Play all audios:
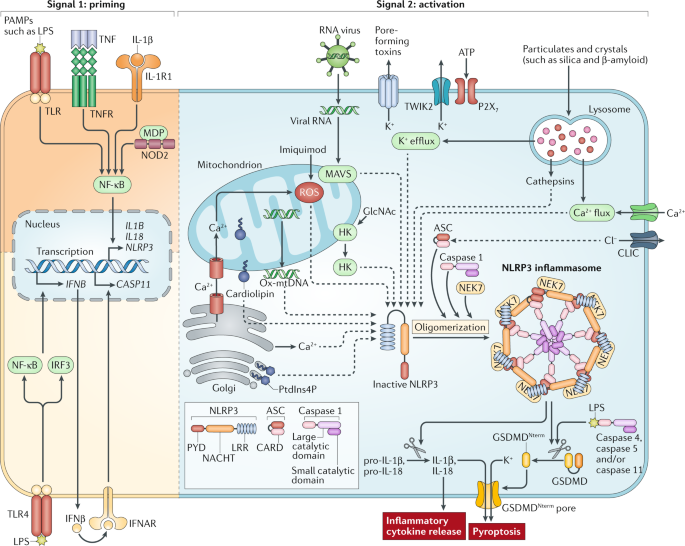
NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3) is an intracellular sensor that detects a broad range of microbial motifs, endogenous danger signals and environmental irritants,
resulting in the formation and activation of the NLRP3 inflammasome. Assembly of the NLRP3 inflammasome leads to caspase 1-dependent release of the pro-inflammatory cytokines IL-1β and
IL-18, as well as to gasdermin D-mediated pyroptotic cell death. Recent studies have revealed new regulators of the NLRP3 inflammasome, including new interacting or regulatory proteins,
metabolic pathways and a regulatory mitochondrial hub. In this Review, we present the molecular, cell biological and biochemical bases of NLRP3 activation and regulation and describe how
this mechanistic understanding is leading to potential therapeutics that target the NLRP3 inflammasome.
This Review was supported by the National Center for Advancing Translational Sciences, US National Institutes of Health (NIH), through grant KL2TR002490 awarded to K.V.S. and by the NIH
through grants AI029564, CA156330, DK094779, AI109965 and AI067798 awarded to J.P.-Y.T. The content is solely the responsibility of the authors and does not necessarily represent the
official views of the NIH.
Department of Medicine, Infectious Diseases, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Oral and Craniofacial Biomedicine Program, School of Dentistry, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Department of Genetics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Institute for Inflammatory Diseases, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Center for Translational Immunology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
All the authors contributed equally to all aspects of the article.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
(LRR domain). In Toll-like receptors (TLRs), the LRR domain mediates the detection of microbial components; it may serve a similar role in certain NLRs (NACHT–LRR proteins). The LRR domain
of NLRs and TLRs is structurally similar. It consists of leucine-rich amino acid strands forming a peptide loop. The loops occur as tandem repeats that together form a coil or solenoid and
contain constant sequences, as well as unique insertions or variable residues for each ligand.
A sensor that combines with the adaptor protein ASC and the protease caspase 1 to form the AIM2 inflammasome. It senses cytosolic double-stranded DNA from bacteria or viruses or from
mislocalized self-DNA and contributes to infection defence.
(P2X7). An ATP-gated cation channel that is expressed by haematopoietic cells and participates in cell proliferation and apoptosis. It belongs to the family of purinoceptors for ATP and is
responsible for the ATP-dependent activation of NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3).
An experimental model of peritonitis in rodents, in which the caecum is ligated and then punctured, thereby forming a small hole. This leads to leakage of intestinal bacteria into the
peritoneal cavity and subsequent peritoneal infection.
The selective removal of mitochondria by macroautophagy under conditions of nutrient starvation or mitochondrial stress.
Cells continuously produce reactive oxygen species (ROS) such as hydrogen peroxide or superoxide anions. Under physiological conditions, mitochondria are the main source, and cellular
antioxidants ensure that the redox equilibrium is maintained. During inflammatory responses (and in cancer), excessive production of ROS leads to a metabolic condition known as oxidative
stress, which can lead to apoptosis and necrosis.
A cytoplasmic bulk degradation system in which cytoplasmic cargo is targeted and is typically sequestered in double-membrane vesicles, leading to subsequent fusion with the lysosome. This
process is essential for the response to starvation because it facilitates the recycling of cellular components. In addition, autophagy can be targeted to intracellular bacteria to restrict
their growth.
A mouse model of crystal-induced peritonitis that activates the NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3) inflammasome.
A lytic, inflammatory form of programmed cell death that is triggered by cleavage of gasdermin D by the inflammatory caspase 1, 4, 5 or 11. It is characterized by cytoplasmic swelling, early
plasma membrane rupture and nuclear condensation. The cytoplasmic content is released into the extracellular space, and this is thought to augment inflammatory and repair responses.
(NETs). Fibrous networks that are released into the extracellular environment by neutrophils. They are composed mainly of DNA but also contain proteins from neutrophil granules. NETs act as
a mesh that traps microorganisms and exposes them to neutrophil-derived effector molecules.
Anyone you share the following link with will be able to read this content: