABSTRACT Over the past several years, important strides have been made in demonstrating protonic ceramic fuel cells (PCFCs). Such fuel cells offer the potential of environmentally
sustainable and cost-effective electric power generation. However, their power outputs have lagged behind predictions based on their high electrolyte conductivities. Here we overcome PCFC
performance and stability challenges by employing a high-activity cathode, PrBa0.5Sr0.5Co1.5Fe0.5O5+δ (PBSCF), in combination with a chemically stable electrolyte, BaZr0.4Ce0.4Y0.1Yb0.1O3
(BZCYYb4411). We deposit a thin dense interlayer film of the cathode material onto the electrolyte surface to mitigate contact resistance, an approach which is made possible by the proton
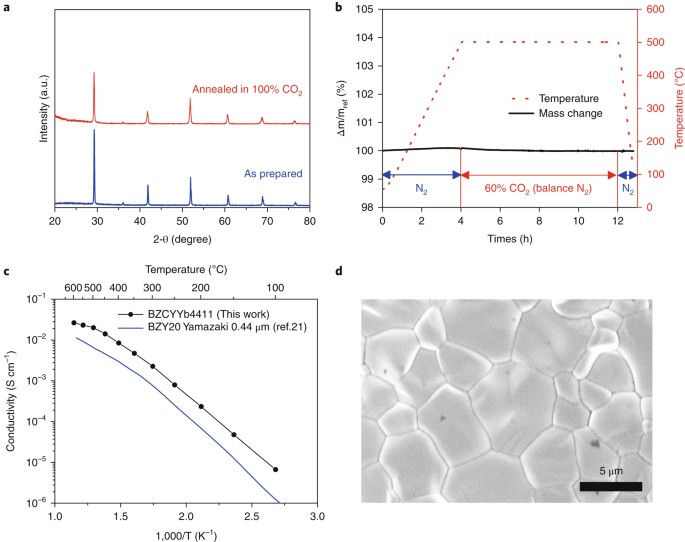
permeability of PBSCF. The peak power densities of the resulting fuel cells exceed 500 mW cm−2 at 500 °C, while also offering exceptional, long-term stability under CO2. Access through your
institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio
journals Get Nature+, our best-value online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 digital issues and online access to articles
$119.00 per year only $9.92 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are
calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS
LOWERING THE OPERATING TEMPERATURE OF PROTONIC CERAMIC ELECTROCHEMICAL CELLS TO <450 °C Article 07 September 2023 IMPROVED MECHANICAL STRENGTH, PROTON CONDUCTIVITY AND POWER DENSITY IN AN
‘ALL-PROTONIC’ CERAMIC FUEL CELL AT INTERMEDIATE TEMPERATURE Article Open access 29 September 2021 REVITALIZING INTERFACE IN PROTONIC CERAMIC CELLS BY ACID ETCH Article 20 April 2022
REFERENCES * Fabbri, E., Pergolesi, D. & Traversa, E. Materials challenges toward proton-conducting oxide fuel cells: a critical review. _Chem. Soc. Rev._ 39, 4355–4369 (2010). Article
Google Scholar * Nguyen, N. T. Q. & Yoon, H. H. Preparation and evaluation of BaZr0.1Ce0.7Y0.1Yb0.1O3-δ (BZCYYb) electrolyte and BZCYYb-based solid oxide fuel cells. _J. Power Sources_
231, 213–218 (2013). Article Google Scholar * Duan, C. et al. Readily processed protonic ceramic fuel cells with high performance at low temperatures. _Science_ 349, 1321–1326 (2015).
Article Google Scholar * Nien, S. H., Hsu, C. S., Chang, C. L. & Hwang, B. H. Preparation of BaZr0.1Ce0.7Y0.2O3–δ based solid oxide fuel cells with anode functional layers by tape
casting. _Fuel Cells_ 11, 178–183 (2011). * Bae, K. et al. Demonstrating the potential of yttrium-doped barium zirconate electrolyte for high-performance fuel cells. _Nat. Commun._ 8, 14553
(2017). Article Google Scholar * Yoo, S., Choi, S., Kim, J., Shin, J. & Kim, G. Investigation of layered perovskite type NdBa1−_x_Sr _x_ Co 2O5+δ (_x_= 0, 0.25, 0.5, 0.75, and 1.0)
cathodes for intermediate-temperature solid oxide fuel cells. _Electrochim. Acta_ 100, 44–50 (2013). Article Google Scholar * Liu, Q. L., Khor, K. A. & Chan, S. H. High-performance
low-temperature solid oxide fuel cell with novel BSCF cathode. _J. Power Sources_ 161, 123–128 (2006). Article Google Scholar * Shao, Z. & Haile, S. M. A high-performance cathode for
the next generation of solid-oxide fuel cells. _Nature_ 431, 170–173 (2004). Article Google Scholar * Choi, S. et al. Highly efficient and robust cathode materials for low-temperature
solid oxide fuel cells: PrBa0.5Sr0.5Co2−_x_Fe _x_ O5+δ. _Sci. Rep_. 3, 2426 (2013). * Fabbri, E., Markus, I., Bi, L., Pergolesi, D. & Traversa, E. Tailoring mixed proton-electronic
conductivity of BaZrO3 by Y and Pr co-doping for cathode application in protonic SOFCs. _Solid State Ion._ 202, 30–35 (2011). Article Google Scholar * Wang, Z. et al. A mixed-conducting
BaPr0.8In0.2O3−δ cathode for proton-conducting solid oxide fuel cells. _Electrochem. Commun._ 27, 19–21 (2013). Article Google Scholar * Han, D., Okumura, Y., Nose, Y. & Uda, T.
Synthesis of La1−_x_Sr _x_ Sc1−_y_Fe _y_ O3−δ (LSSF) and measurement of water content in LSSF, LSCF and LSC hydrated in wet artificial air at 300°C. _Solid State Ion._ 181, 1601–1606 (2010).
Article Google Scholar * Grimaud, A. et al. Hydration and transport properties of the Pr2-_x_Sr _x_ NiO4+δ compounds as H+-SOFC cathodes. _J. Mater. Chem._ 22, 16017–16025 (2012). Article
Google Scholar * Grimaud, A. et al. Hydration properties and rate determining steps of the oxygen reduction reaction of perovskite-related oxides as H+-SOFC cathodes. _J. Electrochem.
Soc._ 159, B683–B694 (2012). * Strandbakke, R. et al. Gd- and Pr-based double perovskite cobaltites as oxygen electrodes for proton ceramic fuel cells and electrolyser cells. _Solid State
Ion._ 278, 120–132 (2015). Article Google Scholar * Fabbri, E., D'Epifanio, A., Di Bartolomeo, E., Licoccia, S. & Traversa, E. Tailoring the chemical stability of Ba(Ce0.8−_x_Zr
_x_ )Y0.2O3−δ protonic conductors for Intermediate Temperature Solid Oxide Fuel Cells (IT-SOFCs). _Solid State Ion._ 179, 558–564 (2008). Article Google Scholar * Yang, L. et al. Enhanced
sulfur and coking tolerance of a mixed ion conductor for SOFCs: BaZr0. 1Ce0. 7Y0.2–_x_Yb _x_ O3–δ. _Science_ 326, 126–129 (2009). Article Google Scholar * Haile, S. M., Staneff, G. &
Ryu, K. H. Non-stoichiometry, grain boundary transport and chemical stability of proton conducting perovskites. _J. Mater. Sci._ 36, 1149–1160 (2001). Article Google Scholar *
Takayama-Muromachi, E. & Navrotsky, A. Energetics of compounds (A2+B4+O3) with the perovskite structure. _J. Solid State Chem._ 72, 244–256 (1988). Article Google Scholar * Ryu, K. H.
& Haile, S. M. Chemical stability and proton conductivity of doped BaCeO3–BaZrO3 solid solutions. _Solid State Ion._ 125, 355–367 (1999). Article Google Scholar * Yamazaki, Y.,
Hernandez-Sanchez, R. & Haile, S. M. High total proton conductivity in large-grained yttrium-doped barium zirconate. _Chem. Mater._ 21, 2755–2762 (2009). Article Google Scholar *
Bozza, F., Arroyo, Y. & Graule, T. Flame spray synthesis of BaZr0.8Y0.2O3–δ electrolyte nanopowders for intermediate temperature proton conducting fuel cells. _Fuel Cells_ 15, 588–594
(2015). * Ling, Y., Yu, J., Zhang, X., Zhao, L. & Liu, X. A cobalt-free Sm0.5Sr0.5Fe0.8Cu0.2O3−δ–Ce0.8Sm0.2O2−δ composite cathode for proton-conducting solid oxide fuel cells. _J. Power
Sources_ 196, 2631–2634 (2011). Article Google Scholar * Kim, J. et al. Triple‐conducting layered perovskites as cathode materials for proton‐conducting solid oxide fuel cells.
_ChemSusChem_ 7, 2811–2815 (2014). * Choi, S., Shin, J. & Kim, G. The electrochemical and thermodynamic characterization of PrBaCo2−_x_ Fe _x_ O5+δ (_x_= 0, 0.5, 1) infiltrated into
yttria-stabilized zirconia scaffold as cathodes for solid oxide fuel cells. _J. Power Sources_ 201, 10–17 (2012). Article Google Scholar * Kim, G. et al. Rapid oxygen ion diffusion and
surface exchange kinetics in PrBaCo2O5+_x_ with a perovskite related structure and ordered A cations. _J. Mater. Chem._ 17, 2500–2505 (2007). Article Google Scholar * Kim, J. H., Cassidy,
M., Irvine, J. T. & Bae, J. Electrochemical investigation of composite cathodes with SmBa0.5Sr0.5Co2O5+δ cathodes for intermediate temperature-operating solid oxide fuel cell. _Chem.
Mater._ 22, 883–892 (2009). * Jun, A. et al. Correlation between fast oxygen kinetics and enhanced performance in Fe doped layered perovskite cathodes for solid oxide fuel cells. _J. Mater.
Chem. A_ 3, 15082–15090 (2015). Article Google Scholar * Kim, J.-H. & Manthiram, A. Layered LnBaCo2O5+δ perovskite cathodes for solid oxide fuel cells: an overview and perspective. _J.
Mater. Chem. A_ 3, 24195–24210 (2015). Article Google Scholar * Jeong, D. et al. Structural, electrical, and electrochemical characteristics of LnBa0.5Sr0.5Co1.5Fe0.5O5+δ (Ln=Pr, Sm, Gd)
as cathode materials in intermediate-temperature solid oxide fuel cells. _Energy Technol._ 5, 1337–1343 (2017). * Kim, J.-H., Prado, F. & Manthiram, A. Characterization of GdBa1−_x_Sr
_x_ Co2O5+δ (0⩽_x_⩽1.0) double perovskites as cathodes for solid oxide fuel cells. _J. Electrochem. Soc._ 155, B1023–B1028 (2008). * Burriel, Mn et al. Anisotropic oxygen ion diffusion in
layered PrBaCo2O5+δ. _Chem. Mater._ 24, 613–621 (2012). Article Google Scholar * Hashimoto, D., Han, D. & Uda, T. Dependence of lattice constant of Ba, Co-contained perovskite oxides
on atmosphere, and measurements of water content. _Solid State Ion._ 262, 687–690 (2014). Article Google Scholar * Yamazaki, Y., Babilo, P. & Haile, S. M. Defect chemistry of
yttrium-doped barium zirconate: a thermodynamic analysis of water uptake. _Chem. Mater._ 20, 6352–6357 (2008). Article Google Scholar * Yamazaki, Y., Yang, C.-K. & Haile, S. M.
Unraveling the defect chemistry and proton uptake of yttrium-doped barium zirconate. _Scr. Mater._ 65, 102–107 (2011). Article Google Scholar * Poetzsch, D., Merkle, R. & Maier, J.
Proton uptake in the H+-SOFC cathode material Ba0.5Sr0.5Fe0.8 Zn0.2O3−δ: transition from hydration to hydrogenation with increasing oxygen partial pressure. _Faraday Discuss._ 182, 129–143
(2015). Article Google Scholar * Zohourian, R., Merkle, R. & Maier, J. Proton uptake into the protonic cathode material BaCo0.4Fe0.4 Zr0.2O3-δ and comparison to protonic electrolyte
materials. _Solid State Ion._ 299, 64–69 (2017). Article Google Scholar * Hildenbrand, N., Boukamp, B. A., Nammensma, P. & Blank, D. H. Improved cathode/electrolyte interface of SOFC.
_Solid State Ion._ 192, 12–15 (2011). Article Google Scholar * Usiskin, R. E., Maruyama, S., Kucharczyk, C. J., Takeuchi, I. & Haile, S. M. Probing the reaction pathway in
(La0.8Sr0.2)0.95MnO3+δ using libraries of thin film microelectrodes. _J. Mater. Chem. A_ 3, 19330–19345 (2015). Article Google Scholar * Newman, J. Resistance for flow of current to a
disk. _J. Electrochem. Soc._ 113, 501–502 (1966). Article Google Scholar * Pechini, M. P. Method of preparing lead and alkaline earth titanates and niobates and coating method using the
same form a capacitor. US Patent 3,330,697 (1967). * Babilo, P., Uda, T. & Haile, S. M. Processing of yttrium-doped barium zirconate for high proton conductivity. _J. Mater. Res._ 22,
1322–1330 (2007). Article Google Scholar Download references ACKNOWLEDGEMENTS This research was funded in part by the US Department of Energy, through ARPA-e Contract DE-AR0000498, via
subcontract from United Technologies Research Center, and by the National Science Foundation, DMR-1505103. Selected facilities used were supported by the National Science Foundation via
Northwestern University’s MRSEC, DMR-1121262. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Materials Science and Engineering, Northwestern University, Evanston, IL, USA Sihyuk Choi, Chris
J. Kucharczyk, Ho-Il Ji & Sossina M. Haile * Applied Physics & Materials Science, California Institute of Technology, Pasadena, CA, USA Chris J. Kucharczyk & Ho-Il Ji * Materials
Science and Engineering, University of Maryland, College Park, MD, USA Yangang Liang, Xiaohang Zhang & Ichiro Takeuchi Authors * Sihyuk Choi View author publications You can also search
for this author inPubMed Google Scholar * Chris J. Kucharczyk View author publications You can also search for this author inPubMed Google Scholar * Yangang Liang View author publications
You can also search for this author inPubMed Google Scholar * Xiaohang Zhang View author publications You can also search for this author inPubMed Google Scholar * Ichiro Takeuchi View
author publications You can also search for this author inPubMed Google Scholar * Ho-Il Ji View author publications You can also search for this author inPubMed Google Scholar * Sossina M.
Haile View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS S.M.H led the development of the concept, guided the experimental design, and
supervised the research. S.C. developed the materials, fabricated the cells, and performed the following experiments and analyses: conductivity, thermogravimetry, fuel cell polarization, and
impedance spectroscopy. Y.L. and X.Z. prepared and characterized PLD microdot electrodes, on which C.J.K. performed electrochemical measurements. I.T. supervised PLD film growth and
characterization. H.-I. J. provided critical suggestions for experimental and analytical methods. S.M.H. and S.C. wrote the paper with contributions from all authors. CORRESPONDING AUTHOR
Correspondence to Sossina M. Haile. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figures 1–15, Supplementary Table 1 and Supplementary References RIGHTS AND PERMISSIONS Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Choi, S., Kucharczyk, C.J., Liang, Y. _et al._ Exceptional power density and stability at intermediate temperatures in protonic ceramic fuel
cells. _Nat Energy_ 3, 202–210 (2018). https://doi.org/10.1038/s41560-017-0085-9 Download citation * Received: 18 August 2017 * Accepted: 18 December 2017 * Published: 12 February 2018 *
Issue Date: March 2018 * DOI: https://doi.org/10.1038/s41560-017-0085-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative