Play all audios:
Leaves of the carnivorous sundew plants (Drosera spp.) secrete mucilage that hosts microorganisms, but whether this microbiota contributes to prey digestion is unclear. We identified the
acidophilic fungus Acrodontium crateriforme as the dominant species in the mucilage microbial communities, thriving in multiple sundew species across the global range. The fungus grows and
sporulates on sundew glands as its preferred acidic environment, and its presence in traps increased the prey digestion process. A. crateriforme has a reduced genome similar to other
symbiotic fungi. During A. crateriforme–Drosera spatulata coexistence and digestion of prey insects, transcriptomes revealed significant gene co-option in both partners. Holobiont expression
patterns during prey digestion further revealed synergistic effects in several gene families including fungal aspartic and sedolisin peptidases, facilitating prey digestion in leaves, as
well as nutrient assimilation and jasmonate signalling pathway expression. This study establishes that botanical carnivory is defined by adaptations involving microbial partners and
interspecies interactions.
Botanical carnivory has evolved independently at least 11 times in the plant kingdom, each group showcasing distinct molecular adaptations to attract, trap and digest insects1. Many
carnivorous plants have served as research models since the era of Charles Darwin2 to understand the evolutionary and molecular basis of carnivorous structures, which are frequently related
to adaptations of leaf organs. These specialised leaves secrete digestive exudates that may also host a diverse array of microorganisms. Although the significance of microorganisms in
vertebrate digestion is widely established3, recent research has suggested symbiotic roles for microorganisms in carnivorous plants4, but the underlying molecular responses through which
microorganisms facilitate or enhance plant carnivory are only just emerging4,5,6.
Plant–microorganism interactions are highly dynamic and can impact plant fitness through many mechanisms7. Previous studies using metabarcoding suggested that the digestive mucilage secreted
by modified leaves, or traps, of carnivorous plants, can be colonised by diverse bacterial and eukaryotic microbial communities (Extended Data Table 1). In bladderwort, corkscrew and
pitcher plants, there were no single dominant species represented, but diverse bacteria can be broadly grouped into major phyla5,8,9,10,11,12. Bacterial diversity and biomass were found to
improve prey decomposition rates in the pitcher plant Darlingtonia californica and increase nitrogen uptake efficiency in host leaves12. Meta-transcriptomic profiling of Genlisea species
revealed non-host transcripts dominated by metazoan hydrolases, suggesting a role in phosphate acquisition6. The composition of the microbiota also appears to be highly time dependent and
influenced by factors such as host plant12, community succession12,13, surrounding environment and prey possibly contributing bacteria14, so there are complex interactions that shape these
microbial communities.
Drosera is a genus known as sundews within the Droseraceae, the second largest carnivorous plant family after Lentibulariaceae15. Sundews have ‘flypaper’ leaf traps with tentacle-like
trichomes16 that secrete sticky mucilage to entrap and envelop prey. Subsequent insect digestion is a well-coordinated process, first by synthesis and secretion of digestive enzymes to break
down organic materials, followed by uptake and assimilation of nutrients with specialised transporters17,18. This complex behaviour is mediated by the jasmonate (JA) signalling pathway that
was present in non-carnivorous plant ancestors19. Recent comparative genomics of carnivorous plants has revealed expansion and clade-specific gene families involved in defence, such as JA
signalling, as well as peptidases and hydrolases20,21,22, which were upregulated during the digestion process suggesting that these genes have been co-opted23 for new roles in carnivory. The
extent to which these genes still retain their ancestral functions remains to be elucidated.
We hypothesize that carnivorous plants harbour microorganisms that positively enhance their digestion process, The digestion process therefore involves a plant–microorganism holobiont24, in
which specific microbial taxa would facilitate digestion within carnivorous plants and others may be neutral or even pathogenic to the host plant. To test this hypothesis, we focus on
Drosera spatulata25 (Fig. 1a), a sundew native to temperate and tropical regions including Taiwan and which has a fully sequenced genome21. The fascinating mechanism of trap movement in
sundews, associated with prey capture and digestion, has attracted significant scientific curiosity. The traps also act as a tractable system to examine responses to diverse stimuli22 in
both natural and laboratory settings. We aimed to characterise the sundew mucilage microbial community and assess their impact on digestion of insect prey. We identified Acrodontium
crateriforme as the dominant fungal species that enhances prey digestion efficiency in D. spatulata, showing that its enzymatic activities synergize with the plant’s own digestive processes.
Our research aims to shed light on the intricate symbiotic interactions between carnivorous plants and their resident microorganisms.
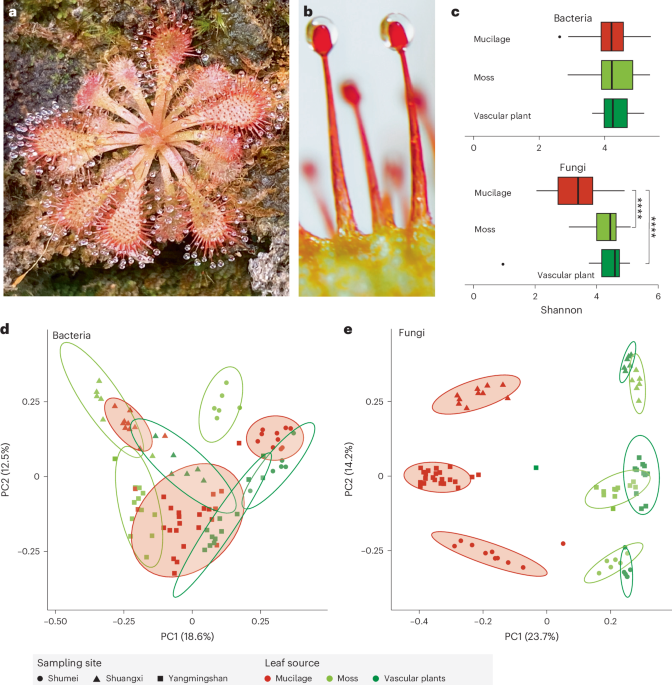
a, D. spatulata. b, Close-up of stalk glands with secreted mucilage. c, Bacterial and fungal species evenness in sundew mucilage (n = 44) versus those of co-occurring moss (n = 24) and
vascular plant leaf surfaces (n = 24). Asterisks denote significant difference from Wilcoxon rank sum test (two sided with multiple testing, ****adjusted P