Play all audios:
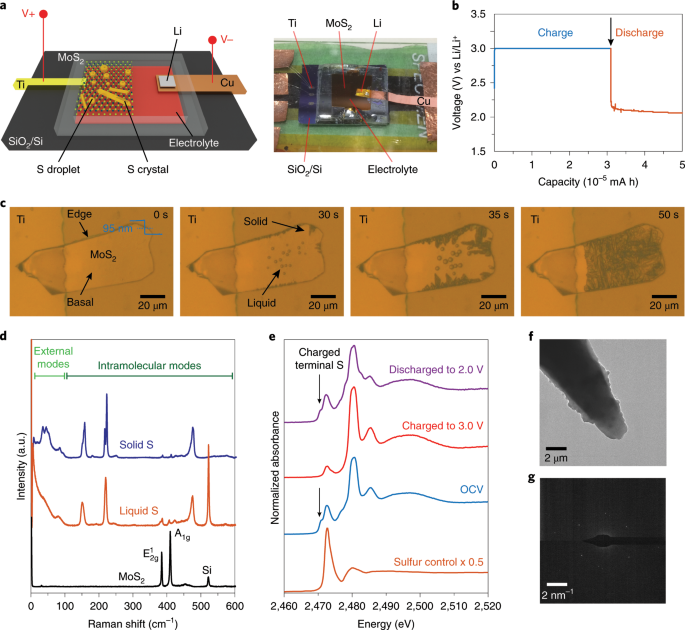
ABSTRACT It has recently been shown that sulfur, a solid material in its elementary form S8, can stay in a supercooled state as liquid sulfur in an electrochemical cell. We establish that
this newly discovered state could have implications for lithium–sulfur batteries. Here, through in situ studies of electrochemical sulfur generation, we show that liquid (supercooled) and
solid elementary sulfur possess very different areal capacities over the same charging period. To control the physical state of sulfur, we studied its growth on two-dimensional layered
materials. We found that on the basal plane, only liquid sulfur accumulates; by contrast, at the edge sites, liquid sulfur accumulates if the thickness of the two-dimensional material is
small, whereas solid sulfur nucleates if the thickness is large (tens of nanometres). Correlating the sulfur states with their respective areal capacities, as well as controlling the growth
of sulfur on two-dimensional materials, could provide insights for the design of future lithium–sulfur batteries. Access through your institution Buy or subscribe This is a preview of
subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value
online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more
Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS:
* Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS HIGH-ENTROPY SULFOSELENIDE AS NEGATIVE ELECTRODES WITH
FAST KINETICS AND HIGH STABILITY FOR SODIUM-ION BATTERIES Article Open access 30 April 2025 A NEAR DIMENSIONALLY INVARIABLE HIGH-CAPACITY POSITIVE ELECTRODE MATERIAL Article 12 December 2022
REALIZING HIGH-CAPACITY ALL-SOLID-STATE LITHIUM-SULFUR BATTERIES USING A LOW-DENSITY INORGANIC SOLID-STATE ELECTROLYTE Article Open access 05 April 2023 DATA AVAILABILITY The data that
support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request. REFERENCES * Bruce, P. G., Freunberger, S. A.,
Hardwick, L. J. & Tarascon, J.-M. Li–O2 and Li–S batteries with high energy storage. _Nat. Mater._ 11, 19–29 (2012). Article CAS Google Scholar * Seh, Z. W., Sun, Y., Zhang, Q. &
Cui, Y. Designing high-energy lithium–sulfur batteries. _Chem. Soc. Rev._ 45, 5605–5634 (2016). Article CAS Google Scholar * Zheng, G. et al. Amphiphilic surface modification of hollow
carbon nanofibers for improved cycle life of lithium sulfur batteries. _Nano Lett._ 13, 1265–1270 (2013). Article CAS Google Scholar * Yao, H. et al. Improving lithium–sulphur batteries
through spatial control of sulphur species deposition on a hybrid electrode surface. _Nat. Commun._ 5, 3943 (2014). Article CAS Google Scholar * Wang, H. et al. High electrochemical
selectivity of edge versus terrace sites in two-dimensional layered MoS2 materials. _Nano Lett._ 14, 7138–7144 (2014). Article CAS Google Scholar * Liu, N. et al. Direct electrochemical
generation of supercooled sulfur microdroplets well below their melting temperature. _Proc. Natl Acad. Sci. USA_ 116, 201817286–201817770 (2019). Google Scholar * Nelson, J. et al. In
operando X-ray diffraction and transmission X-ray microscopy of lithium sulfur batteries. _J. Am. Chem. Soc._ 134, 6337–6343 (2012). Article CAS Google Scholar * Zhang, L., Sun, D., Feng,
J., Cairns, E. J. & Guo, J. Revealing the electrochemical charging mechanism of nanosized Li2S by in situ and operando X-ray absorption spectroscopy. _Nano Lett._ 17, 5084–5091 (2017).
Article CAS Google Scholar * Zhang, L. et al. The synergetic interaction between LiNO3 and lithium polysulfides for suppressing shuttle effect of lithium-sulfur batteries. _Energy Storage
Mater._ 11, 24–29 (2018). Article Google Scholar * Wu, Y. & Liu, N. Visualizing battery reactions and processes by using in situ and in operando microscopies. _Chem_ 4, 438–465
(2018). Article CAS Google Scholar * Stephenson, T., Li, Z., Olsen, B. & Mitlin, D. Lithium ion battery applications of molybdenum disulfide (MoS2) nanocomposites. _Energy Environ.
Sci._ 7, 209–231 (2013). Article Google Scholar * Ghazi, Z. A. et al. MoS2/Celgard separator as efficient polysulfide barrier for long‐life lithium–sulfur batteries. _Adv. Mater._ 29,
1606817 (2017). Article Google Scholar * Lei, T. et al. Multi‐functional layered WS2 nanosheets for enhancing the performance of lithium–sulfur batteries. _Adv. Energy Mater._ 7, 1601843
(2017). Article Google Scholar * Seh, Z. W. et al. Two-dimensional layered transition metal disulphides for effective encapsulation of high-capacity lithium sulphide cathodes. _Nat.
Commun._ 5, 5017 (2014). Article CAS Google Scholar * Babu, G., Masurkar, N., Salem, Al. H. & Arava, L. M. R. Transition metal dichalcogenide atomic layers for lithium polysulfides
electrocatalysis. _J. Am. Chem. Soc._ 139, 171–178 (2016). Article Google Scholar * Zhang, Q. et al. Understanding the anchoring effect of two-dimensional layered materials for
lithium–sulfur batteries. _Nano Lett._ 15, 3780–3786 (2015). Article CAS Google Scholar * Wang, H. et al. Graphene-wrapped sulfur particles as a rechargeable lithium–sulfur battery
cathode material with high capacity and cycling stability. _Nano Lett._ 11, 2644–2647 (2011). Article CAS Google Scholar * Ji, L. et al. Graphene oxide as a sulfur immobilizer in high
performance lithium/sulfur cells. _J. Am. Chem. Soc._ 133, 18522–18525 (2011). Article CAS Google Scholar * Chen, H. et al. High‐quality graphene microflower design for high‐performance
Li–S and Al‐ion batteries. _Adv. Energy Mater._ 7, 1700051 (2017). Article Google Scholar * Su, D., Cortie, M. & Wang, G. Fabrication of N‐doped graphene–carbon nanotube hybrids from
Prussian blue for lithium–sulfur batteries. _Adv. Energy Mater._ 7, 1602014 (2017). Article Google Scholar * Choi, S. et al. WO3 nanolayer coated 3D-graphene/sulfur composites for high
performance lithium/sulfur batteries. _J. Mater. Chem. A_ 7, 4596–4603 (2019). Article CAS Google Scholar * Sun, J. et al. Entrapment of polysulfides by a black-phosphorus-modified
separator for lithium-sulfur batteries. _Adv. Mater._ 28, 9797–9803 (2016). Article CAS Google Scholar * Nims, C., Cron, B., Wetherington, M., Macalady, J. & Cosmidis, J. Low
frequency Raman spectroscopy for micron-scale and in vivo characterization of elemental sulfur in microbial samples. _Sci. Rep._ 9, 1–12 (2019). Article CAS Google Scholar * Pascal, T. A.
et al. X-ray absorption spectra of dissolved polysulfides in lithium–sulfur batteries from first-principles. _J. Phys. Chem. Lett._ 5, 1547–1551 (2014). Article CAS Google Scholar *
Levin, B. D. A. et al. Characterization of sulfur and nanostructured sulfur battery cathodes in electron microscopy without sublimation artifacts. _Microsc. Microanal._ 23, 155–162 (2017).
Article CAS Google Scholar * Kim, H. et al. In situ TEM observation of electrochemical lithiation of sulfur confined within inner cylindrical pores of carbon nanotubes. _Adv. Energy
Mater._ 5, 1501306 (2015). Article Google Scholar * Hamada, S., Nakazawa, Y. & Shirai, T. Nucleation in liquid sulfur droplets. _Bull. Chem. Soc. Jpn._ 43, 3096–3101 (2006). Article
Google Scholar * Mugele, F. & Baret, J.-C. Electrowetting: from basics to applications. _J. Phys.: Condens. Matter_ 17, R705–R774 (2005). CAS Google Scholar * Pollack, M. G., Fair, R.
B. & Shenderov, A. D. Electrowetting-based actuation of liquid droplets for microfluidic applications. _Appl. Phys. Lett._ 77, 1725–1726 (2000). Article CAS Google Scholar * Yang,
Y., Zheng, G. & Cui, Y. A membrane-free lithium/polysulfide semi-liquid battery for large-scale energy storage. _Energy Environ. Sci._ 6, 1552–1558 (2013). Article CAS Google Scholar
* Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data Analysis for X-Ray Absorption Spectroscopy Using IFEFFIT. _J. Synchrotron Radiat._ 12, 537–541 (2005). Article CAS Google
Scholar * Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. _J. Comput. Phys._ 117, 1–19 (1995). Article CAS Google Scholar * Sresht, V. et al. Quantitative
modeling of MoS2–solvent interfaces: predicting contact angles and exfoliation performance using molecular dynamics. _J. Phys. Chem. C._ 121, 9022–9031 (2017). Article CAS Google Scholar
* Rajput, N. N. et al. Elucidating the solvation structure and dynamics of lithium polysulfides resulting from competitive salt and solvent interactions. _Chem. Mater._ 29, 3375–3379 (2017).
Article CAS Google Scholar * Merlet, C. et al. On the molecular origin of supercapacitance in nanoporous carbon electrodes. _Nat. Mater._ 11, 306–310 (2012). Article CAS Google Scholar
* Wang, Z., Yang, Y., Olmsted, D. L., Asta, M. & Laird, B. B. Evaluation of the constant potential method in simulating electric double-layer capacitors. _J. Chem. Phys._ 141, 184102
(2014). Article Google Scholar * Laturia, A., Van de Put, M. L. & Vandenberghe, W. G. Dielectric properties of hexagonal boron nitride and transition metal dichalcogenides: from
monolayer to bulk. _npj 2D Mater. Appl._ 2, 4106 (2018). Article Google Scholar Download references ACKNOWLEDGEMENTS We acknowledge support from the Department of Energy, Office of Basic
Energy Sciences, Division of Materials Science and Engineering under contract DE-AC02-76SF00515. Part of this work was performed at the Stanford Nano Shared Facilities (SNSF) and Stanford
Nanofabrication Facilities (SNF), supported by the National Science Foundation under award ECCS-1542152. R.A.V. acknowledges support from the National Academy of Sciences Ford Foundation
Fellowship and the National Science Foundation Graduate Research Fellowship Program (NSF GRFP, grant number: DGE – 1656518). We acknowledge C. Su and J. Li from MIT for performing the DFT
calculations. A.Y. acknowledges D. Zakhidov for his assistance with polarized Raman measurements and Y. Ye, Z. Wang and R. Xu for helpful discussions. AUTHOR INFORMATION Author notes * Di
Chen Present address: The Future Laboratory, Tsinghua University, Beijing, China * These authors contributed equally: Ankun Yang, Guangmin Zhou. AUTHORS AND AFFILIATIONS * Department of
Materials Science and Engineering, Stanford University, Stanford, CA, USA Ankun Yang, Guangmin Zhou, Rafael A. Vilá, Allen Pei, Xiaoyun Yu, Xueli Zheng, Chun-Lan Wu, Bofei Liu, Hao Chen, Yan
Xu, Di Chen, Yanxi Li & Yi Cui * Department of Chemical Engineering, Stanford University, Stanford, CA, USA Xian Kong & Jian Qin * Department of Electrical Engineering, Stanford
University, Stanford, CA, USA Yecun Wu * Advanced Light Source, Lawrence Berkeley National Laboratory, Berkeley, CA, USA Sirine Fakra * Department of Applied Physics, Stanford University,
Stanford, CA, USA Harold Y. Hwang * Stanford Institute for Materials and Energy Sciences, SLAC National Accelerator Laboratory, Menlo Park, CA, USA Harold Y. Hwang & Yi Cui * Department
of Physics, Stanford University, Stanford, CA, USA Steven Chu * Department of Molecular and Cellular Physiology, Stanford University, Stanford, CA, USA Steven Chu Authors * Ankun Yang View
author publications You can also search for this author inPubMed Google Scholar * Guangmin Zhou View author publications You can also search for this author inPubMed Google Scholar * Xian
Kong View author publications You can also search for this author inPubMed Google Scholar * Rafael A. Vilá View author publications You can also search for this author inPubMed Google
Scholar * Allen Pei View author publications You can also search for this author inPubMed Google Scholar * Yecun Wu View author publications You can also search for this author inPubMed
Google Scholar * Xiaoyun Yu View author publications You can also search for this author inPubMed Google Scholar * Xueli Zheng View author publications You can also search for this author
inPubMed Google Scholar * Chun-Lan Wu View author publications You can also search for this author inPubMed Google Scholar * Bofei Liu View author publications You can also search for this
author inPubMed Google Scholar * Hao Chen View author publications You can also search for this author inPubMed Google Scholar * Yan Xu View author publications You can also search for this
author inPubMed Google Scholar * Di Chen View author publications You can also search for this author inPubMed Google Scholar * Yanxi Li View author publications You can also search for this
author inPubMed Google Scholar * Sirine Fakra View author publications You can also search for this author inPubMed Google Scholar * Harold Y. Hwang View author publications You can also
search for this author inPubMed Google Scholar * Jian Qin View author publications You can also search for this author inPubMed Google Scholar * Steven Chu View author publications You can
also search for this author inPubMed Google Scholar * Yi Cui View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS A.Y. and Y.C. conceived and
designed the experiments. A.Y. and G.Z. carried out device fabrication, imaging and electrochemical measurements. X.K. and J.Q. performed MD simulations. R.A.V. performed TEM
characterizations. A.P. performed COMSOL simulations. X.Z. and S.F. performed in situ XAS measurements. Y.W., C.-L.W. and B.L. assisted in material preparation. X.Y., H.C., Y.X., D.C. and
Y.L. assisted in electrochemical measurements. H.Y.H., S.C. and Y.C. supervised the project and all authors contributed to data discussions. A.Y. and Y.C. analysed the data and wrote the
paper with input from all authors. CORRESPONDING AUTHOR Correspondence to Yi Cui. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION
PEER REVIEW INFORMATION Nature Nanotechnology thanks Yuyan Shao, Guoxiu Wang and Reza Shahbazian-Yassar for their contribution to the peer review of this work. PUBLISHER’S NOTE Springer
Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figs. 1-21 and
Table 1. SUPPLEMENTARY VIDEO 1 Sulfur generation on MoS2 shows distinct growth behaviors on the basal plane and the edges. Specifically, liquid sulfur droplets were generated on the basal
plane of the MoS2 while solid sulfur crystal was generated on the edges of the MoS2 flake. Play speed is 3× of the actual speed. SUPPLEMENTARY VIDEO 2 Cryogenic electron microscopy (Cryo-EM)
Selected Area Electron Diffraction (SAED) of solid sulfur indicates its high crystallinity. The sample was tilted between -30° and 30°. Play speed is 5× of the actual speed. SUPPLEMENTARY
VIDEO 3 Liquid and solid sulfur generation on the edges of 2D flake. Liquid droplets were observed on the edges at the initial stage and quickly turned into solid by the emerging crystals
from the edges. Play speed is 5× of the actual speed. SUPPLEMENTARY VIDEO 4 Sulfur generation on monolayer MoS2. Liquid droplets were dynamically generated on monolayer MoS2 without
crystallization. The MoS2 flake in the middle was not connected to the Ti electrode, so no sulfur was generated. Play speed is 20× of the actual speed. SUPPLEMENTARY VIDEO 5 Liquid sulfur
generation on MoS2 window. The edges of the MoS2 flake were completely covered to suppress crystal growth. Play speed is 10× of the actual speed. SUPPLEMENTARY VIDEO 6 Solid sulfur
generation on MoS2 window. The edges of the MoS2 flake were partially left open to initiate crystal growth. Play speed is 10× of the actual speed. RIGHTS AND PERMISSIONS Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yang, A., Zhou, G., Kong, X. _et al._ Electrochemical generation of liquid and solid sulfur on two-dimensional layered materials with
distinct areal capacities. _Nat. Nanotechnol._ 15, 231–237 (2020). https://doi.org/10.1038/s41565-019-0624-6 Download citation * Received: 10 June 2019 * Accepted: 12 December 2019 *
Published: 27 January 2020 * Issue Date: March 2020 * DOI: https://doi.org/10.1038/s41565-019-0624-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative