Play all audios:
ABSTRACT Initiation of development requires differential gene expression and metabolic adaptations. Here we show in the nematode-trapping fungus, _Arthrobotrys flagrans_, that both are
achieved through a dual-function G-protein-coupled receptor (GPCR). _A. flagrans_ develops adhesive traps and recognizes its prey, _Caenorhabditis elegans_, through nematode-specific
pheromones (ascarosides). Gene-expression analyses revealed that ascarosides activate the fungal GPCR, GprC, at the plasma membrane and together with the G-protein alpha subunit GasA,
reprograms the cell. However, GprC and GasA also reside in mitochondria and boost respiration. This dual localization of GprC in _A. flagrans_ resembles the localization of the cannabinoid
receptor CB1 in humans. The _C. elegans_ ascaroside-sensing GPCR, SRBC66 and GPCRs of many fungi are also predicted for dual localization, suggesting broad evolutionary conservation. An
SRBC64/66-GprC chimaeric protein was functional in _A. flagrans_, and _C. elegans_ SRBC64/66 and DAF38 share ascaroside-binding sites with the fungal GprC receptor, suggesting
400-million-year convergent evolution. SIMILAR CONTENT BEING VIEWED BY OTHERS THE NEMATODE-TRAPPING FUNGUS _ARTHROBOTRYS OLIGOSPORA_ DETECTS PREY PHEROMONES VIA G PROTEIN-COUPLED RECEPTORS
Article 22 April 2024 FATAL ATTRACTION OF _CAENORHABDITIS ELEGANS_ TO PREDATORY FUNGI THROUGH 6-METHYL-SALICYLIC ACID Article Open access 15 September 2021 DISTINCT NEUROPEPTIDE-RECEPTOR
MODULES REGULATE A SEX-SPECIFIC BEHAVIORAL RESPONSE TO A PHEROMONE Article Open access 31 August 2021 MAIN G-protein-coupled receptors (GPCRs) are widespread in all eukaryotes and represent
the largest receptor family. Work on these proteins was honoured with the Nobel prize in 20121. In humans, GPCRs are important drug targets2. GPCRs typically perceive external signals and
transmit the signal from the plasma membrane through coupled G-proteins to different cellular actions3. Besides this canonical signalling, starting at the cytoplasmic membrane, localization
of some receptors in other organelles in human cells suggests additional functions. One example is the human cannabinoid receptor CB1 which was found at mitochondria where it controls
respiration4. Lower eukaryotes, such as yeast or filamentous fungi, use GPCRs for nutrient sensing but also for communication before mating and interkingdom communication in pathogenic or
symbiotic interactions5,6. Microbial interactions often rely on complex chemical signal exchange for recognition. In the case of predatory relationships, recognition should be followed by
avoidance or defence reactions. Therefore, it is advantageous for the predator to sense prey-specific molecules with important functions for the prey because this will reduce the chance to
escape recognition during evolution. However, such a dual function of molecules requires receptors for the same molecule in both organisms, predator and prey. In the case of
nematode-trapping fungi, nematode-derived ascaroside pheromones serve this function7,8. They control many developmental processes in nematodes and are hijacked as signalling molecules by the
fungal predator9,10. We study the predatory fungus _A. flagrans_ (formerly _Duddingtonia flagrans_) and the model nematode _Caenorhabditis elegans_. _A. flagrans_ produces adhesive trapping
networks and overcomes the _C. elegans_ defence also via small secreted proteins8,10,11,12,13. RESULTS THREE GPCRS AND TWO G-PROTEIN ALPHA SUBUNITS OF _A. FLAGRANS_ CONTROL TRAP FORMATION
In _C. elegans_, eight ascaroside-sensing G-protein-dependent receptors (SRBC64, 66, SRG36, 37, DAF37, 38, SRX43 and 44) have been described, but in fungi, information on ascaroside-sensing
receptors is lacking14,15. To identify GPCRs involved in the control of trap formation and thereby potentially in ascaroside sensing, we analysed the genome of _A. flagrans_ by standard
protein Blast and identified 14 putative receptor-encoding genes using _Aspergillus nidulans_ and _Saccharomyces cerevisiae_ GPCRs as baits. Due to similarities to characterized GPCRs, some
proteins are not likely to sense ascarosides (Extended Data Table 1). Therefore, we focused our work on six candidates, GprA–F. Sequence analyses revealed a putative signal peptide only in
GprC (first 30 amino acids at the N terminus) (https://ipsort.hgc.jp). In addition, GprC contains a putative mitochondrial targeting signal (MTS) cleaved after the 82nd amino acid
(possibility of 98.41%) (https://ihg.helmholtz-munich.de/ihg/mitoprot.html). The probabilities for mitochondrial targeting were much lower for GprD, E and F (predicted to be cleaved at the
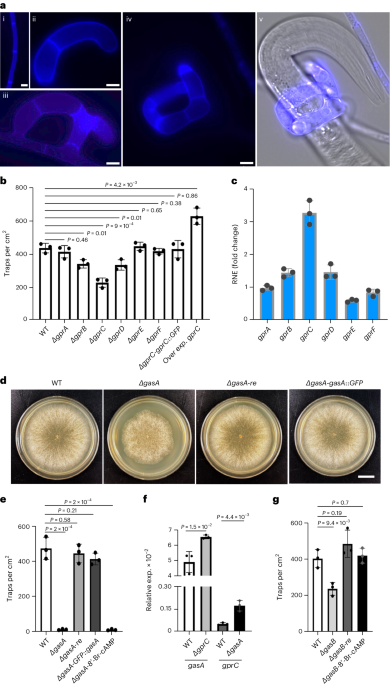
13th, 13th and 34th amino acids with possibilities of 2.58, 43.86 and 34.12%, respectively). To functionally characterize the six GPCRs, we deleted the corresponding genes, _gprA–F_
(Extended Data Fig. 1a–e). The most drastic reduction in trap numbers occurred after deletion of _gprC_, whereas deletion of _gprB_ or _gprD_ had smaller effects (Fig. 1a,b). Another
indication for a role in a signalling process can be their expression at the gene level. This was studied in starved fungal mycelia before and after exposure to nematodes by quantitative PCR
with reverse transcription (RT–qPCR). Significant upregulation in the presence of nematodes was found in the case of _gprC_ (Fig. 1c). Furthermore, trap number was significantly increased
after overexpression of the _gprC_ gene using the _oliC_ promoter from _A. nidulans_ (Fig. 1b). These lines of evidence strongly suggest a function of GprC, and possibly GprB and D, in
nematode (ascaroside) sensing. Typically, GPCR-dependent signalling cascades consist of downstream G-proteins connected to other signalling modules such as MAP kinase cascades. Ultimately,
activation of the signalling cascades leads to differential gene regulation. To identify putative G-proteins downstream of GprC, we studied the role of three G-protein alpha subunits of
_gasA_ (_dfl_009501_), _gasB_ (_dfl_000801_) and _gasC_ (_dfl_009358_) in trap morphogenesis. Deletion of _gasA_ resulted in complete loss of trap formation, and recomplementation with a
wild-type (WT) copy restored the WT phenotype (Fig. 1d,e and Extended Data Fig. 2a,b). An N-terminally GFP-tagged GasA version also recomplemented the mutant phenotype, suggesting that the
fusion protein (used for localization in experiments below) is biologically functional (Fig. 1e). Deletion of _gprC_ caused upregulation of _gasA_, and deletion of _gasA_ resulted in _gprC_
induction (Fig. 1f). Previously, it was shown that the MAP kinases Fus3, Slt2 and Hog1 are involved in trap formation and are probably downstream of a G-protein16,17,18. Another possibility
for G-protein-dependent signalling is through changes of the cAMP level19. To distinguish between the two possibilities, MAP kinase versus cAMP signalling by GprC and GasA, we tried to
rescue the _gasA-_mutant phenotype by adding the cAMP analogue 8’-Br-cAMP. No stimulation of trap formation in the mutant was observed (Fig. 1e). The analogue did not influence trap
formation in WT. This result suggests that GprC–GasA channel the signal into MAP kinase pathways or use other signalling cascades. Deletion of _gasB_ also affected trap initiation (Fig. 1g
and Extended Data Fig. 2c,d), but _gasC_ appeared to play no role (data not shown). The _gasB-_deletion phenotype could be rescued by 8’-Br-cAMP, suggesting that GasB uses the cAMP pathway
for signal transduction (Fig. 1g). GPRC INTERACTS WITH GASA AT THE CYTOPLASMIC MEMBRANE AND AT MITOCHONDRIA Next, we tested the hypothesis that GprC and GasA interact at the protein level.
First, we aimed at localizing GprC in _A. flagrans_ and tagged GprC at its C terminus. GprC-GFP was expressed using the constitutively active and quite strong _oliC_ promoter. The fusion
protein localized at filamentous structures inside the fungal compartments and not, or at least not visibly, at the cytoplasmic membrane. Costaining with mitotracker revealed that the
intracellular structures were mitochondria20 (Fig. 2a). The tagged protein was able to complement the _gprC-_deletion phenotype, suggesting that the fluorescent protein tag does not
interfere with the function (Fig. 1b). Mitochondrial morphology was not obviously different from mitochondria in WT despite the overexpression of GprC. When the same GprC-GFP construct was
expressed from its natural promoter, the localization pattern was more complex. Whereas the protein appeared at mitochondria in hyphal tips, the protein localized at the cytoplasmic membrane
in compartments away from the tip. To investigate whether dual localization reflects dual action of the receptor, we tested for interaction of GprC with GasA and used a bifluorescent
complementation system (split GFP). GprC was tagged at the C terminus with the C-terminal half of GFP. GasA was tagged N terminally with the N-terminal half of GFP. Neither construct alone
resulted in fluorescent _A. flagrans_ strains, but the combination of the two highlighted the plasma membrane and mitochondria in the same way as the GprC-GFP fusion protein when nematodes
were present (Fig. 2a). In traps, GprC was found at the cytoplasmic membrane and in mitochondria, but a gradient in the localization was not observed. In addition, traps contained some
autofluorescent signals observed in the GFP channel (Fig. 2b). To confirm the obtained localization and interaction results, we isolated mitochondria from _A. flagrans_ expressing GprC-GFP
and tested them for the presence of GprC (Fig. 2c and Extended Data Fig. 3)21. The protein amount was normalized to the volume of the original protein extract. After fractionation,
mitochondria were enriched in pellets 1 and 2 (P1, 400_g_; P2, 11,000_g_) and the cytoplasmic membrane in pellet 3 (P3, 100,000_g_). GprC-GFP (~90 kDa) was detected in the mitochondrial and
the plasma membrane fractionation. The fusion protein of all three pellets was completely degraded after digestion with proteinase K (P1, 2 and 3 + K), suggesting that the mitochondrial GprC
protein resides in the outer mitochondrial membrane. As a positive marker for mitochondria, we targeted GFP to the mitochondrial matrix using the N-terminal region (40 amino acids) of
citrate synthase (CitA), established in _A. nidulans_20. The CitA(N)-GFP fusion protein has a molecular mass of 33.2 kDa and after import, 30.2 kDa. Both bands were visible, plus a
degradation product (in P1). After treatment of proteinase K, the 30.2 kDa band remained, whereas the non-imported fusion protein (33.2 kDa) band disappeared. This showed that the
cytoplasmic protein variant was digested (CitA(N)::GFPc) and the mitochondrial one (CitA(N)::GFPi) was protected. GprC–GasA protein interaction was confirmed using a yeast two-hybrid (Y2H)
assay. The C-terminal tail (111 amino acids) of GprC appeared to interact slightly stronger with GasA than full-length GprC (Fig. 2d). GPRC–GASA SIGNALLING CONTROLS GENE EXPRESSION AND
MITOCHONDRIAL RESPIRATION To understand which genes may be controlled by _C. elegans_, the interkingdom signalling process between fungus and nematode has to be understood. _C. elegans_ is
lured into the fungal mycelium and into the traps by 6-methyl-salicylic acid (6-MSA) and small, volatile molecules that mimic a sexual partner and/or food8,22,23. In addition, _A. flagrans_
produces polyketide derivatives, arthrosporols and 6-MSA as inhibitors of trap formation in the absence of nematodes8,24. If nematodes are present and ascarosides reach a certain threshold
concentration, arthrosporol and 6-MSA productions are inhibited and traps are formed. Hence, ascarosides repress the expression of the polyketide synthase gene, _artA_, required for
arthrosporol and 6-MSA biosynthesis. The expression of the _artA-_cluster genes was quantified by RT–qPCR (Extended Data Fig. 2e). The presence of nematodes reduced the expression level of
_artA–D_ slightly, in comparison with WT without nematodes. In the _gasA_-deletion strain, the absence or presence of nematodes did not affect the expression levels. The differences were not
very pronounced because in older hyphal compartments, the _artA_-gene cluster is again activated to inhibit excessive trap formation8. To obtain more convincing results, the expression of
_artA_ was studied in a promoter–reporter assay which allows for cellular resolution of expression. The _artA_ promoter was fused to the mCherry- and a histone-encoding gene. In WT, strong
mCherry signals were observed in nuclei of vegetative hyphae. After the addition of nematodes or ascaroside #18, the signals disappeared (Fig. 3a). We used ascaroside #18 because it is
commercially available and was also shown to induce trap formation25. In the _gasA-_ and the _gprC-_deletion strains, the _artA_ promoter did not respond to _C. elegans_ or ascaroside #18.
These results suggest a canonical function of GprC and GasA. Next, we tested whether MAP kinases would be phosphorylated and thereby activated. The characterized MakB (DFL_000344), which
participates in hyphal fusion, and homologues of MakA (Slt2) (DFL_005546) and HogA (DFL_000806) were analysed for their phosphorylation status with antibodies against the phospho-p38 MAPK
and the phospho-p44/42 MAPK17,18,26. Same amounts of extracted protein of uninduced and induced mycelia from WT and the _gasA-_ and the _gprC-_deletion strains were processed for western
blotting. After 3 h of induction by nematodes, HogA and MakB (48.2 and 40.7 kDa) were phosphorylated in WT and mutant strains, which was not seen in uninduced hyphae (Fig. 3b). A weak
phosphorylation level of MakA (47.6 kDa) was observed in all samples. This result suggests that the GprC–GasA signalling is independent of the three MAP kinases. Mitochondrial localization
of GprC suggested direct effects on respiration. This hypothesis was tested by staining the fungal hyphae for the presence of reactive oxygen species (ROS) (Fig. 3c). ROS are a byproduct of
respiration. Growing hyphal tips showed weak fluorescence of mitochondria, which increased when the hyphae were exposed to _C. elegans_. In the absence of GprC or GasA, the increase in
fluorescence was much smaller and not significant. The fluorescent signal was very weak or not detectable in hyphal compartments away from the tip. These results suggest stimulation of
respiration in mitochondria at the hyphal tip through GprC–GasA signalling. To further confirm this effect on mitochondria, we measured the oxygen consumption rates (OCR) of fungal hyphae
and fungal hyphae incubated with _C. elegans_. Indeed, WT hyphae consumed more oxygen after induction with nematodes as compared with noninduced hyphae. The oxygen consumption rate was
unaffected by nematodes in the ∆_gprC_ or the ∆_gasA_ mutant strains (Fig. 3d). Taken together, the results suggest two functions of the GPCR protein and the G-protein alpha subunit at the
cytoplasmic membrane and in mitochondria, respectively. Although there is currently no information about the cellular path for mitochondrial targeting, direct transfer from the ER membrane
to the mitochondrial membrane is one possibility27,28. _A. FLAGRANS_ GPRC AND _C. ELEGANS_ SRBC64/66 SHARE THE ASCAROSIDE-BINDING SITE Next, we asked how the ascaroside-sensing GPCRs in _C.
elegans_ and _A. flagrans_ could have evolved. The predatory lifestyle of these fungi dates back more than 400 million years, and fungi could have acquired ascaroside receptors from
nematodes by horizonal gene transfer or by convergent evolution29. In _C. elegans_, SRBC64 recognizes ascaroside #1 but not ascaroside #5. Likewise, ascaroside #1 was more effective in trap
induction in _A. oligospora_ than ascaroside #5 (ref. 7). Therefore, we hypothesized that the fungal GPCR should be similar to _C. elegans_ SRBC64/66. However, none of the 14 fungal GPCRs
shares extended sequence similarities to SRBC64/66, ruling out horizontal gene transfer. Nevertheless, sequence comparisons of GprC and the ascaroside-sensing GPCRs of _C. elegans_ revealed
two short conserved sequence motifs (S and R), suggesting conservation of ascaroside binding and/or signalling (Fig. 4a). Next, we tested whether any of the _C. elegans_ receptors could
complement the lack of GprC in _A. flagrans_. Four candidates were successfully amplified from _C. elegans_ complementary DNA, whereas we failed to clone SRG36/37 and SRX43/44. The four
candidates, SRBC64/66 and Daf37/38 were expressed in _A. flagrans_ under the control of the _gprC_ promoter, but none of the transgenic strains recovered trap formation to WT levels. One
reason for the failure of complementation could be specific downstream signalling components in the fungus and the nematode. Therefore, we constructed chimaeric proteins, always keeping the
last three transmembrane helices to enable intracellular fungal signalling. The combination of the first four transmembrane (TM) helices of SRBC64/66 and DAF38, but neither DAF37 nor Octr-1
(a non-ascaroside receptor of _C. elegans_ as a negative control)30, with the three fungal TM helices resulted in functional receptors (Fig. 4b). The expression of all GPCR genes (_gprC_
variants, chimaeric version or _C. elegans_ receptor genes) in _A. flagrans_ was confirmed by RT–PCR (Extended Data Fig. 2f). Comparison of these results with the distribution of the S and
the R motifs in the sequences revealed the need for both motifs in the receptor. Interestingly, SRBC66 (probability of 82.92% for mitochondrial localization) and SRBC64 (with lower
probability of 29.71% for mitochondrial localization) were also predicted by mitoprot and ipsort for dual localization at the cytoplasmic membrane and in mitochondria. This was not the case
for DAF37 and DAF38. To further validate the hypothesis that the receptors in _A. flagrans_ and in _C. elegans_ evolved by convergence, we performed docking studies on receptor models of
GprC, SRBC64 and DAF37 to identify and compare the ascaroside-binding sites in the fungal and nematode receptors. TMs 2 and 3 in DAF37 are longer and more tilted than the corresponding
helices in the other two receptors. The top five binding poses of ascarosides #1, #2, #3, #5 and #18 were analysed using Autodock Vina31,32, resulting in a total of 25 different binding
poses (Fig. 5a–c and Extended Data Table 2). In addition, we tested the binding affinity of the putative ligands, glucose and sucrose, as many Gpr1 homologues are known to be nutrient
sensors33. Models of GprB and GprD were also used. The estimated binding energies of the best predicted poses are shown in Extended Data Table 2. Missing dynamics, such as loop movement or
induced fit mechanism, may affect the result. Therefore, it is crucial to avoid overinterpretation and instead prioritize the various binding poses. All putative ligands occupied the
classical orthosteric binding sites of GPCRs, with TMs 1 and 4 not involved in binding. Even though there are seven TM domains (Fig. 5a), only five of them work actively in binding ligands
(Extended Data Table 3), which is typical for GPCRs34. The loop connecting TMs 4 and 5 is called extracellular loop 2 (ECL2) and has been shown to be involved in ligand binding in many
GPCRs34,35,36. The ECL2 in GprC and SRBC64 coordinated ascarosides, whereas in DAF37 it did not bind any of the 20 poses and is much longer than in the other two receptors. SRBC64 and DAF37
are members of the solo families srbc and srw, respectively37. To compare GPCRs across different families, several generic residue numbering schemes have been introduced38. The
Ballesteros–Weinstein numbering scheme39 uses the most conserved residue for each TM helix individually and denotes it as number 50. For example, Pro2.53 denotes a proline residue in TM2,
which is three residues behind the most conserved residue. In the three receptors, we found Asp2.50 which is highly conserved between different GPCRs in many organisms (Fig. 5a–c). In most
GPCRs, the highly conserved residue in TM6 is a proline, which is present in GprC and SRBC64. DAF37 has a tryptophan instead. Regarding the most conserved residues, the length of ECL2 and
the shape of TMs 2 and 3, the two receptors GprC and SRBC64 are more similar to each other than to DAF37. By counting all receptor residues located within 3.5 Å of one of the 20 binding
poses, we obtained an ensemble of coordinating residues for all three receptors (Extended Data Table 3). Due to the steric hindrance of the conical receptor, a binding pocket on the
intracellular side is unlikely (Fig. 5d–f). To test the predicted binding sites for function, we performed mutagenesis studies on certain amino acids. In GprC, two asparagines in TM2 were
selected, separated by a helical loop. Mutation of the more intracellular N2.53 to alanine showed no effect on receptor function (neg. mutation), whereas mutation of N2.57 resulted in loss
of function (pos. mutation), leading us to conclude that the orthosteric binding site on the extracellular side is occupied by ascarosides in GprC (Figs. 4b and 5a). In SRBC64, the
asparagine N2.60 affected the function of the receptor after mutation, confirming the extracellular orthosteric binding site for SRBC64 (Fig. 5b). To compare the binding pockets, we looked
at similar residues in the same relative position in a TM. Apart from aromatic residues in ECL2, a polar residue in 2.60 and a basic residue in 2.61 or 2.63, no motifs of the same chemical
nature were observed in the same position (Fig. 5g–i). On the other hand, the R-motif is present in all three receptors. In GprC, the arginine R6.45 points to the binding site, a mutation to
alanine leads to a drastic drop in binding affinity (Extended Data Table 2) and it has been experimentally confirmed to be essential for receptor function (Fig. 4b). In SRBC64 and DAF37,
the R-motif is not involved in binding (Fig. 5a–c). The S-motif is only present in GprC and in SRBC64. In fact, in TM2 it is in the exact same position (S2.41). It is far from the binding
site but has been experimentally confirmed to be essential for function (Fig. 4b). In the model of the chimaeric protein DAF37-GprC, which did not rescue the GprC function, the S-motif is
missing, and a completely different binding site between the TMs 1, 2 and 7 is occupied. The chimaeric receptor SRBC64-GprC coordinated ascarosides between TM helices 3, 5, 6 and 7 (Fig.
5j,k). Since the S-motif is in the same relative position (S2.41) in the rescued chimaeric receptor, we hypothesize that it is essential for signalling. DISCUSSION We show that a
G-protein-coupled receptor protein of _A. flagrans_ exhibits dual localization and function (Fig. 6). Whether both localizations are also required for the fungal–nematode interaction,
however, remains to be determined. Although there are few examples for GPCR localizations in endosomes40 and mitochondria41, the functions of the proteins at the different places are not yet
well understood42. Our results show that this property of GPCRs is conserved in evolution from fungi to humans and appears to be of much greater importance than so far anticipated.
GPCR-dependent signalling cascades have been broadly studied in fungi because they are implicated in many environmental cues and are often crucial for host interactions in organisms ranging
from plants to nematodes to humans. Our discovery of a dual-function GPCR in _A. flagrans_ led us to analyse several GPCRs of other fungi. We identified GPCRs with predicted dual
localization using the mitoprot server in the ascomycetes _A. nidulans_ (GprH, 74% probability for mitochondrial localization)_, Aspergillus fumigatus_ and _Metharhizium anisopliae_ as well
as in the basidiomycetes _Cryptococcus neoformans_ and _Ustilago maydis_. In nerve and striated muscle cells, cannabinoid receptors inhibit respiration of mitochondria4,43. The effect of
GPCR activation in mitochondria of _A. flagrans_ appears to be opposite and respiration is activated. It will be the challenge of future research to unravel the connection between GPCR
signalling and the respiratory chain and its function(s) in fungal growth and pathogenicity. METHODS STRAINS AND CULTURE CONDITIONS _A. flagrans_ (CBS 349.94) was obtained from the CBS-KNAW
culture collection (Westerdijk Institute, the Netherlands) and cultured at 28 °C on potato dextrose agar (PDA) for normal growth and on low-nutrient agar (LNA) (1 g l−1 KCl, 0.2 g
MgSO4·7H2O, 0.4 mg MnSO4·4H2O, 0.88 mg ZnSO4·7H2O, 3 mg FeCl3·6H2O, 10 g agar, pH 5.5) for fungal starvation. N2 _C. elegans_ was obtained from Prof. Dr Ralf Baumeister (University of
Freiburg) and used as the WT. Standard cultivation and synchronization methods were used for _C. elegans_ (https://doi.org/10.1895/wormbook.1.101.1) and _S. cerevisiae_. All strains are
listed in Supplementary Table 1 and Extended Data Table 4. PROTOPLAST TRANSFORMATION OF _A. FLAGRANS_ _A. flagrans_ was cultured on a 9 cm PDA Petri dish at 28 °C for 7 days. Mycelia were
scratched off the agar and inoculated in 150 ml PDB medium and incubated for 24 h at 28 °C. The mycelium was collected and washed with MN solution (0.3 mol l−1 MgSO4, 0.3 mol l−1 NaCl) and
~0.5 g of mycelia (wet weight) was collected and suspended in 5 ml MN buffer containing 4 mg ml−1 kitalase (Fujifilm Wako Chemicals) and 20 mg ml−1 VinoTaste Pro (Novozymes), followed by
incubation at 30 °C for 2 h. Quality of protoplasts was checked microscopically. Subsequently, undigested mycelia were removed by filtering the protoplast through two layers of miracloth
tissue. Protoplasts were precipitated at 2,400_g_ for 15 min. After carefully removing the supernatant, protoplasts were washed with 50 ml KTC buffer (1.2 mol l−1 KCl, 10 mmol l−1 Tris-HCl
pH 7.5 and 50 mmol l−1 CaCl2) and the resulting pellet was resuspended in 500 μl KTC solution. For transformation, 100 µl protoplast suspension (2 × 107) were mixed with 3 μg DNA and
incubated for 2 min on ice. Then, 1 ml of PTC (10 mmol l−1 Tris-HCl pH 7.5, 50 mmol l−1 CaCl2, 60% w/v polyethylene glycol 6000) was added and incubated at room temperature for 20 min. PDSSA
(10 ml; 24 g l−1 potato dextrose broth, 0.6 mol l−1 sucrose, 0.3 g l−1 peptone, 0.3 g l−1 yeast extract, 8 g l−1 agar) was added to the transformation mixture and the mixture poured onto
PDA plates supplemented with 100 μg ml−1 hygromycin-B or 150 μg ml−1 geneticin (G418) and incubated at 28 °C for 5–7 days. PLASMID CONSTRUCTION Q5 High-Fidelity DNA polymerase for PCR and
restriction enzymes were purchased from New England Biolabs. Plasmids were assembled using the NEBuilder HiFi DNA Assembly Cloning kit (New England Biolabs). Standard transformation
procedures and plasmid isolation for _Escherichia coli_ were used. To create a C-terminal mCherry fusion of histone H2B under the control of the _artA_ promoter, the corresponding fragment
of ~1.5 kb including the promoter sequences was amplified by PCR, using _A. flagrans_ genomic DNA as template. The backbone of the plasmid containing H2B and mCherry was amplified and
assembled with the promoter11. Gene deletions were obtained by homologous recombination. Around 1 kb flanks homologous to the 5’ and 3’ regions of the targeted gene were amplified by PCR
using _A. flagrans_ genomic DNA as template. Both fragments containing 25 bp overlapping regions to the neighbouring fragment were assembled, with a hygromycin-B or geneticin resistance
cassette in between, into the pJET1.2 vector (Thermo Fisher, digested with _Eco_RV) using the NEBuilder HiFi DNA Assembly Cloning kit. For chimaeric protein recomplementation experiments,
the region encoding the first four TM helices in GPCRs was amplified from cDNA of _C. elegans_ and the region encoding the last three TM helices of GprC was amplified from genomic DNA of _A.
flagrans_. The two half fragments were assembled under the control of the native promoter of _gprC_ and introduced into the deletion strain of _gprC_. The full length of the GPCR genes was
amplified from cDNA of _C. elegans_ and ligated with the backbone containing 1.5 kb fragments upstream and downstream of the _gprC_ open reading frame (ORF). TM helices were predicted by the
server Phyre2 (http://www.sbg.bio.ic.ac.uk/~phyre2/html/page.cgi?id=index) and TMHMM 2.0 (https://services.healthtech.dtu.dk/services/TMHMM-2.0/). For site-directed mutagenesis, mutated
_gprC_ genes with 1.5 kb left and right borders were assembled into the pJET1.2 vector containing the G418 cassette12. The _gprC_ expression cassette was amplified from two fragments: (1)
the first half fragments containing 1.5 kb left border and the sequences from the start codon until the mutated spot (the reverse primer contains the mutated gene sequence) and (2) the other
half fragments containing 1.5 kb right border and the sequences from the mutated spot until the stop codon (the forward primer contains the mutated gene sequence). All amino acids were
mutated into alanine using codon GCT. For the C-terminal GFP fusion of GprC, the _gprC_ ORF region excluding the stop codon or 120 bp of _citA_ gene was amplified from gDNA of _A. flagrans_
and _A. nidulans_ individually. The backbone containing GFP and the hygromycin-B cassette was amplified from PNH21. The genes were expressed under the control of the constitutive _A.
nidulans oliC_ promoter. To express the _gprC_ or _gasA_ gene natively, the 1.5 kb fragments upstream of the gene ORFs were used as promoters. For the bifluorescent complementation
experiment, the GFP gene was split into N-terminal and C-terminal fragments as in _Botrytis cinerea_, and linkers were used between GFP fragments and the _A. flagrans_ genes44. The
C-terminal half of the GFP-encoding DNA fragment was fused at the 3′ end of _gprC_ and the N-terminal half of GFP-encoding fragment, at the 5′ end of the _gasA_ gene. Both constructs were
under their native promoters. The _gprC-GFPC_ and the _GFPN-gasA_ fragments were ligated with the backbones containing G418 and hygromycin-B resistance cassettes, respectively. All plasmids
are listed in Supplementary Table 2. RNA EXTRACTION, RT–QPCR AND CDNA SYNTHESIS To induce traps for RNA extraction, 106 _A. flagrans_ spores were incubated on LNA covered with a cellophane
membrane for 24 h at 28 °C. Individuals (104) of a mixed _C. elegans_ population were added to the membrane and co-incubated at 28 °C for 24 h to induce trap formation. The uninduced group
was treated with the same volume of double-distilled H2O. Afterwards, mycelia were collected from cellophane membranes on LNA and ground in liquid N2. Total RNA was extracted with Trizol
reagent (Invitrogen). DNase digestion was performed using the Turbo DNA-free kit (Invitrogen) and RNA was diluted to 50 ng μl−1. The SensiFast SYBR and fluorescein One Step kit (Bioline) was
used for the RT–qPCR analysis on a CFX Connect Real-Time PCR Detection System (Bio-Rad). Each reaction mixture contained 0.2 μM oligonucleotides (Supplementary Table 3; Eurofins Genomics
Europe) and 100 ng of RNA in a 20 μl total volume. Melting curve analysis was performed to assess the specific amplification of DNA. Fold changes were calculated using the formula 2−(ΔΔCt),
with ΔΔCt being ΔCt (treatment)−ΔCt (control), ΔCt is Ct (target gene)−Ct (actin) and Ct is the threshold cycle. The gamma actin orthologue DFL_002353 was used as internal reference gene for
normalization. RT–qPCR was performed with three biological replicates. The First-Strand cDNA Synthesis kit (Thermo Fisher) was used for cDNA synthesis and RT–PCR. FRACTIONATION OF
MITOCHONDRIA AND PLASMA MEMBRANE _A. flagrans_ protoplast was applied to the Yeast Mitochondria Isolation Kit (Sigma-Aldrich) and mitochondria were isolated as described in the
manufacturer’s protocol using detergent lysis. In the first centrifugation step of 400_g_, mitochondria were obtained in the supernatant (S1) and in the pellet (P1). We used the supernatant
for the second centrifugation of 11,000_g_, where mitochondria sedimented in the pellet (P2). The second supernatant (S2) was used for the third centrifugation at 100,000_g_, where the
plasma membrane was sedimented in the pellet (P3). All pellet samples were used for digestion by proteinase K. Proteins were analysed in a western blot using anti-GFP antibody (11814460001,
Roche) and anti-mouse IgG (Fab specific)-peroxidase antibody (A2304, Sigma-Aldrich). The protein amount was normalized to the volume in the original protein extract. Before blocking, the
nitrocellulose membrane was stained using Ponceau S (0.1% Ponceau red dye, 5% glacial acetic acid) for 15 min. YEAST TWO-HYBRID ASSAY This work followed the user manual of the Matchmaker
Gold yeast two-hybrid system (Clontech). Genes of _gprC, gasA_ and the 111 amino acid C-terminal tail of _gprC_ were amplified from the cDNA of _A. flagrans_. The PCR products were ligated
into pGBKT7 and pGADT7 vectors. In the constructs, BD and AD domains were fused at the N terminus of GasA and the C terminus of GprC fragments individually. Yeast strains AH109 and Y187 were
used for transformation of BD and AD vectors. The two strains were mated for interaction detection. Dilution series of strains were grown on SD-LW (leucine−, tryptophan−) and SD-LWH
(leucine−, tryptophan− and histidine−) agar plates for 3–5 days. MEASUREMENT OF OXYGEN CONSUMPTION RATE The analysis was performed with an XF24 extracellular flux analyser (Seahorse
XFe24)45. Spores (~103) of the _A. flagrans_ WT and mutant strains suspended in 10 μl liquid low-nutrient medium were incubated in XF24 Islet capture microplates for 24 h at 28 °C. Then, ~50
nematode adults were added in wells for induction of 6 h, while the same volume of sterile water was used as a control (uninduced). After that, free living worms were washed off carefully.
Then, the wells were filled with 200 μl liquid low-nutrient medium for OCR detection. Wells with 210 μl liquid low-nutrient medium were used as control. APPLICATION OF 8’-BROMO-CAMP
8’-bromo-cAMP (Sigma) was used as the analogue of cAMP, with 1 mg dissolved into 20 μl 1 M ammonia as stock solution of 50 mg ml−1 (122.52 mM). The working concentration was 5 mM suspended
in the melted LNA medium, which was cooled down to the proper temperature. PROTEIN EXTRACTION AND IMMUNOBLOTTING Around 106 spores of _A. flagrans_ were inoculated on cellophane on LNA
plates and incubated for 5 days at 28 °C. For induction, 10,000 N2 nematodes were applied on the grown hyphae for co-incubation of 3 h at 28 °C. Afterwards, worms were washed off with
double-distilled H2O. The mycelia were collected and immediately frozen in liquid nitrogen for protein extraction. Mycelia from four LNA plates were collected into Eppendorf tubes and ground
in liquid nitrogen. Protein extraction buffer (500 μl; 20 mM Tris-HCl, pH 8.0, 0.05% Triton-X-100, 150 mM NaCl) containing 1 mM PMSF was added into each tube and incubated on ice for 20
min. The samples were centrifuged at 17,000_g_ at 4 °C. The supernatants were collected. The protein concentration was measured using the Bradford protein assay and all the samples were
adjusted to the same concentration with protein extraction buffer. Samples with 5× SDS loading buffer and 10 mM dithiothreitol were denatured at 95 °C for 10 min. Then, denatured samples
were loaded onto a 10% SDS polyacrylamide gel and blotted to a nitrocellulose membrane. For immunodetection, anti-phospho-p38 MAP kinase (Tyr180/Tyr182) antibodies (9211, Cell Signaling
Technology; dilution 1:1,000) against phosphorylated HogA, anti-phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) antibodies (9101, Cell Signaling Technology; dilution 1:1,000) against
phosphorylated MakA/MakB, anti-Histone H3 (DFL_003537) (ab1791, abcam; dilution 1:2,000) antibodies, and anti-rabbit IgG (whole molecular)-peroxidase antibody (A0545, Sigma-Aldrich; dilution
1:10,000) and anti-mouse IgG (Fab specific)-peroxidase antibody (A2304, Sigma-Aldrich; dilution 1:10,000) were used. MICROSCOPY To induce trap formation for microscopy, ~104 spores of _A.
flagrans_ were inoculated on thin LNA on top of microscopic slides and ~200 individuals of _C. elegans_ were added. The compound Ascr#18 (MedChenExpress) was used for induction.
Co-incubation was performed at 28 °C in darkness for 20 h. To visualize ROS, we used the CellROX orange reagent (Invitrogen). Conventional fluorescence images were captured at room
temperature using a Zeiss Plan-Apochromat ×63/1.4 Oil DIC, EC Plan-Neofluar ×40/0.75, EC Plan-Neofluar ×20/0.50 or EC Plan-Neofluar ×10/0.30 objective attached to a Zeiss AxioImager Z.1 and
AxioCamMR. Images were collected using ZEN 2012 Blue Edition. The Zeiss LSM 900 with Airyscan2 was used for confocal microscopy. Colour images were acquired using the AxioCam 105 colour.
Confocal images were captured at room temperature using a Leica HCX PL APO ×63/1.4 oil objective attached to a Leica TC SP5 and conventional photomultiplier tube detectors (Leica). Images
were collected using the AxioVision software. Cell fluorescence was measured using ImageJ. (https://theolb.readthedocs.io/en/latest/imaging/measuring-cell-fluorescence-using-imagej.html).
CTCF (corrected total cell fluorescence) = integrated density − (area of selected cell × mean fluorescence of background readings). MODELLING AND DOCKING Models for GprC, SRBC64 and DAF37
are available in the AlphaFold Protein Structure Database46. They showed high pLDDT scores (>0.85) for the TM helices, which in principle allows docking studies47. Mutant receptors and
chimaeric proteins were modelled using the AF2 web server with five models per output, 24 max_recycles and 2 num_ensemble. For protein–ligand docking with Autodock Vina, the transmembrane
portions of the predicted GPCR structures were prepared using the ‘prepare_receptor’ tool of the ADFR suite48. Ligands were constructed using Avogadro49 and geometry optimized using Orca at
a B3LYP/def2-TZVP level of theory. Following the default settings of the ‘prepare_ligand’ tool in the ADFR suite, all single bonds that are not included in the ascarylose ring were set to be
rotable, resulting in 7–11 DOFs for the four ascarosides. Atomic charges of the ligand atoms were determined using the Gasteiger charge model50. After processing the pdbqt files, docking
was performed with a box size of 40 Å around the centre of mass of the receptor and an exhaustiveness of 200 and 0.1 Å spacing. Binding affinities and structures of the first five binding
poses were used. STATISTICS AND REPRODUCIBILITY Group sizes are described in the figure legends. Unless specifically noted, each experiment was repeated three or more times independently.
Data were collected from three biological repeats, unless otherwise noted. Data shown in graphs or plots represent mean ± s.d., as indicated in figure legends. Plotted data points are shown.
Details are given in the above methods and in source data files. Data diagrams and statistical analyses were performed with GraphPad Prism 8.0 and IBM SPSS Statistics 19. For statistical
analysis, a two-sided unpaired Student’s _t_-test was performed, with _P_ < 0.05 considered significant. REPORTING SUMMARY Further information on research design is available in the
Nature Portfolio Reporting Summary linked to this article. DATA AVAILABILITY All data generated or analysed during this study are included in this published article or provided as source
data files. The _Arthrobotrys flagrans_ genome database used in this study is available at the National Center for Biotechnology Information GenBank under the accession number PRJNA494930.
References to this accession number can be found throughout this paper. Source data are provided with this paper. REFERENCES * Lefkowitz, R. J. A brief history of G-protein coupled receptors
(Nobel lecture). _Angew. Chem. Int. Ed. Engl._ 52, 6366–6378 (2013). Article CAS PubMed Google Scholar * Yang, D. et al. G protein-coupled receptors: structure- and function-based drug
discovery. _Signal Transduct. Target. Ther._ 6, 7 (2021). Article CAS PubMed PubMed Central Google Scholar * Weis, W. I. & Kobilka, B. K. The molecular basis of G protein-coupled
receptor activation. _Annu. Rev. Biochem._ 87, 897–919 (2018). Article CAS PubMed PubMed Central Google Scholar * Benard, G. et al. Mitochondrial CB1 receptors regulate neuronal energy
metabolism. _Nat. Neurosci._ 15, 558–564 (2012). Article CAS PubMed Google Scholar * Versele, M., Lemaire, K. & Thevelein, J. M. Sex and sugar in yeast: two distinct GPCR systems.
_EMBO Rep._ 2, 574–579 (2001). Article CAS PubMed PubMed Central Google Scholar * Kou, Y., Tan, Y. H., Ramanujam, R. & Naqvi, N. I. Structure–function analyses of the Pth11 receptor
reveal an important role for CFEM motif and redox regulation in rice blast. _New Phytol._ 214, 330–342 (2017). Article CAS PubMed Google Scholar * Hsueh, Y. P., Mahanti, P., Schroeder,
F. C. & Sternberg, P. W. Nematode-trapping fungi eavesdrop on nematode pheromones. _Curr. Biol._ 23, 83–86 (2013). Article CAS PubMed Google Scholar * Yu, X. et al. Fatal attraction
of _Caenorhabditis elegans_ to predatory fungi through 6-methyl-salicylic acid. _Nat. Commun._ 12, 5462 (2021). Article CAS PubMed PubMed Central Google Scholar * Butcher, R. A.
Small-molecule pheromones and hormones controlling nematode development. _Nat. Chem. Biol._ 13, 577–586 (2017). Article CAS PubMed PubMed Central Google Scholar * Fischer, R. &
Requena, N. Small-secreted proteins as virulence factors in nematode-trapping fungi. _Trends Microbiol._ 30, 616–617 (2022). Article Google Scholar * Youssar, L. et al. Intercellular
communication is required for trap formation in the nematode-trapping fungus _Duddingtonia flagrans_. _PLoS Genet._ 15, e1008029 (2019). Article CAS PubMed PubMed Central Google Scholar
* Wernet, N., Wernet, V. & Fischer, R. The small-secreted cysteine-rich protein CyrA is a virulence factor of _Duddingtonia flagrans_ during the _Caenorhabditis elegans_ attack. _PLoS
Pathog._ 17, e1010028 (2021). Article CAS PubMed PubMed Central Google Scholar * Lin, H. C. et al. Key processes required for the different stages of fungal carnivory by a
nematode-trapping fungus. _PLoS Biol._ 21, e3002400 (2023). Article CAS PubMed PubMed Central Google Scholar * Kim, K. et al. Two chemoreceptors mediate developmental effects of dauer
pheromone in _C. elegans_. _Science_ 326, 994–998 (2009). Article CAS PubMed PubMed Central Google Scholar * Park, J. Y., Joo, H. J., Park, S. & Paik, Y. K. Ascaroside pheromones:
chemical biology and pleiotropic neuronal functions. _Int. J. Mol. Sci._ 20, 3898 (2019). Article CAS PubMed PubMed Central Google Scholar * Zhen, Z. et al. MAP kinase Slt2 orthologs
play similar roles in conidiation, trap formation, and pathogenicity in two nematode-trapping fungi. _Fungal Genet. Biol._ 116, 42–50 (2018). Article CAS PubMed Google Scholar * Kuo, C.
Y., Chen, S. A. & Hsueh, Y. P. The high osmolarity glycerol (hog) pathway functions in osmosensing, trap morphogenesis and conidiation of the nematode-trapping fungus _Arthrobotrys
oligospora_. _J. Fungi_ 27, E191 (2020). Article Google Scholar * Chen, S. A., Lin, H. C., Schroeder, F. C. & Hsueh, Y. P. Prey sensing and response in a nematode-trapping fungus is
governed by the MAPK pheromone response pathway. _Genetics_ 217, iyaa008 (2021). Article PubMed Google Scholar * Chen, S. A., Lin, H. C. & Hsueh, Y. P. The cAMP-PKA pathway regulates
prey sensing and trap morphogenesis in the nematode-trapping fungus _Arthrobotrys oligospora_. _G3_ 12, jkac217 (2022). Article CAS PubMed PubMed Central Google Scholar * Suelmann, R.
& Fischer, R. Mitochondrial movement and morphology depend on an intact actin cytoskeleton in _Aspergillus nidulans_. _Cell Motil. Cytoskel._ 45, 42–50 (2000). Article CAS Google
Scholar * Streng, C. et al. Fungal phytochrome chromophore biosynthesis at mitochondria. _EMBO J._ 40, e108083 (2021). Article CAS PubMed PubMed Central Google Scholar * Hsueh, Y. P.
et al. Nematophagous fungus _Arthrobotrys oligospora_ mimics olfactory cues of sex and food to lure its nematode prey. _eLife_ 6, e20023 (2017). Article PubMed PubMed Central Google
Scholar * Wang, B. L. et al. Integrated metabolomics and morphogenesis reveal volatile signaling of the nematode-trapping fungus _Arthrobotrys oligospora_. _Appl. Environ. Microbiol._ 84,
e02749–02717 (2018). Article CAS PubMed PubMed Central Google Scholar * Zhang, H. X. et al. Morphology regulatory metabolites from _Arthrobotrys oligospora_. _J. Nat. Prod._ 75,
1419–1423 (2012). Article CAS PubMed Google Scholar * Huang, J., Zheng, X., Tian, M. & Zhang, K. Ammonia and nematode ascaroside are synergistic in trap formation in _Arthrobotrys
oligospora_. _Pathogens_ 12, 1114 (2023). Article CAS PubMed PubMed Central Google Scholar * Wernet, V., Wäckerle, J. & Fischer, R. The STRIPAK component SipC is involved in
morphology and cell-fate determination in the nematode-trapping fungus _Duddingtonia flagrans_. _Genetics_ 220, iyab153 (2022). Article PubMed Google Scholar * Pfanner, N., Warscheid, B.
& Wiedemann, N. Mitochondrial proteins: from biogenesis to functional networks. _Nat. Rev. Mol. Cell Biol._ 20, 267–284 (2019). Article CAS PubMed PubMed Central Google Scholar *
Wozny, M. R. et al. In situ architecture of the ER–mitochondria encounter structure. _Nature_ 618, 188–192 (2023). Article CAS PubMed PubMed Central Google Scholar * Yang, Y., Yang, E.,
An, Z. & Liu, X. Evolution of nematode-trapping cells of predatory fungi of the Orbiliaceae based on evidence from rRNA-encoding DNA and multiprotein sequences. _Proc. Natl Acad. Sci.
USA_ 104, 8379–8384 (2007). Article CAS PubMed PubMed Central Google Scholar * Sun, J., Singh, V., Kajino-Sakamoto, R. & Aballay, A. Neuronal GPCR controls innate immunity by
regulating noncanonical unfolded protein response genes. _Science_ 332, 729–732 (2011). Article CAS PubMed PubMed Central Google Scholar * Eberhardt, J., Santos-Martins, D., Tillack, A.
F. & Forli, S. AutoDock Vina 1.2.0: new docking methods, expanded force field, and Python bindings. _J. Chem. Inf. Model._ 61, 3891–3898 (2021). Article CAS PubMed PubMed Central
Google Scholar * Trott, O. & Olson, A. J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. _J. Comput.
Chem._ 31, 455–461 (2010). Article CAS PubMed PubMed Central Google Scholar * Lemaire, K., Van de Velde, S., Van Dijck, P. & Thevelein, J. M. Glucose and sucrose act as agonist and
mannose as antagonist ligands of the G protein-coupled receptor Gpr1 in the yeast _Saccharomyces cerevisiae_. _Mol. Cell_ 16, 293–299 (2004). Article CAS PubMed Google Scholar * Chan,
H. C. S., Li, Y., Dahoun, T., Vogel, H. & Yuan, S. New binding sites, new opportunities for GPCR drug discovery. _Trends Biochem. Sci._ 44, 312–330 (2019). Article CAS PubMed Google
Scholar * Wheatley, M. et al. Extracellular loops and ligand binding to a subfamily of family A G-protein-coupled receptors. _Biochem. Soc. Trans._ 35, 717–720 (2007). Article CAS PubMed
Google Scholar * Ragnarsson, L., Andersson, A., Thomas, W. G. & Lewis, R. J. Extracellular surface residues of the alpha1B-adrenoceptor critical for G protein-coupled receptor
function. _Mol. Pharmacol._ 87, 121–129 (2015). Article PubMed Google Scholar * Vidal, B. et al. An atlas of _Caenorhabditis elegans_ chemoreceptor expression. _PLoS Biol._ 16, e2004218
(2018). Article PubMed PubMed Central Google Scholar * Isberg, V. et al. GPCRDB: an information system for G protein-coupled receptors. _Nucleic Acids Res._ 42, D422–D425 (2014). Article
CAS PubMed Google Scholar * Ballesteros, J. A. & Weinstein, H. Integrated methods for the construction of three-dimensional models and computational probing of structure–function
relations in G protein-coupled receptors. _Methods Neurosci._ 25, 366–428 (1995). Article CAS Google Scholar * Irannejad, R. et al. Conformational biosensors reveal GPCR signalling from
endosomes. _Nature_ 495, 534–538 (2013). Article CAS PubMed Google Scholar * Hebert-Chatelain, E. et al. A cannabinoid link between mitochondria and memory. _Nature_ 539, 555–559 (2016).
Article CAS PubMed Google Scholar * Mohammad Nezhady, M. A., Rivera, J. C. & Chemtob, S. Location bias as emerging paradigm in GPCR biology and drug discovery. _iScience_ 23, 101643
(2020). Article CAS PubMed PubMed Central Google Scholar * Mendizabal-Zubiaga, J. et al. Cannabinoid CB(1) receptors are localized in striated muscle mitochondria and regulate
mitochondrial respiration. _Front. Physiol._ 7, 476 (2016). Article PubMed PubMed Central Google Scholar * Schumacher, J. Tools for _Botrytis cinerea_: new expression vectors make the
gray mold fungus more accessible to cell biology approaches. _Fungal Genet Biol._ 49, 483–497 (2012). Article CAS PubMed Google Scholar * Bonnighausen, J. et al. Disruption of the GABA
shunt affects mitochondrial respiration and virulence in the cereal pathogen _Fusarium graminearum_. _Mol. Microbiol._ 98, 1115–1132 (2015). Article PubMed Google Scholar * Varadi, M. et
al. AlphaFold Protein Structure Database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. _Nucleic Acids Res._ 50, D439–D444 (2022). Article
CAS PubMed Google Scholar * Lee, S. et al. Evaluating GPCR modeling and docking strategies in the era of deep learning-based protein structure prediction. _Comput. Struct. Biotechnol.
J._ 21, 158–167 (2023). Article CAS PubMed Google Scholar * Ravindranath, P. A., Forli, S., Goodsell, D. S., Olson, A. J. & Sanner, M. F. AutoDockFR: advances in protein-ligand
docking with explicitly specified binding site flexibility. _PLoS Comput. Biol._ 11, e1004586 (2015). Article PubMed PubMed Central Google Scholar * Hanwell, M. D. et al. Avogadro: an
advanced semantic chemical editor, visualization, and analysis platform. _J. Cheminform._ 4, 17 (2012). Article CAS PubMed PubMed Central Google Scholar * Gasteiger, J. & Marsili,
M. Iterative partial equalization of orbital electronegativity—a rapid access to atomic charges. _Tetrahedron_ 36, 3219–3228 (1980). Article CAS Google Scholar Download references
ACKNOWLEDGEMENTS X.H. was supported by the China Scholarship Council (CSC). R.F. and M.S. were supported for the project by the Deutsche Forschungsgemeinschaft DFG Fi 459/26-1 and
STR1784/1-1. We thank E. Wohlmann for technical assistance. FUNDING Open access funding provided by Karlsruher Institut für Technologie (KIT). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Department of Microbiology, Institute for Applied Biosciences, Karlsruhe Institute of Technology (KIT) - South Campus, Karlsruhe, Germany Xiaodi Hu, Mai Wang, Lars Schuhmacher, Maria C.
Stroe, Birgit Schreckenberger & Reinhard Fischer * Department of Theoretical Chemical Biology, Institute for Physical Chemistry, Karlsruhe Institute of Technology (KIT) - South Campus,
Karlsruhe, Germany David S. Hoffmann & Marcus Elstner Authors * Xiaodi Hu View author publications You can also search for this author inPubMed Google Scholar * David S. Hoffmann View
author publications You can also search for this author inPubMed Google Scholar * Mai Wang View author publications You can also search for this author inPubMed Google Scholar * Lars
Schuhmacher View author publications You can also search for this author inPubMed Google Scholar * Maria C. Stroe View author publications You can also search for this author inPubMed Google
Scholar * Birgit Schreckenberger View author publications You can also search for this author inPubMed Google Scholar * Marcus Elstner View author publications You can also search for this
author inPubMed Google Scholar * Reinhard Fischer View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS X.H. planned and performed most of the
experiments and analysed the data. M.W. was responsible for the GPCR deletions. D.S.H. and M.E. were responsible for prediction of protein structures and analysed molecular docking. L.S.,
M.C.S. and B.S. were responsible for cell fractionation. R.F. planned and supervised the experiments, analysed the data, wrote the manuscript and acquired the funds for the project.
CORRESPONDING AUTHOR Correspondence to Reinhard Fischer. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature
Microbiology_ thanks Gustavo Goldman, Xingzhong Liu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. EXTENDED DATA EXTENDED DATA FIG. 1 DELETION OF SIX GPCR GENES IN _A.
FLAGRANS._ (A) Colonies of WT and _gprA-F_-deletion strains. Scale bar, 1 cm. (B) Confirmation of mutants by PCR. ORF fragments were amplified with primers of gprX ORF_f and ORF_r (or
ORFin_r), L (left flanking borders) with ko_up_for (or ex_for) and hyg_rev, and R (right flanking borders) with hyg_for and ko_down_rev (or ex_rev). PCR gel pictures are representative of
three independent repeats. (C) Confirmation of mutants by PCR. Left lanes show bands from the WT strain and right ones from the corresponding mutants. Fragments of _gprA_ were amplified with
primers of gprA_ex_for and gprA_ex_rev, _gprB_ with gprB_ex_for and gprB_orf_rev or hyg_rev, _gprC_ with gprCORF_for, gprCORF_rev, hyg_for and hyg_rev, _gprD_ with gprD_ex_for and
gprD_orf_rev or hyg_rev, _gprE_ with gprEORF_for, gprEORF_rev, hyg_for and hyg_rev, _gprF_ with gprF_ex_for and gprF_ex_rev. PCR gel pictures are representative of three independent repeats.
(D) Scheme of the deletion strategy of _gprC_. Arrows and the dark blue line indicate the used oligonucleotides for PCR and the probe for the Southern blot. (E) Southern blot analysis of WT
and the ∆_gprC_ mutant. Genomic DNA was digested with _Eco_RV and the blot hybridized with the probe indicated in (D). The blot is representative of three independent repeats. EXTENDED DATA
FIG. 2 DELETION OF _GASA_ AND EXPRESSION ANALYSIS. (A) Scheme of the deletion strategy of _gasA_. (B) Confirmation of _gasA_ deletion by PCR and Southern blot. The fragments after digestion
of the genomic DNA with _Xba_I and the probe are indicated in (A). PCR gel picture and blot are representative of three independent repeats. (C) Scheme of the deletion strategy of _gasB_.
(D) Confirmation of _gasB_ deletion by PCR and Southern blot. The expected fragments and the probe are indicated in (C). PCR gel picture and blot are representative of three independent
repeats. (E) The expression changes of the _artA_-gene cluster in nematode-induced hyphae of WT and the _gasA_-deletion strain compared with un−induced hyphae. RT–qPCR results are displayed
as _fold change_. Bars and error bars indicate means ± s.d. of three biological replicates. The gamma actin orthologue DFL_002353 was used for normalization. (F) Confirmation of _gprC_
re−complemented strains by RT–PCR and the amplified fragments of 374 bp showing the expression of various versions of mutated _gprC_ genes. Strains are from left to right: (1) WT, (2) _gprC_
mutant, (3) _gprC_ re-complemented strain and the chimeric protein re-complemented strains (4) _SRBC64-gprC-re_, (5) _SRBC64__N2.60A__-gprC-re_, (6) full length _SRBC66-re_ with an
unspecific band, (7) _SRBC66-gprC-re_, (8) _DAF37-gprC-re_, (9) _DAF38-gprC-re_, (10) _Octr-1-gprC-re_, and (11) mutated _gprC__R6.45A__-re_, (12) _gprC__S2.41A__-re_, (13)
_gprC__N2.57A__-re_ and (14) _gprC__N2.53A__-re_. The PCR gel picture is representative of three independent repeats. EXTENDED DATA FIG. 3 GPRC RESIDES IN MITOCHONDRIA AND THE CYTOPLASMIC
MEMBRANE. The GprC-GFP and CitA(N)-GFP expressing strains were cultivated overnight in PDB medium and harvested for cellular fractionation. Crude extract (CE), supernatants (S1 & 2),
pellets (P1, 2 & 3) and the proteinase K digested pellets (P1 + K, P2 + K and P3 + K) were analyzed in a Western blot using an anti-GFP antibody. GprC-GFP is about 90 kDa. CitA(N)-GFP
appeared as cytoplasmic fusion protein (around 33.2 kDa) and a shorter imported version (around 30.2 kDa) in the mitochondria which remained after protease K digestion, plus a smaller
degradation product. Both membranes were stained with Ponceau S (PS) before the Western blot. Blots are representative of three independent repeats. SUPPLEMENTARY INFORMATION SUPPLEMENTARY
INFORMATION Supplementary Tables 1–3. REPORTING SUMMARY SOURCE DATA SOURCE DATA FIG. 1 Statistical source data. SOURCE DATA FIG. 2 Source data western blots. SOURCE DATA FIG. 3 Source data
PCR and Southern blots. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Hu, X., Hoffmann, D.S., Wang, M. _et al._ GprC of the nematode-trapping fungus _Arthrobotrys flagrans_ activates mitochondria and reprograms fungal cells for nematode
hunting. _Nat Microbiol_ 9, 1752–1763 (2024). https://doi.org/10.1038/s41564-024-01731-9 Download citation * Received: 27 July 2023 * Accepted: 14 May 2024 * Published: 14 June 2024 * Issue
Date: July 2024 * DOI: https://doi.org/10.1038/s41564-024-01731-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative