Play all audios:
ABSTRACT Genes specifying long non-coding RNAs (lncRNAs) occupy a large fraction of the genomes of complex organisms. The term ‘lncRNAs’ encompasses RNA polymerase I (Pol I), Pol II and Pol
III transcribed RNAs, and RNAs from processed introns. The various functions of lncRNAs and their many isoforms and interleaved relationships with other genes make lncRNA classification and
annotation difficult. Most lncRNAs evolve more rapidly than protein-coding sequences, are cell type specific and regulate many aspects of cell differentiation and development and other
physiological processes. Many lncRNAs associate with chromatin-modifying complexes, are transcribed from enhancers and nucleate phase separation of nuclear condensates and domains,
indicating an intimate link between lncRNA expression and the spatial control of gene expression during development. lncRNAs also have important roles in the cytoplasm and beyond, including
in the regulation of translation, metabolism and signalling. lncRNAs often have a modular structure and are rich in repeats, which are increasingly being shown to be relevant to their
function. In this Consensus Statement, we address the definition and nomenclature of lncRNAs and their conservation, expression, phenotypic visibility, structure and functions. We also
discuss research challenges and provide recommendations to advance the understanding of the roles of lncRNAs in development, cell biology and disease. SIMILAR CONTENT BEING VIEWED BY OTHERS
GENE REGULATION BY LONG NON-CODING RNAS AND ITS BIOLOGICAL FUNCTIONS Article 22 December 2020 TRANSCRIPTION REGULATION BY LONG NON-CODING RNAS: MECHANISMS AND DISEASE RELEVANCE Article 19
January 2024 THE RNA ATLAS EXPANDS THE CATALOG OF HUMAN NON-CODING RNAS Article 17 June 2021 INTRODUCTION Research on long non-coding RNAs (lncRNAs), a previously unsuspected major output of
genomes of complex organisms, has been dogged by uncertainty and controversy from its beginning. lncRNAs have the unfortunate distinction of being named for what they are not, rather than
what they are. This loose description has its origins in the belief that the main role of RNA is to act as the intermediate between a gene and a protein, with other ‘housekeeping’ non-coding
RNAs such as ribosomal RNAs (rRNAs), transfer RNAs (tRNAs), small nucleolar RNAs (snoRNAs), spliceosomal RNAs and other small nuclear RNAs (snRNAs) being ancillary to this function. Broad
recognition of RNA as a regulatory molecule occurred in the early years of the first decade of the twenty-first century with the unexpected discovery of large numbers of small interfering
RNAs (siRNAs), microRNAs (miRNAs) and small PIWI-interacting RNAs (piRNAs) that regulate — through Argonaute family proteins — gene expression at transcriptional, post-transcriptional and
translational levels in eukaryotes1, although there were examples of other small regulatory RNAs in the literature, especially in bacteria2. A few long regulatory RNAs, notably _meiRNA_ in
the fission yeast _Schizosaccharomyces pombe_, _hsrω_, RNA on the X1 (_roX1_) and _roX2_ in _Drosophila melanogaster_, and _H19_ and X-inactive-specific transcript (_XIST_) in mammals, had
also been reported in the preceding years3,4,5,6,7, but were regarded more as oddities than early examples of a general phenomenon. Moreover, the small regulatory RNAs did not disturb the
conceptual framework that most genes encode proteins, but rather fitted comfortably into it. It was later found, however, that while some miRNAs are generated from the introns of pre-mRNAs8,
non-coding primary transcripts of miRNAs and of snoRNAs can also have functions9,10 and that rRNAs, tRNAs and snoRNAs are processed to generate small regulatory RNAs, including
miRNAs11,12,13,14, in some cases contributing to transgenerational epigenetic inheritance15. A bigger surprise, and challenge to the reigning understanding of genetic information, came in
the early and middle years of the first decade of the twenty-first century, when global transcriptomic analyses, intended to better define the proteome, revealed that most of the genome of
animals and plants is dynamically transcribed into longer RNAs that have little or no protein-coding potential16,17,18,19. This surprise was compounded by the associated finding that the
number, and to a large extent the repertoire, of protein-coding genes is similar in animals of widely different developmental and cognitive complexity — the nematode worm _Caenorhabditis
elegans_ (comprising ~1,000 somatic cells) and humans (~30 × 1012 somatic cells20) both have ~20,000 protein-coding genes — which was termed the ‘g-value paradox’21. By contrast, the extent
of non-coding DNA, and consequently the transcription of non-coding RNAs, has increased with increasing developmental complexity22. Understandably, the common initial reaction of the
molecular biology community was to suspect that these unusual RNAs are transcriptional noise, because of their generally low levels of sequence conservation, low levels of expression and low
visibility in genetic screens. Since then, however, there has been an explosion in the number of publications reporting the dynamic expression and biological functions of lncRNAs, aided by
extensive technology development that has enabled their identification and characterization, although only a minority of lncRNAs have confident annotations and very few have mechanistic
information. The realization that the genomes of plants and animals express large numbers of lncRNAs requires a framework for their classification and understanding of their functions and,
more profoundly, a reassessment of the amount and type of information required to programme the development of complex organisms. PURPOSE OF THIS CONSENSUS STATEMENT In this Consensus
Statement we present a current and coherent picture of the roles of lncRNAs in cell and developmental biology, identify the key issues in understanding their functions and chart the path
forward. We address lncRNA definition, nomenclature, conservation, expression, phenotypic visibility, functional assays and molecular mechanisms encompassing lncRNA connections to chromatin
architecture, epigenetic processes, enhancer function and biomolecular condensates, as well as the roles of lncRNAs outside the nucleus. We argue that loci expressing lncRNAs should be
recognized as bona fide genes and discuss lncRNA structure–function relationships as the means to parse mechanisms and pathways. Finally, we identify the current challenges and offer
recommendations for understanding the relationship of lncRNAs to genome architecture, gene regulation and cellular organization. The authors of this Consensus Statement were suggested by
recommendations of colleagues. Consensus was reached by group e-mail and discussion. DEFINITION AND NOMENCLATURE OF LNCRNAS lncRNAs have been arbitrarily defined as non-coding transcripts of
more than 200 nucleotides (200 nt), which is a convenient size cut-off in biochemical and biophysical RNA purification protocols that deplete most infrastructural RNAs, such as 5S rRNAs,
tRNAs, snRNAs and snoRNAs, as well as miRNAs, siRNAs and piRNAs23. This definition also excludes some other well-known short RNAs such as the primate-specific snaRs (~80–120 nt), which
associate with nuclear factor 90 (ref. 24); Y RNAs (~100 nt), which act as scaffolds for ribonucleoprotein (RNP) complexes25; vault RNAs (88–140 nt), which are involved in transferring
extracellular stimuli into intracellular signals26; and promoter-associated RNAs and non-canonical small RNAs produced by post-transcriptional processing27,28,29. Other non-coding RNAs lie
close to the 200-nt border, such as _7SK_ (~330 nt in vertebrates), which controls transcription poising and termination, including at enhancers30,31, and _7SL_ (~300 nt), which is an
integral component of the signal recognition particle that targets proteins to cell membranes32 and the evolutionary ancestor of the widespread primate Alu (~280 nt) and rodent B1 (~135 nt)
small interspersed nuclear elements33,34,35. Given this grey zone of sizes, we support the suggestion that non-coding RNAs be divided into three categories36: (1) small RNAs (less than 50
nt); (2) RNA polymerase III (Pol III) transcripts (such as tRNAs, 5S rRNA, _7SK_, _7SL_, and Alu, vault and Y RNAs37), Pol V transcripts in plants and small Pol II transcripts such as (most)
snRNAs and intron-derived snoRNAs38,39 (~50–500 nt); and (3) lncRNAs (more than 500 nt), which are mostly generated by Pol II. Many lncRNAs are spliced and polyadenylated, which has led to
their description as ‘mRNA-like’. However, other lncRNAs are not polyadenylated or 7-methylguanosine capped19,40,41,42, are expressed from Pol I (5.8S, 28S and 18S rRNAs) or Pol III
promoters, or are processed from precursors, including from introns and repetitive elements, leading to the more agnostic descriptor ‘transcripts of unknown function’43. With respect to
protein-coding genes, lncRNAs can be ‘intergenic’, antisense or intronic. They are also derived from ‘pseudogenes’, which occur commonly in metazoan genomes44, with more than 10,000
pseudogenes identified in the mouse genome45 and almost 15,000 identified in the human genome46, some of which have been shown to be functional44,47. lncRNAs also include circular RNAs
generated by back-splicing of coding and non-coding transcripts, also with demonstrated functions48, and _trans_-acting regulatory RNAs derived from sequences that conventionally act as the
3′ untranslated regions of mRNAs49. There have been many attempts at nomenclature and classification of lncRNAs, by the HUGO Gene Nomenclature Committee, the GENCODE consortium and others,
predominantly based on their genomic position and orientation relative to protein-coding genes46,50,51,52,53. Linking to nearby genes has been useful, as it provides context and has
sometimes provided clues to lncRNA function, for example in regulating the expression of these genes, as is often the case with enhancers (see later), although enhancer activity should not
be assumed to be directed to the most proximal genes. Many early studies focused on long intergenic non-coding RNAs (lincRNAs), whose sequences do not trespass on nearby protein-coding loci,
owing to the need to distinguish their function from that of proteins. However, many other lncRNAs overlap protein-coding loci or are expressed from enclosed introns. Moreover, the
traditional view of genomes as linear arrangements of discrete protein-coding genes fails to accommodate the discovery that eukaryotic transcription, best characterized in human and model
organisms, is a fuzzy continuum54, with ‘genes’ within genes, genes interleaved with other genes and non-coding transcripts overlapping or originating within them18,43,55, together posing a
growing problem for genome annotations. In both humans and _D. melanogaster_, for example, many protein-coding genes have 5′ exons that are incorporated into mRNA in early embryogenesis and
lie hundreds of kilobases upstream of the usual first exon, bypassing many other genes in the intervening region56. Indeed, any base may be exonic, intronic or ‘intergenic’, depending on the
transcriptional output of the cell at any point in its developmental trajectory or physiological state55. For this reason, unless a lncRNA is antisense to a protein-coding gene, we
recommend naming lncRNAs for their own sake with allusion to a discerned characteristic or function (as has been traditional for proteins), such as _XIST_, antisense IGF2R non-protein-coding
RNA57 (_AIRN_), HOX antisense intergenic RNA58 (_HOTAIR_), _Gomafu_ (‘spotted pattern’ in Japanese; also known as _Miat_)59, _COOLAIR_ (referring to plant vernalization)60 and
auxin-regulated promoter loop61 (_APOLO_), for easy recollection, preferably accompanied by complete exon–intron structures and genomic coordinates. If no biological context is available, we
recommend naming the lncRNA according to the GENCODE system46. The wide range of functions of ‘non-coding’ RNAs precludes straightforward classification as specific RNA classes, with some
acting locally and some at a distance, or both62. In the absence of more specific categorization, we recommend retention of the general descriptor ‘lncRNA’, noting that most have some type
of regulatory or architectural, often related, role in cell and developmental biology, and because there are so many historical articles that use this term or variations thereof. Non-coding
RNAs come in all shapes and sizes, and the territory is huge, covering most of the genome and a plethora of functions. Some RNAs have dual functions as coding and regulatory RNAs, and some,
perhaps many, cytosolic lncRNAs encode small peptides63,64,65,66. Protein-coding loci also express lncRNAs through alternative splicing67,68,69, and, surprisingly, the major transcript
produced by ~17% of human protein-coding loci is non-coding70. Indeed, both lncRNA genes and mRNA genes can produce transcripts that function following different levels of processing.
Unspliced transcripts, spliced transcripts, circular RNAs, intronic RNAs and stable small RNAs generated from them can all have a function48,71,72. Any RNA can be regulatory, and any locus
can encode both protein-coding and regulatory RNAs. Well in excess of 100,000 human lncRNAs have been recorded52,73, many of which are specific to the primate lineage74. This is a vastly
incomplete list due to the limited analysis of different cells at different developmental stages (see later). There are now hundreds of thousands of catalogued lncRNAs and dozens of
databases (and databases of databases) with curated information75,76,77,78,79,80. Over the past decade, there have been ~50,000 publications with ‘long non-coding RNA’ as a key term and more
than 2,000 publications reporting validated lncRNA functions81, although most have yet to be followed up in any detail. From here on, we focus on lncRNAs derived from Pol II primary
transcription units (and use the term in that context), as opposed to other non-coding RNAs that are expressed from Pol I or Pol III promoters, processed from introns (which, it should be
noted, constitute a major fraction of the non-coding RNA in mammals and other organisms41,82,83,84) or formed by back-splicing, although many of the same considerations apply. CONSERVATION
OF LNCRNAS Most lncRNAs are less conserved among species than the mRNA sequences encoding the proteome. Initially, most of the mammalian genome (which included most lncRNA loci) was thought
to be evolving neutrally, using the yardstick of the rate of divergence of common ‘ancient repeats’ (derived from transposons) between the human and mouse genomes, on the assumption that
these sequences are non-functional and representative of the original distribution in the ancestor85. However, there is increasing evidence that transposable elements are widely co-opted as
functional elements of gene expression and structure, forming promoters, regulatory networks, exons and splice junctions in protein-coding genes and lncRNAs86,87,88,89, and therefore cannot
be used as indices of neutral evolution. Regulatory sequences, including promoters and lncRNAs, are known to evolve rapidly due to more relaxed structure–function constraints than
protein-coding sequences and due to positive selection during adaptive radiation85,90,91,92. Many lncRNAs are cell lineage specific. Indeed, given their association with developmental
enhancers (see later), variation in the complement and sequences of lncRNAs may be a major factor in species diversity. Loci expressing lncRNAs exhibit many of the characteristics of
protein-coding genes, including promoters, multiple exons, alternative splicing, characteristic chromatin signatures, regulation by morphogens and conventional transcription factors, altered
expression in cancer and other diseases74,93,94,95,96,97,98, and a range of half-lives similar to those of mRNAs99. The promoters of lncRNAs exhibit levels of conservation comparable to
those of protein-coding genes18,74. lncRNAs also have conserved exon structures, splice junctions and sequence patches18,74,93,97, and they retain orthologous functions despite rapid
sequence evolution100,101,102. Indeed, low sequence conservation can be misleading. The lncRNA telomerase RNA template component (_TERC_), which is required for telomere maintenance — a
vital cellular function — differs widely in size and sequence, but has conserved structural topology from yeast to mammals, albeit with some variation, and a conserved catalytic
core103,104,105,106,107,108 (see also later). X chromosome dosage compensation in _Drosophila_ spp. requires the formation of a nuclear domain through phase separation by the lncRNAs _roX1_
and _roX2_ interacting with the intrinsically disordered region (IDR) of a specific partner protein, male sex lethal 2 (MSL2). Replacing the IDR of the mammalian orthologue of MSL2 with that
of the _D. melanogaster_ protein and expression of _roX2_ is sufficient to nucleate ectopic X chromosome dosage compensation in mammalian cells, showing that the _roX_–MSL2 IDR interaction
is the primary determinant of compartmentalization of the X chromosome and that such interactions are preserved over vast evolutionary distances109. Similar processes are involved in the
regulation of X chromosome dosage compensation in placental mammals by _XIST_, which performs several functions, including repulsion of euchromatic factors, scaffolding of new
heterochromatic factors and reorganization of chromosome structure110,111,112,113. EXPRESSION Although there are exceptions (such as metastasis-associated lung adenocarcinoma transcript 1
(_MALAT1_; also known as _NEAT2_), which is one of the most abundant Pol II transcripts in vertebrate cells114, and nuclear paraspeckle assembly transcript 1 (_NEAT1_); see later), lncRNAs
generally show more restricted expression patterns than mRNAs74,115, and are often highly cell specific116, which is consistent with a role in the definition of cell state and developmental
trajectory. They also have specific subcellular locations, often nuclear, although a large fraction is cytoplasmic75. Although it is sometimes asserted that there are a few hundred cell
types in a human, broad classifications obscure the fact that each cell occupies a precise place in a developmental ontogeny, illustrated by the differential expression of HOX genes in
superficially similar skin cells in different regions of the body117, and by the expression of lncRNAs in various regions of the brain118,119,120,121 and at different stages of
development122. lncRNAs are also dynamically expressed during differentiation of mammalian stem, muscle, mammary gland, immune and neural cells, among many others81,116, with a transition
during development from broadly expressed and conserved lncRNAs towards an increasing number of lineage-specific and organ-specific lncRNAs123. lncRNA expression can also be strongly
influenced by environmental factors, a feature that is especially prominent in plants124,125,126, which include a range of stress responses in animals and drug resistance in
cancer127,128,129,130,131,132,133. The restricted expression of lncRNAs in different cells at different stages of development and their generally low copy number (owing to their regulatory
nature) accounts for their sparse representation in bulk-tissue RNA sequencing datasets134, whereas many lncRNAs are relatively easy to detect in particular cells118. The undersampling of
lncRNAs is now being rectified by targeted capture98,135, advanced imaging136,137,138, spatial transcriptomics139 and, in some cases, single-cell sequencing120,121,140, which make it clear
that, whereas ~20,000 human lncRNA loci have been identified by GENCODE46 and ~30,000 by the FANTOM consortium141, there is likely at least an order of magnitude more. Due to the high
complexity and the variation in transcription initiation and termination sites, expression levels and splicing, comprehensive characterization of transcriptomes is extremely challenging. A
recent study showed that the low expression of a lncRNA can be essential for its functional role by ensuring specificity to its regulated targets, suggesting that low abundance levels may be
an essential feature of how lncRNAs work142. To fully catalogue the universe of lncRNAs, and properly record their exon–intron organization and splice variants, high-depth sequencing will
need to be performed on cells at all stages of differentiation and development, undergoing different neural, immunological and other physiological processes, and in various disease states.
This is a huge task, but we recommend that future gene expression profiling should include full transcript analysis not just of mRNAs but also of small RNAs and lncRNAs that are intergenic,
antisense and intronic to the annotated genes, and their stoichiometry143. PHENOTYPIC VISIBILITY Like miRNAs, most lncRNAs have not been identified in genetic screens. There are two reasons
for this. First, most genetic screens historically focused on protein-coding mutations, which often have severe consequences that are easy to track; by contrast, regulatory mutations often
have subtle consequences that affect quantitative traits. Second, it is difficult to identify causal mutations among the many variations that occur in non-coding sequences. Indeed, most
variations that influence human quantitative traits and complex disorders occur in non-coding regions, which are replete with genes expressing lncRNAs144,145 that are transcribed in cell
types relevant to the associated trait141,146. There are exceptions of lncRNAs that have been identified genetically, notably the _roX1 _and _roX2_ RNAs involved in X chromosome activation
in male fruitflies5, mammalian parentally imprinted _H19_, _Airn_ and _Kcnq1ot1_ RNAs in mice6,57,147,148 and others such as _Tug1_ in mice149, _MAENLI_ (ref. 150) and _HELLP_ (named for
‘haemolysis, elevated liver enzyme levels and low platelet count’; also known as _HELLPAR_)151, which are associated with disorders or developmental processes. In _Arabidopsis thaliana_,
non-coding intronic single-nucleotide polymorphisms important for flowering-time adaptation were found to alter the splicing of the lncRNA _COOLAIR_152. Many lncRNAs have been associated
with the cause and progression of cancers, through altered expression of and/or mutations (including translocation breakpoints) in lncRNAs that act as oncogenes or tumour
suppressors153,154,155. Other lncRNAs are involved in human genetic disorders81,156,157, including DiGeorge syndrome and other neurodevelopmental and craniofacial defects158,159,160.
Phenylketonuria, one of the first documented human genetic disorders, caused mostly by mutations in the enzyme phenylalanine hydroxylase, is caused also by mutations in a lncRNA that can be
treated by modified RNA mimics161. A route to analysing lncRNA biological function is to silence or delete, or (less commonly) ectopically express, lncRNAs that have been identified in RNA
sequencing datasets, usually as being differentially expressed. There have been problems with the interpretation of such experiments, however, particularly the difficulty of disentangling
the loss of lncRNA expression from the loss of DNA regulatory elements162,163, which has been addressed by strategies such as inserting polyadenylation sites for early transcription
termination or transcription repression by CRISPR interference (CRISPRi), replacement of the lncRNA with a reporter gene that leaves the promoter intact or deletion of lncRNA exons (although
loss of downstream regulatory elements cannot be ruled out), antisense-mediated blockade of lncRNA splice sites, CRISPR–Cas13 targeting of the lncRNA (rather than its DNA sequence) and
transgene rescue163,164. There are now many studies that have demonstrated the biological roles of lncRNAs163, and high-throughput loss-of-function reverse genetic screens are increasing the
search speed, identifying, for example, lncRNAs that are required for mammalian cell growth and migration, brain, skeletal, lung, muscle and heart development, immune function, epidermal
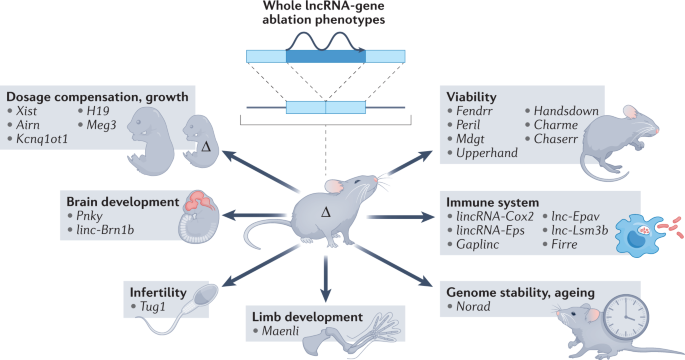
homeostasis and cancer drug responses or lncRNAs that have fitness effects81,165,166,167,168,169,170 (Fig. 1). CRISPRi-mediated transcription repression of more than 16,000 lncRNAs in seven
human cell lines identified almost 500 lncRNAs required for normal cellular proliferation, 89% of which were expressed in only one cell type167. Phenotypic consequences of mutations in
regulatory RNAs, like some protein-coding mutations, may be context dependent and not evident in laboratory conditions, and may be obscured by the robustness of biological systems171. Loss
of _Malat1_, which localizes in nuclear speckles and associates with splicing factors, has no major phenotypes in mice114,172,173,174; however, it does affect cancer progression and synapse
formation, among other physiological and pathophysiological processes175,176. _Neat1_, which is required for the assembly and function of enigmatic, mammal-specific nuclear organelles called
‘paraspeckles’177,178,179, does not appear to be required for normal development in mice but is important for the differentiation of reproduction-related female tissues such as corpus
luteum and mammary gland180. Deletion of brain cytoplasmic RNA 1 (_BC1_), a highly expressed brain lncRNA, is seemingly harmless in mice but results in behavioural changes that would be
lethal in the wild181. So extensive phenotyping is important, especially for cognitive functions. Organoid models may help to identify phenotypes in vitro182,183. Functional annotation of
lncRNAs can also be undertaken by molecular phenotyping184. Analysis of expression patterns, lncRNA–chromatin interactions and other molecular indices following CRISPR–Cas13-mediated
depletion of more than 400 lncRNAs in culture indicated that lncRNAs regulate many genes involved in development, cell cycle and cellular adhesion, among other processes185. BIOLOGICAL
FUNCTIONS OF LNCRNAS Characterized examples have indicated that RNAs participate in virtually all levels of genome organization, cell structure and gene expression, through RNA–RNA, RNA–DNA
and RNA–protein interactions, often involving repeat elements88,186,187, including small interspersed nuclear elements in 3′ untranslated regions188. These interactions are involved in the
regulation of chromatin architecture and transcription (see later), splicing (especially by antisense lncRNAs)189,190,191, protein translation and localization188,192,193, and other forms of
RNA processing, editing, localization and stability194,195. Many lncRNAs are involved in the regulation of cell differentiation and development in animals and plants23,81,116,124,196. They
also have roles in physiological processes such as (in mammals) the p53-mediated response to DNA damage197, V(D)J recombination and class switch recombination in immune cells198, cytokine
expression199, endotoxic shock200, inflammation and neuropathic pain201,202,203, cholesterol biosynthesis and homeostasis204,205, growth hormone and prolactin production206, glucose
metabolism207,208, cellular signal transduction and transport pathways209,210,211,212, synapse function213,214 and learning215, and have roles in the response to various biotic and abiotic
stresses in plants124,125. There is also an emerging association of lncRNAs with the cell membrane216 and with ribozymes217. Presently, a growing number of lncRNAs have their own stories,
and the literature is becoming replete with them. However, several convergent themes are emerging, which explain lncRNA ubiquity and importance in differentiation and development: the
association of lncRNAs with chromatin-modifying proteins; the expression of lncRNAs from developmental ‘enhancers’; and the formation of RNA-nucleated phase-separated coacervates. CONTROL OF
CHROMATIN ARCHITECTURE Epigenetic modifications of chromatin supervise differentiation and development in complex organisms218. DNA methylation is known to be directed by small non-coding
RNAs in plants219, and the RNAi pathway is required for heterochromatin formation and epigenetic gene silencing in fungi and animals220. The mammalian de novo DNA (cytosine
5)-methyltransferase 3A (DNMT3A) and DNMT3B, but not the maintenance DNA methylase DNMT1, bind siRNAs with high affinity221. In turn, DNMT1 (which restores methylation at hemimethylated CpG
dinucleotides following DNA replication) binds lncRNAs to alter DNA methylation patterns at their cognate loci222,223,224, but this is still largely unexplored territory. There are more than
100 different histone modifications that are differentially established by enzymes at a myriad of different positions in plant and animal genomes to control gene expression during
development. The most studied are Polycomb repressive complex 1 (PRC1) and PRC2, which catalyse monoubiquitylation of histone H2A Lys119 (ref. 225) and dimethylation and trimethylation of
histone H3 Lys27 (H3K27), respectively, but in mammals neither complex contains sequence-specific DNA-binding proteins218. Early studies suggested that PRC2 and/or the associated H3K9
methyltransferase G9a are recruited during mouse X chromosome inactivation by _Xist_186, and the control of parental imprinting in mice by _Airn_226 and _Kcnq1ot1_ (ref. 227), although these
associations involve complexities and uncertainties228,229. A subsequent survey of more than 3,300 lncRNAs in human cells showed that ~20% (but only ~2% of mRNAs) interact with PRC2, and
that other lncRNAs are associated with other chromatin-modifying complexes230. Moreover, depletion of a selection of these RNAs caused derepression of genes normally silenced by PRC2 (ref.
230). PRC2 associates with many RNAs228,231,232, more than 9,000 in embryonic stem cells233. There are conflicting reports of whether these associations are nonspecific
(‘promiscuous’)228,234 or specific high-affinity interactions with different RNAs232,235, although these alternatives are not mutually exclusive229. Some recent studies have shown that RNA
is required for PRC2 chromatin occupancy, PRC2 function and cell state definition236, and that the interaction of PRC2 with RNA can regulate transcription elongation232. PRC1 function also
appears to be controlled by RNA237,238. However, deconvoluting RNA–protein interactions is complicated by the low affinity of many antibodies used in pulldown assays and the fact that PRC2,
for example, has at least two subunits that bind RNA228. The recent development of denaturing crosslinked immunoprecipitation (dCLIP), which is based on high-affinity biotin–streptavidin
pulldowns, has indicated that PRC2 interacts with G-rich RNA motifs, including RNA G-quadruplexes, to achieve specificity of RNA-mediated recruitment232,239,240. Other lncRNAs associate with
the gene-activating Trithorax complexes (which methylate H3K4), including enhancer RNAs involved in the maintenance of stem cell fates and lineage specification241,242,243,244,245. H3K9
dimethylation is regulated by lncRNAs during the formation of long-term memory in mice246. lncRNAs also control methylation of a number of non-histone proteins involved in animal cell
signalling, gene expression and RNA processing247. Many other proteins involved in modulating chromatin architecture, including HOX proteins, pioneer transcription factors such as NANOG,
OCT4 (also known asPOU5F1), SOX2 and other high mobility group (HMG) proteins, and proteins of SWI/SNF chromatin remodelling complexes, have only vague or promiscuous DNA sequence
specificity248,249,250,251, which indicates that other factors are involved in determining their targets at different stages of cell differentiation and development. Moreover, binding-site
selection by the zinc-finger transcription factor CTCF, which, together with cohesin complexes, anchors chromosome loops252, was shown to be controlled by the lncRNA just proximal to _Xist_
(_Jpx_) during early cell differentiation, thereby regulating chromatin topology on a genome-wide scale253. CTCF binds thousands of RNAs, including _Xist_, _Jpx_ and the lncRNA _Xist_
antisense RNA (_Tsix_), which targets CTCF to the X inactivation centre254. There is abundant evidence that RNA may guide chromatin remodelling complexes, although accessibility dictated by
DNA and histone modifications (which are also likely directed by regulatory RNAs) may also have a role. The _D. melanogaster_ Hox protein Bicoid (which controls anterior–posterior
patterning) binds RNA through its homeodomain255. SOX2 binds RNA with high affinity through its HMG domain256,257, as do other members of the HMGB family257,258,259. During mouse
embryogenesis, the _Sox2_ locus expresses also an overlapping lncRNA260, and there are well-documented examples of lncRNAs that interact with SOX2 to regulate pluripotency, neurogenesis,
neuronal differentiation and brain development257,261,262,263,264. SWI/SNF nucleosome remodelling complexes are directed to specific sites in chromatin or are antagonized by lncRNAs,
including _XIST_ and enhancer RNAs, in a wide range of differentiation processes and cancers251,265,266,267,268,269,270. The lncRNA _MaTAR25_, which is overexpressed in mammary cancers, acts
in _trans_ to regulate the tensin 1 gene through interaction with the transcription co-activator PURB271. The master transcription factor myoblast determination protein (MYOD), which can
reprogramme mammalian fibroblasts into muscle cells and is central to muscle differentiation in vivo, is regulated by lncRNAs272,273,274, as are other aspects of muscle gene expression275.
The pioneer transcription factor CBP also binds RNAs, including those transcribed from enhancers, to stimulate histone acetylation and consequently transcription276. Some transcription
factors (OCT4, NANOG, SOX2 and SOX9) are also regulated by lncRNAs, including pseudogene-derived lncRNAs277,278,279,280,281, and reciprocally regulate the expression of lncRNAs282.
Enhancer-derived lncRNAs also regulate the expression of the nuclear hormone receptor ESR1 (ref. 283) and of CCAAT/enhancer-binding protein-α (CEBPA)284. ENHANCER ACTION Enhancers are
non-coding genomic loci that control the spatiotemporal expression of other genes during development. There appear to be ~400,000 (±100,000) enhancers in the mammalian genome285,286,287,288,
sometimes clustered into ‘super-enhancers’ or ‘enhancer jungles’288,289,290,291. Enhancers are thought to function by juxtaposing transcription factors bound at the enhancer promoters with
the promoters of target genes292,293. There is no question that enhancer action alters chromatin topology and may be responsible for the formation of chromatin-loop domains that act as local
transcription and splicing hubs294,295. Enhancers are transcribed in the cells in which they are active141,289,296,297,298,299, which has led to uncertainty about whether the resulting RNAs
are by-products of the binding of transcription factors or have a role in enhancer activity298. The latter appears to be the case. The epigenetic landscape of and the features of
transcription initiation at the promoters of protein-coding genes and enhancers are almost indistinguishable296,297,298,299,300. Enhancers express bidirectional promoter-associated short
RNAs301,302,303, termed ‘eRNAs’, although such short RNAs are not specific to enhancers, as similar bidirectional transcripts are produced from the promoters of protein-coding genes304,305.
Also analogously to mRNAs produced from protein-coding genes, enhancers express long (non-coding) RNAs (confusingly also referred to as ‘eRNAs’298,306), and transcription is considered the
best molecular indicator of enhancer activity in developmental processes296,297,306,307,308 and cancers288. Moreover, enhancer-lncRNA splicing has been shown to modulate enhancer
activity309,310. Although the extent of congruency of combined genetic and high-depth transcriptomic data is uncertain, as their availability is still limited, the data suggest that many if
not most lncRNAs are derived from enhancers141,298 and that lncRNAs are required for enhancer activity163,284,311,312,313,314, examples including the lncRNAs _Evf2_ (also known as
_Dlx6os1_)315, _Firre_316, _Peril_317, _Upperhand_ (also known as _Hand2os1_)318 and _Maenli_150 in mice. Enhancer RNA function is fertile ground for investigation, but if enhancer loci are
considered bona fide ‘genes’, the g-value paradox (the perceived lack of increase in gene number with developmental complexity) is resolved. It also means that a key development in the
evolution of complex organisms was the use of RNA to organize developmental trajectories319. It appears that “every cell type expresses precise lncRNA signatures to control lineage-specific
regulatory programs”270, and that cell state during ontogeny is likely directed by lncRNAs. FORMATION OF BIOMOLECULAR CONDENSATES The past decade has seen the growing appreciation of the
role of biomolecular condensates, or phase-separated domains (PSDs), in the organization of cells and chromatin. These condensates are highly dynamic assemblies with high local
concentrations of macromolecules, a feature that promotes functional interactions. The condensates usually contain both RNA and proteins320,321,322, the latter having IDRs, which are the
major sites of post-translational modifications323. IDRs interact with and are tunable by many partners324. The fraction of the proteome containing IDRs has expanded with cellular and
developmental complexity323, and nearly all proteins involved in the regulation of development, including most transcription factors, histones, histone-modifying proteins, other
chromatin-binding proteins, RNA-binding proteins, splicing factors, nuclear hormone receptors, cytoskeletal proteins and membrane receptors, contain IDRs323,325,326,327,328,329,330,331,332.
RNA is crucial for the form, composition and function of phase-separated RNA–protein condensates320,321,322. Specific ‘architectural’ lncRNAs333 associate with nuclear condensates of
different half-lives and functionalities, including in centrosomes334, nucleoli335 (the lncRNAs _SLERT_138 and _LETN_336), nuclear speckles (the lncRNA _MALAT1_ (refs. 173,337)) rich in
RNA-processing factors, speckle-related condensates that contain the lncRNA _Gomafu_ in mice338,339 and paraspeckles (the lncRNA _NEAT1_ (refs. 340,341)) (Fig. 2), in vertebrates as well as
polyadenylation complexes342 and other condensates in plants343. RNP condensates also include cytoplasmic membraneless organelles such as P-granules344,345, subcellular-localized
translational messenger RNP assemblies346 and synaptic compartments320,322,347. The mammalian cytoplasmic lncRNA _NORAD_, which is induced by DNA damage and required for genome stability,
prevents aberrant mitosis by sequestering Pumilio proteins (which bind many RNAs to regulate stem cell fate, development and neurological functions) into PSDs through its repeat
sequences137,348. It has been proposed that RNAs have a central role in organizing the genome and gene expression by the formation of spatial compartments and transcriptional
condensates349,350,351,352,353. Phase separation appears to drive chromatin long-range interactions and to be required for the action of enhancers and super-enhancers328,351,354,355,356,357
as well as for transcription, transcription factors and polyadenylation complexes342,358,359,360,361, although transcription factor hubs have been reported to operate in the absence of
detectable phase separation362. PSDs scaffolded by lncRNAs, including repeat-rich RNAs363,364, mediate the formation of heterochromatin353,365,366, euchromatin367, Polycomb bodies368 and
alternative splicing369. lncRNAs are a substantial component of rapidly renaturing, repeat-rich RNA (technically termed ‘CoT-1 RNA’), and high-resolution imaging shows many repeat-containing
RNAs bound to chromatin, indicating that the collective presence of thousands of lncRNAs serves to counter chromatin condensation364. High-resolution imaging also shows the localization of
many lncRNAs in compartments in the nucleus that resemble PSDs136,353. These data all suggest that there are thousands of low copy number lncRNAs involved in the organization of chromosome
territories. LNCRNA STRUCTURE–FUNCTION RELATIONSHIPS lncRNAs generally range in size from around 1 kb to longer than 100 kb (refs. 370,371) and have a modular structure372,373,374,375. They
are often multi-exonic and highly alternatively spliced (Fig. 3a), a feature that was not obvious before the advent of high-depth sequencing98. They also contain a higher proportion of GC–AG
splice sites376 and are therefore less efficiently spliced than protein-coding transcripts377,378, which are properties associated with alternative splicing379. Alternative splicing has,
unsurprisingly, been shown to alter the function of lncRNAs42,152,380,381. Some lncRNAs also exhibit common motifs and motif combinations101. At least 18% of the human genome is conserved
among mammals at the level of predicted RNA structure382, and similar and potentially paralogous RNA structures occur at many places throughout the genome383,384. Chemical probing has shown
that lncRNAs, including _Xist_, form complex multidomain structures108,385,386,387,388,389, with chemical data matching data predicted by evolutionary conservation of secondary structure389.
Moreover, lncRNAs with similar _k_-base oligonucleotide (short motif) content have related functions despite their lack of general homology, implying that small sequence elements are also
key determinants of lncRNA function390. Many lncRNA exons are derived from transposable elements187,391. The most highly conserved sequences in _Xist_, which has been intensively studied,
are its repeats7, whereas its unique sequences have evolved rapidly392, and many of its biological functions, including recruitment of gene-repressive complexes and gene silencing, are
mediated through its modular repeat elements142,186,388,393,394,395,396,397,398,399. Transposable element-derived sequences participate in many RNA–protein interactions369,400,401, which
leads to the conclusion that repeat structures are common building blocks of lncRNAs87,391,396 and essential components of their function391. The molecular mechanisms of lncRNA action are
unclear. In most well-characterized cases of RNA regulation, such as RNAi, snoRNAs, CRISPR and telomerase, RNA acts as a guide to target effector protein complexes to complementary RNA or
DNA sequences. Data on selected lncRNAs (for example, _HOTAIR_, _roX1_, _roX2_, _Meg3_, _Tug1_, _PARTICLE_ (also known as _PARTCL_), _PAPAS_ and _KHPS1_) indicates that they form triplex
structures with DNA at purine-rich GA stretches to recruit chromatin modifiers to specific loci across the genome402,403,404,405,406,407,408, with evidence that triplex formation by lncRNAs
is a widespread phenomenon409,410,411. Others, especially antisense lncRNAs, appear to function through RNA–DNA hybrid formation61,412,413, but detail is presently lacking. lncRNA RNP
structure and function have been well characterized in only one instance, the telomerase complex, which has been studied for decades. Telomerase reverse transcriptase (TERT) catalyses the
addition of telomere repeats to chromosome ends, and other proteins in the complex provide nuclear localization, stability or recruitment to telomeres or to Cajal bodies. The lncRNA _TERC_
provides the scaffold for assembly of the RNP and the template for DNA polymerization by TERT, and mutations in TERT and _TERC_ are major contributors to the aetiology of cancer and the
cause of hereditary disorders such as dyskeratosis congenita103,104,105,106,107,414,415,416. By contrast, while we know the phenotypes caused by the loss of some lncRNAs, we know almost
nothing about how most of them work, although, considering that as recently as 2010 the very existence of pervasive transcription was still a matter of contention417,418,419 and the sheer
number of lncRNAs, substantial progress has been made. It is assumed, in our view reasonably, that generally lncRNAs will engage in multilateral interactions similarly to _TERC_ and the
telomerase complex108, and there is some evidence to support this assumption in cases such as _XIST_ (Fig. 3b), but the assumption has not yet been rigorously tested. There are promising
discoveries, such as the demonstration that conserved pseudoknots in lncRNA _Meg3_ are essential for stimulation of the p53 pathway420. There is also growing evidence of discrete structural
organization in lncRNAs421. Nonetheless, there is a long journey ahead to understand the structure and function of the many thousands of lncRNAs, and their splice variants, in the context of
their associated RNP complexes and biomolecular condensates in both the nucleus and the cytoplasm. CHALLENGES If the complex ontogenies of animals and, to a lesser extent plants, require a
large number of RNAs to guide the epigenetic decisions at each cell division, then it is not surprising that many lncRNAs have common protein-binding modules and specific targeting sequences
that vary between different stages of development. The challenge is to define which lncRNAs and modules within them interact with effector proteins and which convey target (DNA or RNA)
specificity. The former is complicated by the multisubunit nature of many RNP complexes, but is being addressed by technologies such as iCLIP422, RAP–MS423, ChIRP-MS388 and iDRiP424.
Determining target specificity is even more difficult, as specific targeting requires only short stretches of nucleotide complementarity given the strength of RNA–RNA and RNA–DNA
interactions425, but it may be tackled by new methods that analyse RNA–chromatin and RNA–RNA interactions, such as GRID-seq426, RADICL-seq427, RIC-seq428 and RD-SPRITE353. Other lncRNAs are
localized in cytoplasmic compartments, whose components also need to be characterized. Understanding the roles of lncRNAs and how they function in dynamic assemblies with other
macromolecules will provide a more comprehensive understanding of cell and developmental biology and of gene–environment interactions. Emerging challenges include understanding the roles of
lncRNAs and RNA modifications in functional plasticity, especially in the brain, and the dysregulation of these lncRNA-mediated pathways in neurological disorders, cancer and other diseases.
RECOMMENDATIONS * 1. In the absence of more specific categorization, we recommend retention of the general descriptor ‘lncRNA’ for non-coding RNAs greater than 500 nt in length. * 2. Unless
a lncRNA is antisense to a protein-coding gene (in which case the designation ‘_gene name_-AS’ should be used), we recommend naming lncRNAs for their own sake with allusion to a discerned
characteristic or function (as has been traditional for proteins), preferably accompanied by complete exon–intron structures and genomic coordinates. If no biological context is available,
we recommend naming the lncRNA according to the GENCODE system46. * 3. We recommend that future gene expression profiling should include full transcript analysis of the isoforms and
stoichiometry of mRNAs, lncRNAs and small RNAs in cells at different stages of differentiation, and in various physiological and disease states, learning and stress conditions. * 4. These
efforts should be complemented by cell-based, organoid-based and in vivo studies using strategies for conditional and tissue-specific or cell type-specific gain-of-function and
loss-of-function of lncRNAs. More broadly, identifying and understanding the roles of lncRNAs and RNA regulatory networks in multicellular development, cell biology and disease will require
the following: * 1. The determination of the interplay between lncRNAs, chromatin modifications, proteins and the genome in the assembly of the nuclear domains essential for chromatin
organization, enhancer function, transcription and splicing. This effort will require the development of antibodies with high specificity for protein–RNA complexes, and of intracellular
RNA-tracking methods429. * 2. The determination of lncRNA localization, structure–function relationships and interactions using a range of sequencing, chemical probing, imaging
methods430,431,432,433 and cryogenic electron microscopy434. * 3. The identification and characterization of the many unknown nuclear and cytoplasmic compartments decorated by specific
lncRNAs. * 4. Harnessing the power of machine learning to interrogate large genomic, epigenomic, transcriptomic, proteomic and phenomic datasets to identify causal links and pathways.
REFERENCES * Ender, C. & Meister, G. Argonaute proteins at a glance. _J. Cell Sci._ 123, 1819–1823 (2010). Article CAS PubMed Google Scholar * Wassarman, K. M., Zhang, A. &
Storz, G. Small RNAs in _Escherichia coli_. _Trends Microbiol._ 7, 37–45 (1999). Article CAS PubMed Google Scholar * Watanabe, Y. & Yamamoto, M. _S. pombe mei2+_ encodes an
RNA-binding protein essential for premeiotic DNA synthesis and meiosis I, which cooperates with a novel RNA species meiRNA. _Cell_ 78, 487–498 (1994). Article CAS PubMed Google Scholar *
Lakhotia, S. C. & Sharma, A. The 93D (hsr-omega) locus of _Drosophila_: non-coding gene with house-keeping functions. _Genetica_ 97, 339–348 (1996). Article CAS PubMed Google Scholar
* Kelley, R. L. et al. Epigenetic spreading of the _Drosophila_ dosage compensation complex from _roX_ RNA genes into flanking chromatin. _Cell_ 98, 513–522 (1999). Article CAS PubMed
Google Scholar * Bartolomei, M. S., Zemel, S. & Tilghman, S. M. Parental imprinting of the mouse H19 gene. _Nature_ 351, 153–155 (1991). Article CAS PubMed Google Scholar * Brown,
C. J. et al. The human _XIST_ gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. _Cell_ 71, 527–542 (1992). Article
CAS PubMed Google Scholar * Rodriguez, A., Griffiths-Jones, S., Ashurst, J. L. & Bradley, A. Identification of mammalian microRNA host genes and transcription units. _Genome Res._
14, 1902–1910 (2004). Article CAS PubMed PubMed Central Google Scholar * He, D. et al. miRNA-independent function of long noncoding pri-miRNA loci. _Proc. Natl Acad. Sci. USA_ 118,
e2017562118 (2021). Article CAS PubMed PubMed Central Google Scholar * Askarian-Amiri, M. E. et al. SNORD-host RNA Zfas1 is a regulator of mammary development and a potential marker for
breast cancer. _RNA_ 17, 878–891 (2011). Article CAS PubMed PubMed Central Google Scholar * Lambert, M., Benmoussa, A. & Provost, P. Small non-coding RNAs derived from eukaryotic
ribosomal RNA. _Noncoding RNA_ 5, 16 (2019). CAS PubMed PubMed Central Google Scholar * Kawaji, H. et al. Hidden layers of human small RNAs. _BMC Genomics_ 9, 157 (2008). Article PubMed
PubMed Central Google Scholar * Krishna, S. et al. Dynamic expression of tRNA-derived small RNAs define cellular states. _EMBO Rep._ 20, e47789 (2019). Article PubMed PubMed Central
Google Scholar * Taft, R. J. et al. Small RNAs derived from snoRNAs. _RNA_ 15, 1233–1240 (2009). Article CAS PubMed PubMed Central Google Scholar * Chen, Q. et al. Sperm tsRNAs
contribute to intergenerational inheritance of an acquired metabolic disorder. _Science_ 351, 397–400 (2016). Article CAS PubMed Google Scholar * Kapranov, P. et al. Large-scale
transcriptional activity in chromosomes 21 and 22. _Science_ 296, 916–919 (2002). Article CAS PubMed Google Scholar * Okazaki, Y. et al. Analysis of the mouse transcriptome based on
functional annotation of 60,770 full-length cDNAs. _Nature_ 420, 563–573 (2002). Article PubMed Google Scholar * Carninci, P. et al. The transcriptional landscape of the mammalian genome.
_Science_ 309, 1559–1563 (2005). Article CAS PubMed Google Scholar * Cheng, J. et al. Transcriptional maps of 10 human chromosomes at 5-nucleotide resolution. _Science_ 308, 1149–1154
(2005). Article CAS PubMed Google Scholar * Sender, R., Fuchs, S. & Milo, R. Revised estimates for the number of human and bacteria cells in the body. _PLoS Biol._ 14, e1002533
(2016). Article PubMed PubMed Central Google Scholar * Hahn, M. W. & Wray, G. A. The g-value paradox. _Evol. Dev._ 4, 73–75 (2002). Article PubMed Google Scholar * Liu, G.,
Mattick, J. S. & Taft, R. J. A meta-analysis of the genomic and transcriptomic composition of complex life. _Cell Cycle_ 12, 2061–2072 (2013). Article CAS PubMed PubMed Central
Google Scholar * Mercer, T. R., Dinger, M. E. & Mattick, J. S. Long noncoding RNAs: insights into function. _Nat. Rev. Genet._ 10, 155–159 (2009). Article CAS PubMed Google Scholar
* Parrott, A. M. et al. The evolution and expression of the snaR family of small non-coding RNAs. _Nucleic Acids Res._ 39, 1485–1500 (2011). Article CAS PubMed Google Scholar * Täuber,
H., Hüttelmaier, S. & Köhn, M. POLIII-derived non-coding RNAs acting as scaffolds and decoys. _J. Mol. Cell Biol._ 11, 880–885 (2019). Article PubMed PubMed Central Google Scholar *
Hahne, J. C., Lampis, A. & Valeri, N. Vault RNAs: hidden gems in RNA and protein regulation. _Cell. Mol. Life Sci._ 78, 1487–1499 (2021). Article CAS PubMed Google Scholar *
Kapranov, P. et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. _Science_ 316, 1484–1488 (2007). Article CAS PubMed Google Scholar * Fejes-Toth,
K. et al. Post-transcriptional processing generates a diversity of 5′-modified long and short RNAs. _Nature_ 457, 1028–1032 (2009). Article CAS PubMed Central Google Scholar * Preker, P.
et al. PROMoter uPstream Transcripts share characteristics with mRNAs and are produced upstream of all three major types of mammalian promoters. _Nucleic Acids Res._ 39, 7179–7193 (2011).
Article CAS PubMed PubMed Central Google Scholar * Castelo-Branco, G. et al. The non-coding snRNA 7SK controls transcriptional termination, poising, and bidirectionality in embryonic
stem cells. _Genome Biol._ 14, R98 (2013). Article PubMed PubMed Central Google Scholar * Flynn, R. A. et al. 7SK-BAF axis controls pervasive transcription at enhancers. _Nat. Struct.
Mol. Biol._ 23, 231–238 (2016). Article CAS PubMed PubMed Central Google Scholar * Gussakovsky, D. & McKenna, S. A. Alu RNA and their roles in human disease states. _RNA Biol._ 18,
574–585 (2021). Article CAS PubMed PubMed Central Google Scholar * Ullu, E. & Tschudi, C. Alu sequences are processed 7SL RNA genes. _Nature_ 312, 171–172 (1984). Article CAS
PubMed Google Scholar * Tsirigos, A. & Rigoutsos, I. Alu and B1 repeats have been selectively retained in the upstream and intronic regions of genes of specific functional classes.
_PLoS Comput. Biol._ 5, e1000610 (2009). Article PubMed PubMed Central Google Scholar * Zhang, X.-O., Gingeras, T. R. & Weng, Z. Genome-wide analysis of polymerase III–transcribed
Alu elements suggests cell-type–specific enhancer function. _Genome Res._ 29, 1402–1414 (2019). Article CAS PubMed PubMed Central Google Scholar * Deng, W. et al. Organization of the
_Caenorhabditis elegans_ small non-coding transcriptome: Genomic features, biogenesis, and expression. _Genome Res._ 16, 20–29 (2006). Article CAS PubMed PubMed Central Google Scholar *
Dieci, G., Conti, A., Pagano, A. & Carnevali, D. Identification of RNA polymerase III-transcribed genes in eukaryotic genomes. _Biochim. Biophys. Acta_ 1829, 296–305 (2013). Article
CAS PubMed Google Scholar * Jawdekar, G. W. & Henry, R. W. Transcriptional regulation of human small nuclear RNA genes. _Biochim. Biophys. Acta_ 1779, 295–305 (2008). Article CAS
PubMed PubMed Central Google Scholar * Kufel, J. & Grzechnik, P. Small nucleolar RNAs tell a different tale. _Trends Genet._ 35, 104–117 (2019). Article CAS PubMed Google Scholar
* Wilusz, J. E., Freier, S. M. & Spector, D. L. 3’ end processing of a long nuclear-retained noncoding RNA yields a tRNA-like cytoplasmic RNA. _Cell_ 135, 919–932 (2008). Article CAS
PubMed PubMed Central Google Scholar * Yin, Q.-F. et al. Long noncoding RNAs with snoRNA ends. _Mol. Cells_ 48, 219–230 (2012). Article CAS Google Scholar * Wu, H. et al. Unusual
processing generates SPA lncRNAs that sequester multiple RNA binding proteins. _Mol. Cells_ 64, 534–548 (2016). Article CAS Google Scholar * Gingeras, T. R. Origin of phenotypes: genes
and transcripts. _Genome Res._ 17, 682–690 (2007). Article CAS PubMed Google Scholar * Cheetham, S. W., Faulkner, G. J. & Dinger, M. E. Overcoming challenges and dogmas to understand
the functions of pseudogenes. _Nat. Rev. Genet._ 21, 191–201 (2020). Article CAS PubMed Google Scholar * Frith, M. C. et al. Pseudo–messenger RNA: phantoms of the transcriptome. _PLoS
Genet._ 2, e23 (2006). Article PubMed PubMed Central Google Scholar * Frankish, A. et al. GENCODE 2021. _Nucleic Acids Res._ 49, D916–D923 (2021). Article CAS PubMed Google Scholar *
Ma, Y. et al. Genome-wide analysis of pseudogenes reveals HBBP1’s human-specific essentiality in erythropoiesis and implication in β-thalassemia. _Dev. Cell_ 56, 478–493 (2021). Article
CAS PubMed Google Scholar * Patop, I. L., Wüst, S. & Kadener, S. Past, present, and future of circRNAs. _EMBO J._ 38, e100836 (2019). Article PubMed PubMed Central Google Scholar
* Mercer, T. R. et al. Expression of distinct RNAs from 3’ untranslated regions. _Nucleic Acids Res._ 39, 2393–2403 (2011). Article CAS PubMed Google Scholar * Wright, M. W. A short
guide to long non-coding RNA gene nomenclature. _Hum. Genomics_ 8, 7 (2014). Article PubMed PubMed Central Google Scholar * Mattick, J. S. & Rinn, J. L. Discovery and annotation of
long noncoding RNAs. _Nat. Struct. Mol. Biol._ 22, 5–7 (2015). Article CAS PubMed Google Scholar * Uszczynska-Ratajczak, B., Lagarde, J., Frankish, A., Guigó, R. & Johnson, R.
Towards a complete map of the human long non-coding RNA transcriptome. _Nat. Rev. Genet._ 19, 535–548 (2018). Article CAS PubMed PubMed Central Google Scholar * Seal, R. L. et al. A
guide to naming human non-coding RNA genes. _EMBO J._ 39, e103777 (2020). Article CAS PubMed PubMed Central Google Scholar * Mattick, J. S. Challenging the dogma: the hidden layer of
non-protein-coding RNAs in complex organisms. _Bioessays_ 25, 930–939 (2003). Article CAS PubMed Google Scholar * Kapranov, P., Willingham, A. T. & Gingeras, T. R. Genome-wide
transcription and the implications for genomic organization. _Nat. Rev. Genet._ 8, 413–423 (2007). Article CAS PubMed Google Scholar * Willingham, A. T. et al. Transcriptional landscape
of the human and fly genomes: nonlinear and multifunctional modular model of transcriptomes. _Cold Spring Harb. Symp. Quant. Biol._ 71, 101–110 (2006). Article CAS PubMed Google Scholar
* Lyle, R. et al. The imprinted antisense RNA at the _Igf2r_ locus overlaps but does not imprint _Mas1_. _Nat. Genet._ 25, 19–21 (2000). Article CAS PubMed Google Scholar * Rinn, J. L.
et al. Functional demarcation of active and silent chromatin domains in human _HOX_ loci by noncoding RNAs. _Cell_ 129, 1311–1323 (2007). Article CAS PubMed PubMed Central Google Scholar
* Sone, M. et al. The mRNA-like noncoding RNA Gomafu constitutes a novel nuclear domain in a subset of neurons. _J. Cell Sci._ 120, 2498–2506 (2007). Article CAS PubMed Google Scholar
* Ietswaart, R., Wu, Z. & Dean, C. Flowering time control: another window to the connection between antisense RNA and chromatin. _Trends Genet._ 28, 445–453 (2012). Article CAS PubMed
Google Scholar * Ariel, F. et al. R-loop mediated _trans_ action of the _APOLO_ long noncoding RNA. _Mol. Cells_ 77, 1055–1065 (2020). Article CAS Google Scholar * Kopp, F. &
Mendell, J. T. Functional classification and experimental dissection of long noncoding RNAs. _Cell_ 172, 393–407 (2018). Article CAS PubMed PubMed Central Google Scholar * Dinger, M.
E., Gascoigne, D. K. & Mattick, J. S. The evolution of RNAs with multiple functions. _Biochimie_ 93, 2013–2018 (2011). Article CAS PubMed Google Scholar * Wu, P. et al. Emerging role
of tumor-related functional peptides encoded by lncRNA and circRNA. _Mol. Cancer_ 19, 22 (2020). Article CAS PubMed PubMed Central Google Scholar * Wright, B. W., Yi, Z., Weissman, J.
S. & Chen, J. The dark proteome: translation from noncanonical open reading frames. _Trends Cell Biol._ 32, 243–258 (2022). Article CAS PubMed Google Scholar * Makarewich, C. A.
& Olson, E. N. Mining for micropeptides. _Trends Cell Biol._ 27, 685–696 (2017). Article CAS PubMed PubMed Central Google Scholar * Hube, F. et al. Alternative splicing of the first
intron of the steroid receptor RNA activator (SRA) participates in the generation of coding and noncoding RNA isoforms in breast cancer cell lines. _DNA Cell Biol._ 25, 418–428 (2006).
Article CAS PubMed Google Scholar * Williamson, L. et al. UV irradiation induces a non-coding RNA that functionally opposes the protein encoded by the same gene. _Cell_ 168, 843–855
(2017). Article CAS PubMed PubMed Central Google Scholar * Grelet, S. et al. A regulated PNUTS mRNA to lncRNA splice switch mediates EMT and tumour progression. _Nat. Cell Biol._ 19,
1105–1115 (2017). Article CAS PubMed PubMed Central Google Scholar * Gonzàlez-Porta, M., Frankish, A., Rung, J., Harrow, J. & Brazma, A. Transcriptome analysis of human tissues and
cell lines reveals one dominant transcript per gene. _Genome Biol._ 14, R70 (2013). Article PubMed PubMed Central Google Scholar * Tuck, A. C. & Tollervey, D. RNA in pieces. _Trends
Genet._ 27, 422–432 (2011). Article CAS PubMed Google Scholar * Chan, S. N. & Pek, J. W. Stable intronic sequence RNAs (sisRNAs): an expanding universe. _Trends Biochem. Sci._ 44,
258–272 (2019). Article CAS PubMed Google Scholar * Fang, S. et al. NONCODEV5: a comprehensive annotation database for long non-coding RNAs. _Nucleic Acids Res._ 46, D308–D314 (2017).
Article PubMed Central Google Scholar * Derrien, T. et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. _Genome Res._
22, 1775–1789 (2012). Article CAS PubMed PubMed Central Google Scholar * Mas-Ponte, D. et al. LncATLAS database for subcellular localization of long noncoding RNAs. _RNA_ 23, 1080–1087
(2017). Article CAS PubMed PubMed Central Google Scholar * Ma, L. et al. LncBook: a curated knowledgebase of human long non-coding RNAs. _Nucleic Acids Res._ 47, D128–D134 (2018).
Article PubMed Central Google Scholar * Volders, P.-J. et al. LNCipedia 5: towards a reference set of human long non-coding RNAs. _Nucleic Acids Res._ 47, D135–D139 (2019). Article CAS
PubMed Google Scholar * Seifuddin, F. et al. lncRNAKB, a knowledgebase of tissue-specific functional annotation and trait association of long noncoding RNA. _Sci. Data_ 7, 326 (2020).
Article CAS PubMed PubMed Central Google Scholar * Jin, J. et al. PLncDB V2.0: a comprehensive encyclopedia of plant long noncoding RNAs. _Nucleic Acids Res._ 49, D1489–D1495 (2020).
Article PubMed Central Google Scholar * RNAcentral Consortium. RNAcentral 2021: secondary structure integration, improved sequence search and new member databases. _Nucleic Acids Res._
49, D212–D220 (2021). Article Google Scholar * Statello, L., Guo, C.-J., Chen, L.-L. & Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. _Nat. Rev. Mol.
Cell Biol._ 22, 96–118 (2021). Article CAS PubMed Google Scholar * St Laurent, G. et al. Intronic RNAs constitute the major fraction of the non-coding RNA in mammalian cells. _BMC
Genomics_ 13, 504 (2012). Article Google Scholar * Gardner, E. J., Nizami, Z. F., Talbot, C. C. & Gall, J. G. Stable intronic sequence RNA (sisRNA), a new class of noncoding RNA from
the oocyte nucleus of _Xenopus tropicalis_. _Genes Dev._ 26, 2550–2559 (2012). Article CAS PubMed PubMed Central Google Scholar * Zhang, Y. et al. Circular intronic long noncoding RNAs.
_Mol. Cells_ 51, 792–806 (2013). Article CAS Google Scholar * Pheasant, M. & Mattick, J. S. Raising the estimate of functional human sequences. _Genome Res._ 17, 1245–1253 (2007).
Article CAS PubMed Google Scholar * Faulkner, G. J. et al. The regulated retrotransposon transcriptome of mammalian cells. _Nat. Genet._ 41, 563–571 (2009). Article CAS PubMed Google
Scholar * Kapusta, A. et al. Transposable elements are major contributors to the origin, diversification, and regulation of vertebrate long noncoding RNAs. _PLoS Genet._ 9, e1003470 (2013).
Article CAS PubMed PubMed Central Google Scholar * Kelley, D. & Rinn, J. Transposable elements reveal a stem cell-specific class of long noncoding RNAs. _Genome Biol._ 13, R107
(2012). Article PubMed PubMed Central Google Scholar * Fueyo, R., Judd, J., Feschotte, C. & Wysocka, J. Roles of transposable elements in the regulation of mammalian transcription.
_Nat. Rev. Mol. Cell Biol._ 23, 481–497 (2022). Article CAS PubMed Google Scholar * Pang, K. C., Frith, M. C. & Mattick, J. S. Rapid evolution of noncoding RNAs: lack of conservation
does not mean lack of function. _Trends Genet._ 22, 1–5 (2006). Article CAS PubMed Google Scholar * Kutter, C. et al. Rapid turnover of long noncoding RNAs and the evolution of gene
expression. _PLoS Genet._ 8, e1002841 (2012). Article CAS PubMed PubMed Central Google Scholar * Quinn, J. J. et al. Rapid evolutionary turnover underlies conserved lncRNA-genome
interactions. _Genes Dev._ 30, 191–207 (2016). Article PubMed PubMed Central Google Scholar * Ponjavic, J., Ponting, C. P. & Lunter, G. Functionality or transcriptional noise?
Evidence for selection within long noncoding RNAs. _Genome Res._ 17, 556–565 (2007). Article CAS PubMed PubMed Central Google Scholar * Guttman, M. et al. Chromatin signature reveals
over a thousand highly conserved large non-coding RNAs in mammals. _Nature_ 458, 223–227 (2009). Article CAS PubMed PubMed Central Google Scholar * Mattick, J. S. The genetic signatures
of noncoding RNAs. _PLoS Genet._ 5, e1000459 (2009). Article PubMed PubMed Central Google Scholar * Cawley, S. et al. Unbiased mapping of transcription factor binding sites along human
chromosomes 21 and 22 points to widespread regulation of noncoding RNAs. _Cell_ 116, 499–509 (2004). Article CAS PubMed Google Scholar * Nitsche, A., Rose, D., Fasold, M., Reiche, K.
& Stadler, P. F. Comparison of splice sites reveals that long noncoding RNAs are evolutionarily well conserved. _RNA_ 21, 801–812 (2015). Article CAS PubMed PubMed Central Google
Scholar * Deveson, I. W. et al. Universal alternative splicing of noncoding exons. _Cell Syst._ 6, 245–255 (2018). Article CAS PubMed Google Scholar * Clark, M. et al. Genome-wide
analysis of long noncoding RNA stability. _Genome Res._ 21, 885–898 (2012). Article Google Scholar * Ulitsky, I., Shkumatava, A., Jan, C. H., Sive, H. & Bartel, D. P. Conserved
function of lincRNAs in vertebrate embryonic development despite rapid sequence evolution. _Cell_ 147, 1537–1550 (2011). Article CAS PubMed PubMed Central Google Scholar * Ross, C. J.
et al. Uncovering deeply conserved motif combinations in rapidly evolving noncoding sequences. _Genome Biol._ 22, 29 (2021). Article CAS PubMed PubMed Central Google Scholar * Degani,
N., Lubelsky, Y., Perry, R. B.-T., Ainbinder, E. & Ulitsky, I. Highly conserved and cis-acting lncRNAs produced from paralogous regions in the center of _HOXA_ and _HOXB_ clusters in the
endoderm lineage. _PLoS Genet._ 17, e1009681 (2021). Article CAS PubMed PubMed Central Google Scholar * Chen, J.-L., Blasco, M. A. & Greider, C. W. Secondary structure of
vertebrate telomerase RNA. _Cell_ 100, 503–514 (2000). Article CAS PubMed Google Scholar * Zhang, Q., Kim, N.-K. & Feigon, J. Architecture of human telomerase RNA. _Proc. Natl Acad.
Sci. USA_ 108, 20325–20332 (2011). Article CAS PubMed PubMed Central Google Scholar * Wang, Y., Yesselman, J. D., Zhang, Q., Kang, M. & Feigon, J. Structural conservation in the
template/pseudoknot domain of vertebrate telomerase RNA from teleost fish to human. _Proc. Natl Acad. Sci. USA_ 113, E5125–E5134 (2016). Article CAS PubMed PubMed Central Google Scholar
* Nguyen, T. H. D. et al. Cryo-EM structure of substrate-bound human telomerase holoenzyme. _Nature_ 557, 190–195 (2018). Article CAS PubMed PubMed Central Google Scholar * Mefford,
M. A., Hass, E. P. & Zappulla, D. C. A 4-base-pair core-enclosing helix in telomerase RNA is essential for activity and for binding to the telomerase reverse transcriptase catalytic
protein subunit. _Mol. Cell Biol._ 40, e00239-20 (2020). Article PubMed PubMed Central Google Scholar * Zappulla, D. C. Yeast telomerase RNA flexibly scaffolds protein subunits: results
and repercussions. _Molecules_ 25, 2750 (2020). Article CAS PubMed PubMed Central Google Scholar * Valsecchi, C. I. K. et al. RNA nucleation by MSL2 induces selective X chromosome
compartmentalization. _Nature_ 589, 137–142 (2020). Article PubMed Google Scholar * Galupa, R. & Heard, E. X-chromosome inactivation: a crossroads between chromosome architecture and
gene regulation. _Annu. Rev. Genet._ 52, 535–566 (2018). Article CAS PubMed Google Scholar * van Bemmel, J. G. et al. The bipartite TAD organization of the X-inactivation center ensures
opposing developmental regulation of Tsix and Xist. _Nat. Genet._ 51, 1024–1034 (2019). Article PubMed PubMed Central Google Scholar * Pandya-Jones, A. et al. A protein assembly mediates
Xist localization and gene silencing. _Nature_ 587, 145–151 (2020). Article CAS PubMed PubMed Central Google Scholar * Jégu, T., Aeby, E. & Lee, J. T. The X chromosome in space.
_Nat. Rev. Genet._ 18, 377–389 (2017). Article PubMed Google Scholar * Eißmann, M. et al. Loss of the abundant nuclear non-coding RNA MALAT1 is compatible with life and development. _RNA
Biol._ 9, 1076–1087 (2012). Article PubMed PubMed Central Google Scholar * Gloss, B. S. & Dinger, M. E. The specificity of long noncoding RNA expression. _Biochim. Biophys. Acta_
1859, 16–22 (2016). Article CAS PubMed Google Scholar * Flynn, R. A. & Chang, H. Y. Long noncoding RNAs in cell-fate programming and reprogramming. _Cell Stem Cell_ 14, 752–761
(2014). Article CAS PubMed PubMed Central Google Scholar * Rinn, J. L. et al. A dermal HOX transcriptional program regulates site-specific epidermal fate. _Genes Dev._ 22, 303–307
(2008). Article CAS PubMed PubMed Central Google Scholar * Mercer, T. R., Dinger, M. E., Sunkin, S. M., Mehler, M. F. & Mattick, J. S. Specific expression of long noncoding RNAs in
the mouse brain. _Proc. Natl Acad. Sci. USA_ 105, 716–721 (2008). Article CAS PubMed PubMed Central Google Scholar * Goff, L. A. et al. Spatiotemporal expression and transcriptional
perturbations by long noncoding RNAs in the mouse brain. _Proc. Natl Acad. Sci. USA_ 112, 6855–6862 (2015). Article CAS PubMed PubMed Central Google Scholar * Liu, S. J. et al.
Single-cell analysis of long non-coding RNAs in the developing human neocortex. _Genome Biol._ 17, 67 (2016). Article PubMed PubMed Central Google Scholar * Bocchi, V. D. et al. The
coding and long noncoding single-cell atlas of the developing human fetal striatum. _Science_ 372, eabf5759 (2021). Article CAS PubMed Google Scholar * Kim, D. H. et al. Single-cell
transcriptome analysis reveals dynamic changes in lncRNA expression during reprogramming. _Cell Stem Cell_ 16, 88–101 (2015). Article CAS PubMed PubMed Central Google Scholar *
Sarropoulos, I., Marin, R., Cardoso-Moreira, M. & Kaessmann, H. Developmental dynamics of lncRNAs across mammalian organs and species. _Nature_ 571, 510–514 (2019). Article CAS PubMed
PubMed Central Google Scholar * Chen, L., Zhu, Q.-H. & Kaufmann, K. Long non-coding RNAs in plants: emerging modulators of gene activity in development and stress responses. _Planta_
252, 92 (2020). Article CAS PubMed PubMed Central Google Scholar * Wierzbicki, A. T., Blevins, T. & Swiezewski, S. Long noncoding RNAs in plants. _Annu. Rev. Plant. Biol._ 72,
245–271 (2021). Article CAS PubMed Google Scholar * Zhao, Y. et al. Natural temperature fluctuations promote COOLAIR regulation of FLC. _Genes. Dev._ 35, 888–898 (2021). Article CAS
PubMed PubMed Central Google Scholar * Lakhotia, S. C. Long non-coding RNAs coordinate cellular responses to stress. _Wiley Interdiscip. Rev. RNA_ 3, 779–796 (2012). Article CAS PubMed
Google Scholar * Kato, M. et al. An endoplasmic reticulum stress-regulated lncRNA hosting a microRNA megacluster induces early features of diabetic nephropathy. _Nat. Commun._ 7, 12864
(2016). Article CAS PubMed PubMed Central Google Scholar * Khan, M. R., Xiang, S., Song, Z. & Wu, M. The p53-inducible long noncoding RNA TRINGS protects cancer cells from necrosis
under glucose starvation. _EMBO J._ 36, 3483–3500 (2017). Article CAS PubMed PubMed Central Google Scholar * Barth, D. A. et al. Long-noncoding RNA (lncRNA) in the regulation of
hypoxia-inducible factor (HIF) in cancer. _Noncoding RNA_ 6, 27 (2020). CAS PubMed PubMed Central Google Scholar * Wang, R. et al. LncRNA GIRGL drives CAPRIN1-mediated phase separation
to suppress glutaminase-1 translation under glutamine deprivation. _Sci. Adv._ 7, eabe5708 (2021). Article CAS PubMed PubMed Central Google Scholar * Connerty, P., Lock, R. B. & de
Bock, C. E. Long non-coding RNAs: major regulators of cell stress in cancer. _Front. Oncol._ 10, 285 (2020). Article PubMed PubMed Central Google Scholar * Liu, K. et al. Long non-coding
RNAs regulate drug resistance in cancer. _Mol. Cancer_ 19, 54 (2020). Article CAS PubMed PubMed Central Google Scholar * Deveson, I. W., Hardwick, S. A., Mercer, T. R. & Mattick,
J. S. The dimensions, dynamics, and relevance of the mammalian noncoding transcriptome. _Trends Genet._ 33, 464–478 (2017). Article CAS PubMed Google Scholar * Mercer, T. R. et al.
Targeted RNA sequencing reveals the deep complexity of the human transcriptome. _Nat. Biotechnol._ 30, 99–104 (2012). Article CAS Google Scholar * Cabili, M. N. et al. Localization and
abundance analysis of human lncRNAs at single-cell and single-molecule resolution. _Genome Biol._ 16, 20 (2015). Article PubMed PubMed Central Google Scholar * Elguindy, M. M. &
Mendell, J. T. NORAD-induced Pumilio phase separation is required for genome stability. _Nature_ 595, 303–308 (2021). Article CAS PubMed PubMed Central Google Scholar * Wu, M. et al.
lncRNA SLERT controls phase separation of FC/DFCs to facilitate Pol I transcription. _Science_ 373, 547–555 (2021). Article CAS PubMed Google Scholar * Asp, M. et al. A spatiotemporal
organ-wide gene expression and cell atlas of the developing human heart. _Cell_ 179, 1647–1660 (2019). Article CAS PubMed Google Scholar * Ma, Q. & Chang, H. Y. Single-cell profiling
of lncRNAs in the developing human brain. _Genome Biol._ 17, 68 (2016). Article PubMed PubMed Central Google Scholar * Hon, C.-C. et al. An atlas of human long non-coding RNAs with
accurate 5′ ends. _Nature_ 543, 199–204 (2017). Article CAS PubMed PubMed Central Google Scholar * Jachowicz, J. W. et al. Xist spatially amplifies SHARP/SPEN recruitment to balance
chromosome-wide silencing and specificity to the X chromosome. _Nat. Struct. Mol. Biol._ 29, 239–249 (2022). Article CAS PubMed PubMed Central Google Scholar * Wu, M., Yang, L.-Z. &
Chen, L.-L. Long noncoding RNA and protein abundance in lncRNPs. _RNA_ 27, 1427–1440 (2021). Article CAS PubMed PubMed Central Google Scholar * Bartonicek, N. et al. Intergenic
disease-associated regions are abundant in novel transcripts. _Genome Biol._ 18, 241 (2017). Article CAS PubMed PubMed Central Google Scholar * de Goede, O. M. et al. Population-scale
tissue transcriptomics maps long non-coding RNAs to complex disease. _Cell_ 184, 2633–2648 (2021). Article PubMed PubMed Central Google Scholar * Nasser, J. et al. Genome-wide enhancer
maps link risk variants to disease genes. _Nature_ 593, 238–243 (2021). Article CAS PubMed PubMed Central Google Scholar * Sleutels, F., Zwart, R. & Barlow, D. P. The non-coding air
RNA is required for silencing autosomal imprinted genes. _Nature_ 415, 810–813 (2002). Article CAS PubMed Google Scholar * Thakur, N. et al. An antisense RNA regulates the bidirectional
silencing property of the _Kcnq1_ imprinting control region. _Mol. Cell Biol._ 24, 7855–7862 (2004). Article CAS PubMed PubMed Central Google Scholar * Young, T. L., Matsuda, T. &
Cepko, C. L. The noncoding RNA taurine upregulated gene 1 is required for differentiation of the murine retina. _Curr. Biol._ 15, 501–512 (2005). Article CAS PubMed Google Scholar *
Allou, L. et al. Non-coding deletions identify _Maenli_ lncRNA as a limb-specific En1 regulator. _Nature_ 592, 93–98 (2021). CAS PubMed Google Scholar * van Dijk, M. et al. HELLP babies
link a novel lincRNA to the trophoblast cell cycle. _J. Clin. Invest._ 122, 4003–4011 (2012). Article PubMed PubMed Central Google Scholar * Li, P., Tao, Z. & Dean, C. Phenotypic
evolution through variation in splicing of the noncoding RNA COOLAIR. _Genes. Dev._ 29, 696–701 (2015). Article CAS PubMed PubMed Central Google Scholar * Huarte, M. The emerging role
of lncRNAs in cancer. _Nat. Med._ 21, 1253–1261 (2015). Article CAS PubMed Google Scholar * Schmitt, A. M. & Chang, H. Y. Long noncoding RNAs in cancer pathways. _Cancer Cell_ 29,
452–463 (2016). Article CAS PubMed PubMed Central Google Scholar * Carlevaro-Fita, J. et al. Cancer LncRNA census reveals evidence for deep functional conservation of long noncoding
RNAs in tumorigenesis. _Commun. Biol._ 3, 56 (2020). Article CAS PubMed PubMed Central Google Scholar * Sparber, P., Filatova, A., Khantemirova, M. & Skoblov, M. The role of long
non-coding RNAs in the pathogenesis of hereditary diseases. _BMC Med. Genomics_ 12, 42 (2019). Article PubMed PubMed Central Google Scholar * Aznaourova, M., Schmerer, N., Schmeck, B.
& Schulte, L. N. Disease-causing mutations and rearrangements in long non-coding RNA gene loci. _Front. Genet._ 11, 527484 (2020). Article CAS PubMed PubMed Central Google Scholar *
Sutherland, H. F. et al. Identification of a novel transcript disrupted by a balanced translocation associated with DiGeorge syndrome. _Am. J. Hum. Genet._ 59, 23–31 (1996). CAS PubMed
PubMed Central Google Scholar * Ang, C. E. et al. The novel lncRNA lnc-NR2F1 is pro-neurogenic and mutated in human neurodevelopmental disorders. _Elife_ 8, e41770 (2019). Article PubMed
PubMed Central Google Scholar * Long, H. K. et al. Loss of extreme long-range enhancers in human neural crest drives a craniofacial disorder. _Cell Stem Cell_ 27, 765–783 (2020). Article
CAS PubMed PubMed Central Google Scholar * Li, Y. et al. A noncoding RNA modulator potentiates phenylalanine metabolism in mice. _Science_ 373, 662–673 (2021). Article CAS PubMed
PubMed Central Google Scholar * Gao, F., Cai, Y., Kapranov, P. & Xu, D. Reverse-genetics studies of lncRNAs — what we have learnt and paths forward. _Genome Biol._ 21, 93 (2020).
Article CAS PubMed PubMed Central Google Scholar * Andergassen, D. & Rinn, J. L. From genotype to phenotype: genetics of mammalian long non-coding RNAs in vivo. _Nat. Rev. Genet._
23, 229–243 (2021). Article PubMed Google Scholar * Zibitt, M. S., Hartford, C. C. R. & Lal, A. Interrogating lncRNA functions via CRISPR/Cas systems. _RNA Biol._ 18, 2097–2106
(2021). Article Google Scholar * Sauvageau, M. et al. Multiple knockout mouse models reveal lincRNAs are required for life and brain development. _Elife_ 2, e01749 (2013). Article PubMed
PubMed Central Google Scholar * Lai, K.-M. V. et al. Diverse phenotypes and specific transcription patterns in twenty mouse lines with ablated lincRNAs. _PLoS ONE_ 10, e0125522 (2015).
Article PubMed PubMed Central Google Scholar * Liu, S. J. et al. CRISPRi-based genome-scale identification of functional long noncoding RNA loci in human cells. _Science_ 355, eaah7111
(2017). Article Google Scholar * Cai, P. et al. A genome-wide long noncoding RNA CRISPRi screen identifies PRANCR as a novel regulator of epidermal homeostasis. _Genome Res._ 30, 22–34
(2019). Article PubMed Google Scholar * Xu, D. et al. A CRISPR/Cas13-based approach demonstrates biological relevance of vlinc class of long non-coding RNAs in anticancer drug response.
_Sci. Rep._ 10, 1794 (2020). Article CAS PubMed PubMed Central Google Scholar * Horlbeck, M. A., Liu, S. J., Chang, H. Y., Lim, D. A. & Weissman, J. S. Fitness effects of
CRISPR/Cas9-targeting of long noncoding RNA genes. _Nat. Biotechnol._ 38, 573–576 (2020). Article CAS PubMed PubMed Central Google Scholar * Cannavò, E. et al. Shadow enhancers are
pervasive features of developmental regulatory networks. _Curr. Biol._ 26, 38–51 (2016). Article PubMed PubMed Central Google Scholar * Hutchinson, J. N. et al. A screen for nuclear
transcripts identifies two linked noncoding RNAs associated with SC35 splicing domains. _BMC Genomics_ 8, 39 (2007). Article PubMed PubMed Central Google Scholar * Nakagawa, S. et al.
_Malat1_ is not an essential component of nuclear speckles in mice. _RNA_ 18, 1487–1499 (2012). Article CAS PubMed PubMed Central Google Scholar * Zhang, B. et al. The lncRNA Malat1 is
dispensable for mouse development but its transcription plays a cis-regulatory role in the adult. _Cell Rep._ 2, 111–123 (2012). Article CAS PubMed PubMed Central Google Scholar *
Zhang, X., Hamblin, M. H. & Yin, K.-J. The long noncoding RNA Malat1: its physiological and pathophysiological functions. _RNA Biol._ 14, 1705–1714 (2017). Article PubMed PubMed
Central Google Scholar * Arun, G., Aggarwal, D. & Spector, D. L. MALAT1 long non-coding RNA: functional implications. _Noncoding RNA_ 6, 22 (2020). CAS PubMed PubMed Central Google
Scholar * Sunwoo, H. et al. MEN epsilon/beta nuclear-retained non-coding RNAs are up-regulated upon muscle differentiation and are essential components of paraspeckles. _Genome Res._ 19,
347–359 (2009). Article CAS PubMed PubMed Central Google Scholar * Clemson, C. M. et al. An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of
paraspeckles. _Mol. Cells_ 33, 717–726 (2009). Article CAS Google Scholar * Mao, Y. S., Sunwoo, H., Zhang, B. & Spector, D. L. Direct visualization of the co-transcriptional assembly
of a nuclear body by noncoding RNAs. _Nat. Cell Biol._ 13, 95–101 (2011). Article CAS PubMed Google Scholar * Nakagawa, S. et al. The lncRNA _Neat1_ is required for corpus luteum
formation and the establishment of pregnancy in a subpopulation of mice. _Development_ 141, 4618–4627 (2014). Article CAS PubMed PubMed Central Google Scholar * Lewejohann, L. et al.
Role of a neuronal small non-messenger RNA: behavioural alterations in BC1 RNA-deleted mice. _Behav. Brain Res._ 154, 273–289 (2004). Article CAS PubMed Google Scholar * Field, A. R. et
al. Structurally conserved primate lncRNAs are transiently expressed during human cortical differentiation and influence cell-type-specific genes. _Stem Cell Rep._ 12, 245–257 (2019).
Article CAS Google Scholar * Liu, S. J. et al. CRISPRi-based radiation modifier screen identifies long non-coding RNA therapeutic targets in glioma. _Genome Biol._ 21, 83 (2020). Article
CAS PubMed PubMed Central Google Scholar * Ramilowski, J. A. et al. Functional annotation of human long noncoding RNAs via molecular phenotyping. _Genome Res._ 30, 1060–1072 (2020).
Article CAS PubMed PubMed Central Google Scholar * Cao, H. et al. Very long intergenic non-coding (vlinc) RNAs directly regulate multiple genes in cis and trans. _BMC Biol._ 19, 108
(2021). Article CAS PubMed PubMed Central Google Scholar * Zhao, J., Sun, B. K., Erwin, J. A., Song, J. J. & Lee, J. T. Polycomb proteins targeted by a short repeat RNA to the mouse
X chromosome. _Science_ 322, 750–756 (2008). Article CAS PubMed PubMed Central Google Scholar * Hacisuleyman, E., Shukla, C. J., Weiner, C. L. & Rinn, J. L. Function and evolution
of local repeats in the _Firre_ locus. _Nat. Commun._ 7, 11021 (2016). Article CAS PubMed PubMed Central Google Scholar * Zucchelli, S. et al. SINEUPs: a new class of natural and
synthetic antisense long non-coding RNAs that activate translation. _RNA Biol._ 12, 771–779 (2015). Article CAS PubMed PubMed Central Google Scholar * Morrissy, A. S., Griffith, M.
& Marra, M. A. Extensive relationship between antisense transcription and alternative splicing in the human genome. _Genome Res._ 21, 1203–1212 (2011). Article CAS PubMed PubMed
Central Google Scholar * Romero-Barrios, N., Legascue, M. F., Benhamed, M., Ariel, F. & Crespi, M. Splicing regulation by long noncoding RNAs. _Nucleic Acids Res._ 46, 2169–2184
(2018). Article CAS PubMed PubMed Central Google Scholar * Pisignano, G. & Ladomery, M. Epigenetic regulation of alternative splicing: how lncRNAs tailor the message. _Noncoding
RNA_ 7, 21 (2021). CAS PubMed PubMed Central Google Scholar * Carrieri, C. et al. Long non-coding antisense RNA controls Uchl1 translation through an embedded SINEB2 repeat. _Nature_
491, 454–457 (2012). Article CAS PubMed Google Scholar * Deforges, J. et al. Control of cognate sense mRNA translation by cis-natural antisense RNAs. _Plant Physiol._ 180, 305–322
(2019). Article CAS PubMed PubMed Central Google Scholar * Peters, N. T., Rohrbach, J. A., Zalewski, B. A., Byrkett, C. M. & Vaughn, J. C. RNA editing and regulation of _Drosophila
4f-rnp_ expression by sas-10 antisense readthrough mRNA transcripts. _RNA_ 9, 698–710 (2003). Article CAS PubMed PubMed Central Google Scholar * Gong, C. & Maquat, L. E. lncRNAs
transactivate STAU1-mediated mRNA decay by duplexing with 3’ UTRs via Alu elements. _Nature_ 470, 284–288 (2011). Article CAS PubMed PubMed Central Google Scholar * Whittaker, C. &
Dean, C. The FLC locus: a platform for discoveries in epigenetics and adaptation. _Annu. Rev. Cell Dev. Biol._ 33, 555–575 (2017). Article CAS PubMed Google Scholar * Huarte, M. et al. A
large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. _Cell_ 142, 409–419 (2010). Article CAS PubMed PubMed Central Google Scholar *
Rothschild, G. et al. Noncoding RNA transcription alters chromosomal topology to promote isotype-specific class switch recombination. _Sci. Immunol._ 5, eaay5864 (2020). Article CAS PubMed
PubMed Central Google Scholar * Fanucchi, S. et al. Immune genes are primed for robust transcription by proximal long noncoding RNAs located in nuclear compartments. _Nat. Genet._ 51,
138–150 (2019). Article CAS PubMed Google Scholar * Vollmers, A. C. et al. A conserved long noncoding RNA, GAPLINC, modulates the immune response during endotoxic shock. _Proc. Natl
Acad. Sci. USA_ 118, e2016648118 (2021). Article CAS PubMed PubMed Central Google Scholar * Atianand, M. K. et al. A long noncoding RNA lincRNA-EPS acts as a transcriptional brake to
restrain inflammation. _Cell_ 165, 1672–1685 (2016). Article CAS PubMed PubMed Central Google Scholar * Hu, G. et al. LincRNA-Cox2 promotes late inflammatory gene transcription in
macrophages through modulating SWI/SNF-mediated chromatin remodeling. _J. Immunol._ 196, 2799–2808 (2016). Article CAS PubMed Google Scholar * Zhao, X. et al. A long noncoding RNA
contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. _Nat. Neurosci._ 16, 1024–1031 (2013). Article CAS PubMed PubMed Central Google Scholar * Ruan, X. et al.
Identification of human long noncoding RNAs associated with nonalcoholic fatty liver disease and metabolic homeostasis. _J. Clin. Invest._ 131, e136336 (2021). Article CAS PubMed PubMed
Central Google Scholar * Hennessy, E. J. et al. The long noncoding RNA CHROME regulates cholesterol homeostasis in primates. _Nat. Metab._ 1, 98–110 (2019). Article CAS PubMed Google
Scholar * Du, Q. et al. MIR205HG Is a long noncoding RNA that regulates growth hormone and prolactin production in the anterior pituitary. _Dev. Cell_ 49, 618–631 (2019). Article CAS
PubMed PubMed Central Google Scholar * Zhang, P., Cao, L., Fan, P., Mei, Y. & Wu, M. LncRNA-MIF, a c-Myc-activated long non-coding RNA, suppresses glycolysis by promoting
Fbxw7-mediated c-Myc degradation. _EMBO Rep._ 17, 1204–1220 (2016). Article CAS PubMed PubMed Central Google Scholar * Zheng, X. et al. LncRNA wires up Hippo and Hedgehog signaling to
reprogramme glucose metabolism. _EMBO J._ 36, 3325–3335 (2017). Article CAS PubMed PubMed Central Google Scholar * McClintock, M. A. et al. RNA-directed activation of cytoplasmic
dynein-1 in reconstituted transport RNPs. _Elife_ 7, e36312 (2018). Article PubMed PubMed Central Google Scholar * Lin, A. et al. The _LINK-A_ lncRNA interacts with PtdIns(3,4,5)P3 to
hyperactivate AKT and confer resistance to AKT inhibitors. _Nat. Cell Biol._ 19, 238–251 (2017). Article CAS PubMed PubMed Central Google Scholar * Sang, L. et al. LncRNA _CamK-A_
regulates Ca2+-signaling-mediated tumor microenvironment remodeling. _Mol. Cells_ 72, 71–83 (2018). Article CAS Google Scholar * Ma, Y., Zhang, J., Wen, L. & Lin, A. Membrane-lipid
associated lncRNA: a new regulator in cancer signaling. _Cancer Lett._ 419, 27–29 (2018). Article CAS PubMed Google Scholar * Wang, F. et al. The long noncoding RNA _Synage_ regulates
synapse stability and neuronal function in the cerebellum. _Cell Death Differ._ 28, 2634–2650 (2021). Article CAS PubMed PubMed Central Google Scholar * Samaddar, S. & Banerjee, S.
Far from the nuclear crowd: cytoplasmic lncRNA and their implications in synaptic plasticity and memory. _Neurobiol. Learn. Mem._ 185, 107522 (2021). Article CAS PubMed Google Scholar *
Wei, W. et al. ADRAM is an experience-dependent long noncoding RNA that drives fear extinction through a direct interaction with the chaperone protein 14-3-3. _Cell Rep._ 38, 110546 (2022).
Article CAS PubMed PubMed Central Google Scholar * Wu, E. et al. Discovery of plasma membrane-associated RNAs through APEX-seq. _Cell Biochem. Biophys._ 79, 905–917 (2021). Article CAS
PubMed Google Scholar * Chen, Y. et al. Hovlinc is a recently evolved class of ribozyme found in human lncRNA. _Nat. Chem. Biol._ 17, 601–607 (2021). Article CAS PubMed Google Scholar
* Schuettengruber, B., Chourrout, D., Vervoort, M., Leblanc, B. & Cavalli, G. Genome regulation by polycomb and trithorax proteins. _Cell_ 128, 735–745 (2007). Article CAS PubMed
Google Scholar * Erdmann, R. M. & Picard, C. L. RNA-directed DNA methylation. _PLoS Genet._ 16, e1009034 (2020). Article CAS PubMed PubMed Central Google Scholar * Djupedal, I.
& Ekwall, K. Epigenetics: heterochromatin meets RNAi. _Cell Res._ 19, 282–295 (2009). Article CAS PubMed Google Scholar * Jeffery, L. & Nakielny, S. Components of the DNA
methylation system of chromatin control are RNA-binding proteins. _J. Biol. Chem._ 279, 49479–49487 (2004). Article CAS PubMed Google Scholar * Mohammad, F., Mondal, T., Guseva, N.,
Pandey, G. K. & Kanduri, C. Kcnq1ot1 noncoding RNA mediates transcriptional gene silencing by interacting with Dnmt1. _Development_ 137, 2493–2499 (2010). Article CAS PubMed Google
Scholar * Di Ruscio, A. et al. DNMT1-interacting RNAs block gene-specific DNA methylation. _Nature_ 503, 371–376 (2013). Article PubMed PubMed Central Google Scholar * Merry, C. R. et
al. DNMT1-associated long non-coding RNAs regulate global gene expression and DNA methylation in colon cancer. _Hum. Mol. Genet._ 24, 6240–6253 (2015). Article CAS PubMed PubMed Central
Google Scholar * Di Croce, L. & Helin, K. Transcriptional regulation by Polycomb group proteins. _Nat. Struct. Mol. Biol._ 20, 1147–1155 (2013). Article PubMed Google Scholar *
Nagano, T. et al. The Air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. _Science_ 322, 1717–1720 (2008). Article CAS PubMed Google Scholar * Pandey,
R. R. et al. Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. _Mol. Cells_ 32, 232–246 (2008). Article CAS Google
Scholar * Davidovich, C. & Cech, T. R. The recruitment of chromatin modifiers by long noncoding RNAs: lessons from PRC2. _RNA_ 21, 2007–2022 (2015). Article CAS PubMed PubMed Central
Google Scholar * Davidovich, C. et al. Toward a consensus on the binding specificity and promiscuity of PRC2 for RNA. _Mol. Cells_ 57, 552–558 (2015). Article CAS Google Scholar *
Khalil, A. M. et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. _Proc. Natl Acad. Sci. USA_ 106, 11667–11672 (2009).
Article CAS PubMed PubMed Central Google Scholar * Beltran, M. et al. The interaction of PRC2 with RNA or chromatin is mutually antagonistic. _Genome Res._ 26, 896–907 (2016). Article
CAS PubMed PubMed Central Google Scholar * Rosenberg, M. et al. Motif-driven interactions between RNA and PRC2 are rheostats that regulate transcription elongation. _Nat. Struct. Mol.
Biol._ 28, 103–117 (2021). Article CAS PubMed PubMed Central Google Scholar * Zhao, J. et al. Genome-wide identification of Polycomb-associated RNAs by RIP-seq. _Mol. Cells_ 40, 939–953
(2010). Article CAS Google Scholar * Davidovich, C., Zheng, L., Goodrich, K. J. & Cech, T. R. Promiscuous RNA binding by Polycomb repressive complex 2. _Nat. Struct. Mol. Biol._ 20,
1250–1257 (2013). Article CAS PubMed PubMed Central Google Scholar * Cifuentes-Rojas, C., Hernandez, A. J., Sarma, K. & Lee, J. T. Regulatory interactions between RNA and Polycomb
repressive complex 2. _Mol. Cells_ 55, 171–185 (2014). Article CAS Google Scholar * Long, Y. et al. RNA is essential for PRC2 chromatin occupancy and function in human pluripotent stem
cells. _Nat. Genet._ 52, 931–938 (2020). Article CAS PubMed Google Scholar * Yap, K. L. et al. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by
Polycomb CBX7 in transcriptional silencing of INK4a. _Mol. Cells_ 38, 662–674 (2010). Article CAS Google Scholar * Rosenberg, M. et al. Denaturing CLIP, dCLIP, pipeline identifies
discrete RNA footprints on chromatin-associated proteins and reveals that CBX7 targets 3′ UTRs to regulate mRNA expression. _Cell Syst._ 5, 368–385 (2017). Article CAS PubMed PubMed
Central Google Scholar * Wang, X. et al. Targeting of Polycomb repressive complex 2 to RNA by short repeats of consecutive guanines. _Mol. Cells_ 65, 1056–1067 (2017). Article CAS Google
Scholar * Beltran, M. et al. G-tract RNA removes Polycomb repressive complex 2 from genes. _Nat. Struct. Mol. Biol._ 26, 899–909 (2019). Article CAS PubMed PubMed Central Google
Scholar * Dinger, M. E. et al. Long noncoding RNAs in mouse embryonic stem cell pluripotency and differentiation. _Genome Res._ 18, 1433–1445 (2008). Article CAS PubMed PubMed Central
Google Scholar * Wang, K. C. et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. _Nature_ 472, 120–124 (2011). Article CAS PubMed PubMed
Central Google Scholar * Yang, Y. W. et al. Essential role of lncRNA binding for WDR5 maintenance of active chromatin and embryonic stem cell pluripotency. _Elife_ 3, e02046 (2014).
Article PubMed PubMed Central Google Scholar * Deng, C. et al. _HoxBlinc_ RNA recruits Set1/MLL complexes to activate _Hox_ gene expression patterns and mesoderm lineage development.
_Cell Rep._ 14, 103–114 (2016). Article CAS PubMed Google Scholar * Subhash, S. et al. H3K4me2 and WDR5 enriched chromatin interacting long non-coding RNAs maintain transcriptionally
competent chromatin at divergent transcriptional units. _Nucleic Acids Res._ 46, 9384–9400 (2018). Article CAS PubMed PubMed Central Google Scholar * Butler, A. A., Johnston, D. R.,
Kaur, S. & Lubin, F. D. Long noncoding RNA NEAT1 mediates neuronal histone methylation and age-related memory impairment. _Sci. Signal._ 12, eaaw9277 (2019). Article PubMed PubMed
Central Google Scholar * Jantrapirom, S. et al. Long noncoding RNA-dependent methylation of nonhistone proteins. _WIREs RNA_ 12, e1661 (2021). Article CAS PubMed Google Scholar * Luo,
Z., Rhie, S. K. & Farnham, P. J. The enigmatic HOX genes: can we crack their code? _Cancers_ 11, 323 (2019). Article CAS PubMed PubMed Central Google Scholar * Grosschedl, R.,
Giese, K. & Pagel, J. HMG domain proteins: architectural elements in the assembly of nucleoprotein structures. _Trends Genet._ 10, 94–100 (1994). Article CAS PubMed Google Scholar *
Tantin, D. Oct transcription factors in development and stem cells: insights and mechanisms. _Development_ 140, 2857–2866 (2013). Article CAS PubMed PubMed Central Google Scholar *
Clapier, C. R., Iwasa, J., Cairns, B. R. & Peterson, C. L. Mechanisms of action and regulation of ATP-dependent chromatin-remodelling complexes. _Nat. Rev. Mol. Cell Biol._ 18, 407–422
(2017). Article CAS PubMed PubMed Central Google Scholar * Li, Y. et al. The structural basis for cohesin–CTCF-anchored loops. _Nature_ 578, 472–476 (2020). Article CAS PubMed PubMed
Central Google Scholar * Oh, H. J. et al. Jpx RNA regulates CTCF anchor site selection and formation of chromosome loops. _Cell_ 184, P6157–P6173 (2021). Article Google Scholar * Kung,
J. T. et al. Locus-specific targeting to the X chromosome revealed by the RNA interactome of CTCF. _Mol. Cells_ 57, 361–375 (2015). Article CAS Google Scholar * Rivera-Pomar, R.,
Niessing, D., Schmidt-Ott, U., Gehring, W. J. & Jacklë, H. RNA binding and translational suppression by bicoid. _Nature_ 379, 746–749 (1996). Article CAS PubMed Google Scholar *
Holmes, Z. E. et al. The Sox2 transcription factor binds RNA. _Nat. Commun._ 11, 1805 (2020). Article CAS PubMed PubMed Central Google Scholar * Cajigas, I. et al. Sox2-_Evf2_ lncRNA
mechanisms of chromosome topological control in developing forebrain. _Development_ 148, dev197202 (2021). Article CAS PubMed PubMed Central Google Scholar * Genzor, P. & Bortvin,
A. A unique HMG-box domain of mouse Maelstrom binds structured RNA but not double stranded DNA. _PLoS ONE_ 10, e0120268 (2015). Article PubMed PubMed Central Google Scholar * Zhao, Z.,
Dammert, M. A., Grummt, I. & Bierhoff, H. lncRNA-Induced nucleosome repositioning reinforces transcriptional repression of RNA genes upon hypotonic stress. _Cell Rep._ 14, 1876–1882
(2016). Article CAS PubMed Google Scholar * Amaral, P. P. et al. Complex architecture and regulated expression of the Sox2ot locus during vertebrate development. _RNA_ 15, 2013–2027
(2009). Article CAS PubMed PubMed Central Google Scholar * Ng, S.-Y., Johnson, R. & Stanton, L. W. Human long non-coding RNAs promote pluripotency and neuronal differentiation by
association with chromatin modifiers and transcription factors. _EMBO J._ 31, 522–533 (2012). Article CAS PubMed Google Scholar * Ng, S.-Y., Bogu, G. K., Soh, B. S. & Stanton, L. W.
The long noncoding RNA RMST interacts with SOX2 to regulate neurogenesis. _Mol. Cells_ 51, 349–359 (2013). Article CAS Google Scholar * Samudyata et al. Interaction of Sox2 with RNA
binding proteins in mouse embryonic stem cells. _Exp. Cell Res._ 381, 129–138 (2019). Article CAS PubMed PubMed Central Google Scholar * Hou, L. et al. Concurrent binding to DNA and RNA
facilitates the pluripotency reprogramming activity of Sox2. _Nucleic Acids Res._ 48, 3869–3887 (2020). Article CAS PubMed PubMed Central Google Scholar * Tang, Y. et al. Linking long
non-coding RNAs and SWI/SNF complexes to chromatin remodeling in cancer. _Mol. Cancer_ 16, 42 (2017). Article PubMed PubMed Central Google Scholar * Jégu, T. et al. Xist RNA antagonizes
the SWI/SNF chromatin remodeler BRG1 on the inactive X chromosome. _Nat. Struct. Mol. Biol._ 26, 96–109 (2019). Article PubMed PubMed Central Google Scholar * Grossi, E. et al. A
lncRNA-SWI/SNF complex crosstalk controls transcriptional activation at specific promoter regions. _Nat. Commun._ 11, 936 (2020). Article CAS PubMed PubMed Central Google Scholar *
Schutt, C. et al. Linc-MYH configures INO80 to regulate muscle stem cell numbers and skeletal muscle hypertrophy. _EMBO J._ 39, e105098 (2020). Article CAS PubMed PubMed Central Google
Scholar * Patty, B. J. & Hainer, S. J. Non-coding RNAs and nucleosome remodeling complexes: an intricate regulatory relationship. _Biology_ 9, 213 (2020). Article CAS PubMed PubMed
Central Google Scholar * Ducoli, L. et al. LETR1 is a lymphatic endothelial-specific lncRNA governing cell proliferation and migration through KLF4 and SEMA3C. _Nat. Commun._ 12, 925
(2021). Article CAS PubMed PubMed Central Google Scholar * Chang, K. C. et al. MaTAR25 lncRNA regulates the Tensin1 gene to impact breast cancer progression. _Nat. Commun._ 11, 6438
(2020). Article CAS PubMed PubMed Central Google Scholar * Caretti, G. et al. The RNA helicases p68/p72 and the noncoding RNA SRA are coregulators of MyoD and skeletal muscle
differentiation. _Dev. Cell_ 11, 547–560 (2006). Article CAS PubMed Google Scholar * Dong, A. et al. A long noncoding RNA, _LncMyoD_, modulates chromatin accessibility to regulate muscle
stem cell myogenic lineage progression. _Proc. Natl Acad. Sci. USA_ 117, 32464–32475 (2020). Article CAS PubMed PubMed Central Google Scholar * Yu, X. et al. Long non-coding RNA
Linc-RAM enhances myogenic differentiation by interacting with MyoD. _Nat. Commun._ 8, 14016 (2017). Article CAS PubMed PubMed Central Google Scholar * Dou, M. et al. The long noncoding
RNA MyHC IIA/X-AS contributes to skeletal muscle myogenesis and maintains the fast fiber phenotype. _J. Biol. Chem._ 295, 4937–4949 (2020). Article CAS PubMed PubMed Central Google
Scholar * Bose, D. A. et al. RNA binding to CBP stimulates histone acetylation and transcription. _Cell_ 168, 135–149 (2017). Article CAS PubMed PubMed Central Google Scholar * Lin,
H., Shabbir, A., Molnar, M. & Lee, T. Stem cell regulatory function mediated by expression of a novel mouse Oct4 pseudogene. _Biochem. Biophys. Res. Commun._ 355, 111–116 (2007). Article
CAS PubMed Google Scholar * Hawkins, P. G. & Morris, K. V. Transcriptional regulation of Oct4 by a long non-coding RNA antisense to Oct4-pseudogene 5. _Transcription_ 1, 165–175
(2010). Article PubMed PubMed Central Google Scholar * Wang, Y. et al. Endogenous miRNA sponge _lincRNA-RoR_ regulates _Oct4, Nanog_, and _Sox2_ in human embryonic stem cell
self-renewal. _Dev. Cell_ 25, 69–80 (2013). Article CAS PubMed Google Scholar * Scarola, M. et al. FUS-dependent loading of SUV39H1 to OCT4 pseudogene-lncRNA programs a silencing complex
with OCT4 promoter specificity. _Commun. Biol._ 3, 632 (2020). Article CAS PubMed PubMed Central Google Scholar * Tariq, A. et al. LncRNA-mediated regulation of SOX9 expression in
basal subtype breast cancer cells. _RNA_ 26, 175–185 (2020). Article CAS PubMed PubMed Central Google Scholar * Sheik Mohamed, J., Gaughwin, P. M., Lim, B., Robson, P. & Lipovich,
L. Conserved long noncoding RNAs transcriptionally regulated by Oct4 and Nanog modulate pluripotency in mouse embryonic stem cells. _RNA_ 16, 324–337 (2010). Article PubMed PubMed Central
Google Scholar * Tomita, S. et al. A cluster of noncoding RNAs activates the ESR1 locus during breast cancer adaptation. _Nat. Commun._ 6, 6966 (2015). Article CAS PubMed Google
Scholar * Setten, R. L., Chomchan, P., Epps, E. W., Burnett, J. C. & Rossi, J. J. CRED9: a differentially expressed elncRNA regulates expression of transcription factor CEBPA. _RNA_ 27,
891–906 (2021). Article CAS PubMed PubMed Central Google Scholar * Shen, Y. et al. A map of the cis-regulatory sequences in the mouse genome. _Nature_ 488, 116–120 (2012). Article CAS
PubMed PubMed Central Google Scholar * Thurman, R. E. et al. The accessible chromatin landscape of the human genome. _Nature_ 489, 75–82 (2012). Article CAS PubMed PubMed Central
Google Scholar * Encode Project Consortium. An integrated encyclopedia of DNA elements in the human genome. _Nature_ 489, 57–74 (2012). Article Google Scholar * Chen, H. & Liang, H. A
high-resolution map of human enhancer RNA loci characterizes super-enhancer activities in cancer. _Cancer Cell_ 38, 701–715 (2020). Article CAS PubMed PubMed Central Google Scholar *
Hnisz, D. et al. Super-enhancers in the control of cell identity and disease. _Cell_ 155, 934–947 (2013). Article CAS PubMed Google Scholar * Pott, S. & Lieb, J. D. What are
super-enhancers? _Nat. Genet._ 47, 8–12 (2015). Article CAS PubMed Google Scholar * Li, S. & Ovcharenko, I. Enhancer jungles establish robust tissue-specific regulatory control in
the human genome. _Genomics_ 112, 2261–2270 (2020). Article CAS PubMed Google Scholar * Ptashne, M. How eukaryotic transcriptional activators work. _Nature_ 335, 683–689 (1988). Article
CAS PubMed Google Scholar * Souaid, C., Bloyer, S. & Noordermeer, D. in _Nuclear Architecture and Dynamics_ Vol. 2 (eds Christophe, L. & Jean-Marc, V.) Ch. 19, 435–456 (Academic
Press, 2018). * Lim, B. & Levine, M. S. Enhancer-promoter communication: hubs or loops? _Curr. Opin. Genet. Dev._ 67, 5–9 (2021). Article CAS PubMed Google Scholar * Zhu, I., Song,
W., Ovcharenko, I. & Landsman, D. A model of active transcription hubs that unifies the roles of active promoters and enhancers. _Nucleic Acids Res._ 49, 4493–4505 (2021). Article CAS
PubMed PubMed Central Google Scholar * Kim, T.-K., Hemberg, M. & Gray, J. M. Enhancer RNAs: a class of long noncoding RNAs synthesized at enhancers. _Cold Spring Harb. Perspect.
Biol._ 7, a018622 (2015). Article PubMed PubMed Central Google Scholar * Arner, E. et al. Transcribed enhancers lead waves of coordinated transcription in transitioning mammalian cells.
_Science_ 347, 1010–1014 (2015). Article CAS PubMed PubMed Central Google Scholar * Li, W., Notani, D. & Rosenfeld, M. G. Enhancers as non-coding RNA transcription units: recent
insights and future perspectives. _Nat. Rev. Genet._ 17, 207–223 (2016). Article CAS PubMed Google Scholar * Arnold, P. R., Wells, A. D. & Li, X. C. Diversity and emerging roles of
enhancer RNA in regulation of gene expression and cell fate. _Front. Cell Dev. Biol._ 7, 377 (2020). Article PubMed PubMed Central Google Scholar * Core, L. J. et al. Analysis of nascent
RNA identifies a unified architecture of initiation regions at mammalian promoters and enhancers. _Nat. Genet._ 46, 1311–1320 (2014). Article CAS PubMed PubMed Central Google Scholar *
Kim, T.-K. et al. Widespread transcription at neuronal activity-regulated enhancers. _Nature_ 465, 182–187 (2010). Article CAS PubMed PubMed Central Google Scholar * Melgar, M. F.,
Collins, F. S. & Sethupathy, P. Discovery of active enhancers through bidirectional expression of short transcripts. _Genome Biol._ 12, R113 (2011). Article CAS PubMed PubMed Central
Google Scholar * Andersson, R. et al. An atlas of active enhancers across human cell types and tissues. _Nature_ 507, 455–461 (2014). Article CAS PubMed PubMed Central Google Scholar
* Seila, A. C. et al. Divergent transcription from active promoters. _Science_ 322, 1849–1851 (2008). Article CAS PubMed PubMed Central Google Scholar * Young, R. S., Kumar, Y.,
Bickmore, W. A. & Taylor, M. S. Bidirectional transcription initiation marks accessible chromatin and is not specific to enhancers. _Genome Biol._ 18, 242 (2017). Article PubMed PubMed
Central Google Scholar * Sartorelli, V. & Lauberth, S. M. Enhancer RNAs are an important regulatory layer of the epigenome. _Nat. Struct. Mol. Biol._ 27, 521–528 (2020). Article CAS
PubMed PubMed Central Google Scholar * Wu, H. et al. Tissue-specific RNA expression marks distant-acting developmental enhancers. _PLoS Genet._ 10, e1004610 (2014). Article PubMed
PubMed Central Google Scholar * Carullo, N. V. N. et al. Enhancer RNAs predict enhancer–gene regulatory links and are critical for enhancer function in neuronal systems. _Nucleic Acids
Res._ 48, 9550–9570 (2020). Article CAS PubMed PubMed Central Google Scholar * Tan, J. Y. & Marques, A. C. The activity of human enhancers is modulated by the splicing of their
associated lncRNAs. _PLoS Comput. Biol._ 18, e1009722 (2022). Article CAS PubMed PubMed Central Google Scholar * Gil, N. & Ulitsky, I. Production of spliced long noncoding RNAs
specifies regions with increased enhancer activity. _Cell Syst._ 7, 537–547 (2018). Article CAS PubMed PubMed Central Google Scholar * Melo, C. A. et al. eRNAs are required for
p53-dependent enhancer activity and gene transcription. _Mol. Cells_ 49, 524–535 (2013). Article CAS Google Scholar * Lam, M. T. Y., Li, W., Rosenfeld, M. G. & Glass, C. K. Enhancer
RNAs and regulated transcriptional programs. _Trends Biochem. Sci._ 39, 170–182 (2014). Article CAS PubMed PubMed Central Google Scholar * Yin, Y. et al. Opposing roles for the lncRNA
Haunt and its genomic locus in regulating HOXA gene activation during embryonic stem cell differentiation. _Cell Stem Cell_ 16, 504–516 (2015). Article CAS PubMed Google Scholar * Isoda,
T. et al. Non-coding transcription instructs chromatin folding and compartmentalization to dictate enhancer-promoter communication and T cell fate. _Cell_ 171, 103–119 (2017). Article CAS
PubMed PubMed Central Google Scholar * Cajigas, I. et al. The _Evf2_ ultraconserved enhancer lncRNA functionally and spatially organizes megabase distant genes in the developing
forebrain. _Mol. Cells_ 71, 956–972 (2018). Article CAS Google Scholar * Lewandowski, J. P. et al. The _Firre_ locus produces a trans-acting RNA molecule that functions in hematopoiesis.
_Nat. Commun._ 10, 5137 (2019). Article PubMed PubMed Central Google Scholar * Groff, A. F., Barutcu, A. R., Lewandowski, J. P. & Rinn, J. L. Enhancers in the Peril lincRNA locus
regulate distant but not local genes. _Genome Biol._ 19, 219 (2018). Article CAS PubMed PubMed Central Google Scholar * Han, X. et al. The lncRNA _Hand2os1/Uph_ locus orchestrates heart
development through regulation of precise expression of _Hand2_. _Development_ 146, dev176198 (2019). Article CAS PubMed Google Scholar * Mattick, J. S. Deconstructing the dogma: a new
view of the evolution and genetic programming of complex organisms. _Ann. N. Y. Acad. Sci._ 1178, 29–46 (2009). Article CAS PubMed Google Scholar * Shin, Y. & Brangwynne, C. P.
Liquid phase condensation in cell physiology and disease. _Science_ 357, eaaf4382 (2017). Article PubMed Google Scholar * Garcia-Jove Navarro, M. et al. RNA is a critical element for the
sizing and the composition of phase-separated RNA–protein condensates. _Nat. Commun._ 10, 3230 (2019). Article PubMed PubMed Central Google Scholar * Roden, C. & Gladfelter, A. S.
RNA contributions to the form and function of biomolecular condensates. _Nat. Rev. Mol. Cell Biol._ 22, 183–195 (2021). Article CAS PubMed Google Scholar * Niklas, K. J., Dunker, A. K.
& Yruela, I. The evolutionary origins of cell type diversification and the role of intrinsically disordered proteins. _J. Exp. Bot._ 69, 1437–1446 (2018). Article CAS PubMed Google
Scholar * Macossay-Castillo, M. et al. The balancing act of intrinsically disordered proteins: enabling functional diversity while minimizing promiscuity. _J. Mol. Biol._ 431, 1650–1670
(2019). Article CAS PubMed PubMed Central Google Scholar * Chen, W. & Moore, M. J. The spliceosome: disorder and dynamics defined. _Curr. Opin. Struct. Biol._ 24, 141–149 (2014).
Article CAS PubMed Google Scholar * Staby, L. et al. Eukaryotic transcription factors: paradigms of protein intrinsic disorder. _Biochem. J._ 474, 2509–2532 (2017). Article CAS PubMed
Google Scholar * Wright, P. E. & Dyson, H. J. Intrinsically disordered proteins in cellular signalling and regulation. _Nat. Rev. Mol. Cell Biol._ 16, 18–29 (2015). Article CAS
PubMed PubMed Central Google Scholar * Hahn, S. Phase separation, protein disorder, and enhancer function. _Cell_ 175, 1723–1725 (2018). Article CAS PubMed Google Scholar * Peng, Z.,
Mizianty, M. J., Xue, B., Kurgan, L. & Uversky, V. N. More than just tails: intrinsic disorder in histone proteins. _Mol. Biosyst._ 8, 1886–1901 (2012). Article CAS PubMed Google
Scholar * Watson, M. & Stott, K. Disordered domains in chromatin-binding proteins. _Essays Biochem._ 63, 147–156 (2019). Article CAS PubMed Google Scholar * Balcerak, A.,
Trebinska-Stryjewska, A., Konopinski, R., Wakula, M. & Grzybowska, E. A. RNA–protein interactions: disorder, moonlighting and junk contribute to eukaryotic complexity. _Open Biol._ 9,
190096 (2019). Article CAS PubMed PubMed Central Google Scholar * Musselman, C. A. & Kutateladze, T. G. Characterization of functional disordered regions within chromatin-associated
proteins. _iScience_ 24, 102070 (2021). Article CAS PubMed PubMed Central Google Scholar * Takeshi, C. & Tetsuro, H. Nuclear bodies built on architectural long noncoding RNAs:
unifying principles of their construction and function. _Mol. Cells_ 40, 889–896 (2017). Google Scholar * Panda, S. et al. Noncoding RNA Ginir functions as an oncogene by associating with
centrosomal proteins. _PLoS Biol._ 16, e2004204 (2018). Article PubMed PubMed Central Google Scholar * Yamazaki, T. & Hirose, T. Control of condensates dictates nucleolar
architecture. _Science_ 373, 486–487 (2021). Article CAS PubMed Google Scholar * Wang, X. et al. Mutual dependency between lncRNA LETN and protein NPM1 in controlling the nucleolar
structure and functions sustaining cell proliferation. _Cell Res._ 31, 664–683 (2021). Article CAS PubMed PubMed Central Google Scholar * Spector, D. L. & Lamond, A. I. Nuclear
speckles. _Cold Spring Harb. Perspect. Biol._ 3, a000646 (2011). Article PubMed PubMed Central Google Scholar * Ishizuka, A., Hasegawa, Y., Ishida, K., Yanaka, K. & Nakagawa, S.
Formation of nuclear bodies by the lncRNA Gomafu-associating proteins Celf3 and SF1. _Genes Cells_ 19, 704–721 (2014). Article CAS PubMed PubMed Central Google Scholar * Tripathi, V. et
al. The nuclear-retained noncoding RNA _MALAT1_ regulates alternative splicing by modulating SR splicing factor phosphorylation. _Mol. Cells_ 39, 925–938 (2010). Article CAS Google
Scholar * Yamazaki, T. et al. Functional domains of NEAT1 architectural lncRNA induce paraspeckle assembly through phase separation. _Mol. Cells_ 70, 1038–1053 (2018). Article CAS Google
Scholar * Fox, A. H., Nakagawa, S., Hirose, T. & Bond, C. S. Paraspeckles: where long noncoding RNA meets phase separation. _Trends Biochem. Sci._ 43, 124–135 (2018). Article CAS
PubMed Google Scholar * Fang, X. et al. Arabidopsis FLL2 promotes liquid–liquid phase separation of polyadenylation complexes. _Nature_ 569, 265–269 (2019). Article CAS PubMed PubMed
Central Google Scholar * Emenecker, R. J., Holehouse, A. S. & Strader, L. C. Emerging roles for phase separation in plants. _Dev. Cell_ 55, 69–83 (2020). Article CAS PubMed PubMed
Central Google Scholar * Brangwynne, C. P. et al. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. _Science_ 324, 1729–1732 (2009). Article
CAS PubMed Google Scholar * Smith, J. et al. Spatial patterning of P granules by RNA-induced phase separation of the intrinsically-disordered protein MEG-3. _Elife_ 5, e21337 (2016).
Article PubMed PubMed Central Google Scholar * Chouaib, R. et al. A dual protein-mRNA localization screen reveals compartmentalized translation and widespread co-translational RNA
targeting. _Dev. Cell_ 54, 773–791 (2020). Article CAS PubMed Google Scholar * Chen, X., Wu, X., Wu, H. & Zhang, M. Phase separation at the synapse. _Nat. Neurosci._ 23, 301–310
(2020). Article CAS PubMed Google Scholar * Tichon, A. et al. A conserved abundant cytoplasmic long noncoding RNA modulates repression by Pumilio proteins in human cells. _Nat. Commun._
7, 12209 (2016). Article CAS PubMed PubMed Central Google Scholar * Mele, M. & Rinn, J. L. “Cat’s cradling” the 3D genome by the act of lncRNA transcription. _Mol. Cells_ 62,
657–664 (2016). Article CAS Google Scholar * Bhat, P., Honson, D. & Guttman, M. Nuclear compartmentalization as a mechanism of quantitative control of gene expression. _Nat. Rev. Mol.
Cell Biol._ 22, 653–670 (2021). Article CAS PubMed Google Scholar * Hnisz, D., Shrinivas, K., Young, R. A., Chakraborty, A. K. & Sharp, P. A. A phase separation model for
transcriptional control. _Cell_ 169, 13–23 (2017). Article CAS PubMed PubMed Central Google Scholar * Henninger, J. E. et al. RNA-mediated feedback control of transcriptional
condensates. _Cell_ 184, 207–225 (2021). Article CAS PubMed Google Scholar * Quinodoz, S. A. et al. RNA promotes the formation of spatial compartments in the nucleus. _Cell_ 184,
5775–5790 (2021). Article CAS PubMed PubMed Central Google Scholar * Sabari, B. R. et al. Coactivator condensation at super-enhancers links phase separation and gene control. _Science_
361, eaar3958 (2018). Article PubMed PubMed Central Google Scholar * Nair, S. J. et al. Phase separation of ligand-activated enhancers licenses cooperative chromosomal enhancer assembly.
_Nat. Struct. Mol. Biol._ 26, 193–203 (2019). Article CAS PubMed PubMed Central Google Scholar * Shrinivas, K. et al. Enhancer features that drive formation of transcriptional
condensates. _Mol. Cells_ 75, 549–561 (2019). Article CAS Google Scholar * Ahn, J. H. et al. Phase separation drives aberrant chromatin looping and cancer development. _Nature_ 595,
591–595 (2021). Article CAS PubMed PubMed Central Google Scholar * Boehning, M. et al. RNA polymerase II clustering through carboxy-terminal domain phase separation. _Nat. Struct. Mol.
Biol._ 25, 833–840 (2018). Article CAS PubMed Google Scholar * Boija, A. et al. Transcription factors activate genes through the phase-separation capacity of their activation domains.
_Cell_ 175, 1842–1855 (2018). Article CAS PubMed Google Scholar * Cho, W.-K. et al. Mediator and RNA polymerase II clusters associate in transcription-dependent condensates. _Science_
361, 412–415 (2018). Article CAS PubMed PubMed Central Google Scholar * Wang, J. et al. Phase separation of OCT4 controls TAD reorganization to promote cell fate transitions. _Cell Stem
Cell_ 28, 1868–1883 (2021). Article CAS PubMed Google Scholar * Chong, S. et al. Imaging dynamic and selective low-complexity domain interactions that control gene transcription.
_Science_ 361, eaar2555 (2018). Article PubMed PubMed Central Google Scholar * Hall, L. L. et al. Stable C0T-1 repeat RNA Is abundant and is associated with euchromatic interphase
chromosomes. _Cell_ 156, 907–919 (2014). Article CAS PubMed PubMed Central Google Scholar * Creamer, K. M., Kolpa, H. J. & Lawrence, J. B. Nascent RNA scaffolds contribute to
chromosome territory architecture and counter chromatin compaction. _Mol. Cells_ 81, 3509–3525 (2021). Article CAS Google Scholar * Strom, A. R. et al. Phase separation drives
heterochromatin domain formation. _Nature_ 547, 241–245 (2017). Article CAS PubMed PubMed Central Google Scholar * Lu, J. Y. et al. Homotypic clustering of L1 and B1/Alu repeats
compartmentalizes the 3D genome. _Cell Res._ 31, 613–630 (2021). Article CAS PubMed PubMed Central Google Scholar * Hilbert, L. et al. Transcription organizes euchromatin via microphase
separation. _Nat. Commun._ 12, 1360 (2021). Article CAS PubMed PubMed Central Google Scholar * Plys, A. J. et al. Phase separation of Polycomb-repressive complex 1 is governed by a
charged disordered region of CBX2. _Genes Dev._ 33, 1–15 (2019). Article Google Scholar * Yap, K. et al. A short tandem repeat-enriched RNA assembles a nuclear compartment to control
alternative splicing and promote cell survival. _Mol. Cells_ 72, 525–540 (2018). Article CAS Google Scholar * Furuno, M. et al. Clusters of internally primed transcripts reveal novel long
noncoding RNAs. _PLoS Genet._ 2, e37 (2006). Article PubMed PubMed Central Google Scholar * St Laurent, G. et al. VlincRNAs controlled by retroviral elements are a hallmark of
pluripotency and cancer. _Genome Biol._ 14, R73 (2013). Article Google Scholar * Guttman, M. & Rinn, J. L. Modular regulatory principles of large non-coding RNAs. _Nature_ 482, 339–346
(2012). Article CAS PubMed PubMed Central Google Scholar * Mercer, T. R. & Mattick, J. S. Structure and function of long noncoding RNAs in epigenetic regulation. _Nat. Struct. Mol.
Biol._ 20, 300–307 (2013). Article CAS PubMed Google Scholar * Rinn, J. L. & Chang, H. Y. Long noncoding RNAs: molecular modalities to organismal functions. _Annu. Rev. Biochem._
89, 283–308 (2020). Article CAS PubMed Google Scholar * Hawkes, E. J. et al. COOLAIR antisense RNAs form evolutionarily conserved elaborate secondary structures. _Cell Rep._ 16,
3087–3096 (2016). Article CAS PubMed PubMed Central Google Scholar * Abou Alezz, M., Celli, L., Belotti, G., Lisa, A. & Bione, S. GC-AG introns features in long non-coding and
protein-coding genes suggest their role in gene expression regulation. _Front. Genet._ 11, 488 (2020). Article PubMed PubMed Central Google Scholar * Tilgner, H. et al. Deep sequencing
of subcellular RNA fractions shows splicing to be predominantly co-transcriptional in the human genome but inefficient for lncRNAs. _Genome Res._ 22, 1616–1625 (2012). Article CAS PubMed
PubMed Central Google Scholar * Mele, M. et al. Chromatin environment, transcriptional regulation, and splicing distinguish lincRNAs and mRNAs. _Genome Res._ 27, 27–37 (2017). Article CAS
PubMed PubMed Central Google Scholar * Garg, K. & Green, P. Differing patterns of selection in alternative and constitutive splice sites. _Genome Res._ 17, 1015–1022 (2007). Article
CAS PubMed PubMed Central Google Scholar * Guo, C.-J. et al. Distinct processing of lncRNAs contributes to non-conserved functions in stem cells. _Cell_ 181, 621–636 (2020). Article
CAS PubMed Google Scholar * Khan, M. R., Wellinger, R. J. & Laurent, B. Exploring the alternative splicing of long noncoding RNAs. _Trends Genet._ 37, 695–698 (2021). Article CAS
PubMed Google Scholar * Smith, M. A., Gesell, T., Stadler, P. F. & Mattick, J. S. Widespread purifying selection on RNA structure in mammals. _Nucleic Acids Res._ 41, 8220–8236 (2013).
Article CAS PubMed PubMed Central Google Scholar * Smith, M. A., Seemann, S. E., Quek, X. C. & Mattick, J. S. DotAligner: identification and clustering of RNA structure motifs.
_Genome Biol._ 18, 244 (2017). Article PubMed PubMed Central Google Scholar * Seemann, S. E. et al. The identification and functional annotation of RNA structures conserved in
vertebrates. _Genome Res._ 27, 1371–1383 (2017). Article CAS PubMed PubMed Central Google Scholar * Novikova, I. V., Hennelly, S. P. & Sanbonmatsu, K. Y. Structural architecture of
the human long non-coding RNA, steroid receptor RNA activator. _Nucleic Acids Res._ 40, 5034–5051 (2012). Article CAS PubMed PubMed Central Google Scholar * Somarowthu, S. et al. HOTAIR
forms an intricate and modular secondary structure. _Mol. Cells_ 58, 353–361 (2015). Article CAS Google Scholar * Spitale, R. C. et al. Structural imprints in vivo decode RNA regulatory
mechanisms. _Nature_ 519, 486–490 (2015). Article CAS PubMed PubMed Central Google Scholar * Chu, C. et al. Systematic discovery of Xist RNA binding proteins. _Cell_ 161, 404–416
(2015). Article CAS PubMed PubMed Central Google Scholar * Lu, Z. et al. RNA duplex map in living cells reveals higher-order transcriptome structure. _Cell_ 165, 1267–1279 (2016).
Article CAS PubMed PubMed Central Google Scholar * Kirk, J. M. et al. Functional classification of long non-coding RNAs by k-mer content. _Nat. Genet._ 50, 1474–1482 (2018). Article
CAS PubMed PubMed Central Google Scholar * Johnson, R. & Guigo, R. The RIDL hypothesis: transposable elements as functional domains of long noncoding RNAs. _RNA_ 20, 959–976 (2014).
Article CAS PubMed PubMed Central Google Scholar * Nesterova, T. B. et al. Characterization of the genomic _Xist_ locus in rodents reveals conservation of overall gene structure and
tandem repeats but rapid evolution of unique sequence. _Genome Res._ 11, 833–849 (2001). Article CAS PubMed PubMed Central Google Scholar * Wutz, A., Rasmussen, T. P. & Jaenisch, R.
Chromosomal silencing and localization are mediated by different domains of Xist RNA. _Nat. Genet._ 30, 167–174 (2002). Article CAS PubMed Google Scholar * Sunwoo, H., Colognori, D.,
Froberg, J. E., Jeon, Y. & Lee, J. T. Repeat E anchors Xist RNA to the inactive X chromosomal compartment through CDKN1A-interacting protein (CIZ1). _Proc. Natl Acad. Sci. USA_ 114,
10654–10659 (2017). Article CAS PubMed PubMed Central Google Scholar * Pintacuda, G. et al. hnRNPK recruits PCGF3/5-PRC1 to the Xist RNA B-repeat to establish polycomb-mediated
chromosomal silencing. _Mol. Cells_ 68, 955–969 (2017). Article CAS Google Scholar * Brockdorff, N. Local tandem repeat expansion in Xist RNA as a model for the functionalisation of
ncRNA. _Noncoding RNA_ 4, 28 (2018). CAS PubMed PubMed Central Google Scholar * Colognori, D., Sunwoo, H., Kriz, A. J., Wang, C.-Y. & Lee, J. T. Xist deletional analysis reveals an
interdependency between Xist RNA and Polycomb complexes for spreading along the inactive X. _Mol. Cells_ 74, 101–117 (2019). Article CAS Google Scholar * Sprague, D. et al. Nonlinear
sequence similarity between the _Xist_ and _Rsx_ long noncoding RNAs suggests shared functions of tandem repeat domains. _RNA_ 25, 1004–1019 (2019). Article CAS PubMed PubMed Central
Google Scholar * Carter, A. C. et al. Spen links RNA-mediated endogenous retrovirus silencing and X chromosome inactivation. _Elife_ 9, e54508 (2020). Article CAS PubMed PubMed Central
Google Scholar * Kelley, D. R., Hendrickson, D. G., Tenen, D. & Rinn, J. L. Transposable elements modulate human RNA abundance and splicing via specific RNA-protein interactions.
_Genome Biol._ 15, 537 (2014). Article PubMed PubMed Central Google Scholar * Percharde, M. et al. A LINE1-nucleolin partnership regulates early development and ESC identity. _Cell_ 174,
391–405 (2018). Article CAS PubMed PubMed Central Google Scholar * Chu, C., Qu, K., Zhong, F. L., Artandi, S. E. & Chang, H. Y. Genomic maps of long noncoding RNA occupancy reveal
principles of RNA-chromatin interactions. _Mol. Cells_ 44, 667–678 (2011). Article CAS Google Scholar * Mondal, T. et al. MEG3 long noncoding RNA regulates the TGF-beta pathway genes
through formation of RNA-DNA triplex structures. _Nat. Commun._ 6, 7743 (2015). Article CAS PubMed Google Scholar * Long, J. et al. Long noncoding RNA Tug1 regulates mitochondrial
bioenergetics in diabetic nephropathy. _J. Clin. Invest._ 126, 4205–4218 (2016). Article PubMed PubMed Central Google Scholar * O’Leary, V. B. et al. PARTICLE, a triplex-forming long
ncRNA, regulates locus-specific methylation in response to low-dose irradiation. _Cell Rep._ 11, 474–485 (2015). Article PubMed Google Scholar * Zhao, Z., Sentürk, N., Song, C. &
Grummt, I. lncRNA _PAPAS_ tethered to the rDNA enhancer recruits hypophosphorylated CHD4/NuRD to repress rRNA synthesis at elevated temperatures. _Genes Dev._ 32, 836–848 (2018). Article
CAS PubMed PubMed Central Google Scholar * Kuo, C.-C. et al. Detection of RNA–DNA binding sites in long noncoding RNAs. _Nucleic Acids Res._ 47, e32 (2019). Article PubMed PubMed
Central Google Scholar * Blank-Giwojna, A., Postepska-Igielska, A. & Grummt, I. lncRNA _KHPS1_ activates a poised enhancer by triplex-dependent recruitment of epigenomic regulators.
_Cell Rep._ 26, 2904–2915 (2019). Article CAS PubMed Google Scholar * Li, Y., Syed, J. & Sugiyama, H. RNA-DNA triplex formation by long noncoding RNAs. _Cell Chem. Biol._ 23,
1325–1333 (2016). Article CAS PubMed Google Scholar * Soibam, B. Super-lncRNAs: identification of lncRNAs that target super-enhancers via RNA:DNA:DNA triplex formation. _RNA_ 23,
1729–1742 (2017). Article CAS PubMed PubMed Central Google Scholar * Farabella, I., Di Stefano, M., Soler-Vila, P., Marti-Marimon, M. & Marti-Renom, M. A. Three-dimensional genome
organization via triplex-forming RNAs. _Nat. Struct. Mol. Biol._ 28, 945–954 (2021). Article CAS PubMed Google Scholar * Niehrs, C. & Luke, B. Regulatory R-loops as facilitators of
gene expression and genome stability. _Nat. Rev. Mol. Cell Biol._ 21, 167–178 (2020). Article CAS PubMed PubMed Central Google Scholar * Xu, C. et al. R-loop resolution promotes
co-transcriptional chromatin silencing. _Nat. Commun._ 12, 1790 (2021). Article CAS PubMed PubMed Central Google Scholar * Zappulla, D. C. & Cech, T. R. Yeast telomerase RNA: A
flexible scaffold for protein subunits. _Proc. Natl Acad. Sci. USA_ 101, 10024–10029 (2004). Article CAS PubMed PubMed Central Google Scholar * Maciejowski, J. & de Lange, T.
Telomeres in cancer: tumour suppression and genome instability. _Nat. Rev. Mol. Cell Biol._ 18, 175–186 (2017). Article CAS PubMed PubMed Central Google Scholar * Chen, H. et al.
Structural insights into yeast telomerase recruitment to telomeres. _Cell_ 172, 331–343 (2018). Article CAS PubMed Google Scholar * Phillips, M. L. Existence of RNA ‘dark matter’ in
doubt. _Nature_ https://doi.org/10.1038/news.2010.1248 (2010). Article PubMed PubMed Central Google Scholar * van Bakel, H., Nislow, C., Blencowe, B. J. & Hughes, T. R. Most “dark
matter” transcripts are associated with known genes. _PLoS Biol._ 8, e1000371 (2010). Article PubMed PubMed Central Google Scholar * Clark, M. B. et al. The reality of pervasive
transcription. _PLoS Biol._ 9, e1000625 (2011). Article CAS PubMed PubMed Central Google Scholar * Uroda, T. et al. Conserved pseudoknots in lncRNA MEG3 are essential for stimulation of
the p53 pathway. _Mol. Cells_ 75, 982–995 (2019). Article CAS Google Scholar * Chillón, I. & Marcia, M. The molecular structure of long non-coding RNAs: emerging patterns and
functional implications. _Crit. Rev. Biochem. Mol. Biol._ 55, 662–690 (2020). Article PubMed Google Scholar * König, J. et al. iCLIP reveals the function of hnRNP particles in splicing at
individual nucleotide resolution. _Nat. Struct. Mol. Biol._ 17, 909–915 (2010). Article PubMed PubMed Central Google Scholar * McHugh, C. A. et al. The Xist lncRNA interacts directly
with SHARP to silence transcription through HDAC3. _Nature_ 521, 232–236 (2015). Article CAS PubMed PubMed Central Google Scholar * Minajigi, A. et al. A comprehensive Xist interactome
reveals cohesin repulsion and an RNA-directed chromosome conformation. _Science_ 349, aab2276 (2015). Article PubMed Google Scholar * Rauzan, B. et al. Kinetics and thermodynamics of DNA,
RNA, and hybrid duplex formation. _Biochemistry_ 52, 765–772 (2013). Article CAS PubMed Google Scholar * Zhou, B. et al. GRID-seq for comprehensive analysis of global RNA–chromatin
interactions. _Nat. Protoc._ 14, 2036–2068 (2019). Article CAS PubMed PubMed Central Google Scholar * Bonetti, A. et al. RADICL-seq identifies general and cell type–specific principles
of genome-wide RNA-chromatin interactions. _Nat. Commun._ 11, 1018 (2020). Article CAS PubMed PubMed Central Google Scholar * Cai, Z. et al. RIC-seq for global in situ profiling of
RNA–RNA spatial interactions. _Nature_ 582, 432–437 (2020). Article CAS PubMed Google Scholar * George, L., Indig, F. E., Abdelmohsen, K. & Gorospe, M. Intracellular RNA-tracking
methods. _Open Biol._ 8, 180104 (2018). Article PubMed PubMed Central Google Scholar * Fazal, F. M. et al. Atlas of subcellular RNA localization revealed by APEX-seq. _Cell_ 178, 473–490
(2019). Article CAS PubMed PubMed Central Google Scholar * Li, P., Zhou, X., Xu, K. & Zhang, Q. C. RASP: an atlas of transcriptome-wide RNA secondary structure probing data.
_Nucleic Acids Res._ 49, D183–D191 (2020). Article PubMed Central Google Scholar * Wang, X.-W., Liu, C.-X., Chen, L.-L. & Zhang, Q. C. RNA structure probing uncovers RNA
structure-dependent biological functions. _Nat. Chem. Biol._ 17, 755–766 (2021). Article CAS PubMed Google Scholar * Cao, H. & Kapranov, P. Methods to analyze the non-coding RNA
interactome — recent advances and challenges. _Front. Genet._ 13, 857759 (2022). Article CAS PubMed PubMed Central Google Scholar * Sanbonmatsu, K. Getting to the bottom of lncRNA
mechanism: structure–function relationships. _Mamm. Genome_ 33, 343–353 (2021). Article PubMed PubMed Central Google Scholar * Wutz, A. et al. Non-imprinted Igf2r expression decreases
growth and rescues the Tme mutation in mice. _Development_ 128, 1881–1887 (2001). Article CAS PubMed Google Scholar * Ballarino, M. et al. Deficiency in the nuclear long noncoding RNA
Charme causes myogenic defects and heart remodeling in mice. _EMBO J._ 37, e99697 (2018). Article PubMed PubMed Central Google Scholar * Rom, A. et al. Regulation of CHD2 expression by
the _Chaserr_ long noncoding RNA gene is essential for viability. _Nat. Commun._ 10, 5092 (2019). Article PubMed PubMed Central Google Scholar * Grote, P. et al. The tissue-specific
lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. _Dev. Cell_ 24, 206–214 (2013). Article CAS PubMed PubMed Central Google Scholar * Gabory, A. et
al. H19 acts as a trans regulator of the imprinted gene network controlling growth in mice. _Development_ 136, 3413–3421 (2009). Article CAS PubMed Google Scholar * Ritter, N. et al.
The lncRNA locus Handsdown regulates cardiac gene programs and is essential for early mouse development. _Dev. Cell_ 50, 644–657 (2019). Article CAS PubMed Google Scholar * Fitzpatrick,
G. V., Soloway, P. D. & Higgins, M. J. Regional loss of imprinting and growth deficiency in mice with a targeted deletion of KvDMR1. _Nat. Genet._ 32, 426–431 (2002). Article CAS
PubMed Google Scholar * Zhou, B. et al. Endogenous retrovirus-derived long noncoding RNA enhances innate immune responses via derepressing RELA expression. _mBio_ 10, e00937-19 (2019).
Article PubMed PubMed Central Google Scholar * Elling, R. et al. Genetic models reveal _cis_ and _trans_ immune-regulatory activities for lincRNA-Cox2. _Cell Rep._ 25, 1511–1524 (2018).
Article CAS PubMed PubMed Central Google Scholar * Jiang, M. et al. Self-recognition of an inducible host lncRNA by RIG-I feedback restricts innate immune response. _Cell_ 173, 906–919
(2018). Article CAS PubMed Google Scholar * Takahashi, N. et al. Deletion of Gtl2, imprinted non-coding RNA, with its differentially methylated region induces lethal
parent-origin-dependent defects in mice. _Hum. Mol. Genet._ 18, 1879–1888 (2009). Article CAS PubMed Google Scholar * Zhou, Y. et al. Activation of paternally expressed genes and
perinatal death caused by deletion of the Gtl2 gene. _Development_ 137, 2643–2652 (2010). Article CAS PubMed PubMed Central Google Scholar * Kopp, F. et al. PUMILIO hyperactivity drives
premature aging of Norad-deficient mice. _Elife_ 8, e42650 (2019). Article PubMed PubMed Central Google Scholar * Andersen, R. E. et al. The long noncoding RNA Pnky is a trans-acting
regulator of cortical development in vivo. _Dev. Cell_ 49, 632–642 (2019). Article CAS PubMed PubMed Central Google Scholar * Lewandowski, J. P. et al. The _Tug1_ lncRNA locus is
essential for male fertility. _Genome Biol._ 21, 237 (2020). Article CAS PubMed PubMed Central Google Scholar * Marahrens, Y., Panning, B., Dausman, J., Strauss, W. & Jaenisch, R.
Xist-deficient mice are defective in dosage compensation but not spermatogenesis. _Genes Dev._ 11, 156–166 (1997). Article CAS PubMed Google Scholar * Hacisuleyman, E. et al. Topological
organization of multichromosomal regions by the long intergenic noncoding RNA _Firre_. _Nat. Struct. Mol. Biol._ 21, 198–206 (2014). Article CAS PubMed PubMed Central Google Scholar *
Maass, P. G., Barutcu, A. R., Weiner, C. L. & Rinn, J. L. Inter-chromosomal contact properties in live-cell imaging and in Hi-C. _Mol. Cells_ 69, 1039–1045 (2018). Article CAS Google
Scholar * West, J. A. et al. Structural, super-resolution microscopy analysis of paraspeckle nuclear body organization. _J. Cell Biol._ 214, 817–830 (2016). Article CAS PubMed PubMed
Central Google Scholar * Wilusz, J. E. et al. A triple helix stabilizes the 3′ ends of long noncoding RNAs that lack poly(A) tails. _Genes Dev._ 26, 2392–2407 (2012). Article CAS PubMed
PubMed Central Google Scholar * Tripathi, V. et al. SRSF1 regulates the assembly of pre-mRNA processing factors in nuclear speckles. _Mol. Biol. Cell_ 23, 3694–3706 (2012). Article CAS
PubMed PubMed Central Google Scholar * Fei, J. et al. Quantitative analysis of multilayer organization of proteins and RNA in nuclear speckles at super resolution. _J. Cell Sci._ 130,
4180–4192 (2017). CAS PubMed PubMed Central Google Scholar * Krause, H. M. New and prospective roles for lncRNAs in organelle formation and function. _Trends Genet._ 34, 736–745 (2018).
Article CAS PubMed Google Scholar * Kretz, M. et al. Control of somatic tissue differentiation by the long non-coding RNA TINCR. _Nature_ 493, 231–235 (2013). Article CAS PubMed
Google Scholar * Aguilar, R. et al. Targeting Xist with compounds that disrupt RNA structure and X inactivation. _Nature_ 604, 160–166 (2022). Article CAS PubMed Google Scholar Download
references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * School of Biotechnology and Biomolecular Sciences, UNSW, Sydney, NSW, Australia John S. Mattick & Marcel E. Dinger * UNSW RNA
Institute, UNSW, Sydney, NSW, Australia John S. Mattick & Marcel E. Dinger * INSPER Institute of Education and Research, São Paulo, Brazil Paulo P. Amaral * RIKEN Center for Integrative
Medical Sciences, Yokohama, Japan Piero Carninci * Human Technopole, Milan, Italy Piero Carninci * Department of Molecular, Cell and Developmental Biology, University of California, Santa
Cruz, Santa Cruz, CA, USA Susan Carpenter * Center for Personal Dynamics Regulomes, Stanford University School of Medicine, Stanford, CA, USA Howard Y. Chang * Department of Dermatology,
Stanford, CA, USA Howard Y. Chang * Department of Genetics, Stanford University School of Medicine, Stanford, CA, USA Howard Y. Chang * Howard Hughes Medical Institute, Stanford University
School of Medicine, Stanford, CA, USA Howard Y. Chang * CAS Center for Excellence in Molecular Cell Science, Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences,
Shanghai, China Ling-Ling Chen * Key Laboratory of RNA Biology, Center for Big Data Research in Health, Institute of Biophysics, Chinese Academy of Sciences, Beijing, China Runsheng Chen *
John Innes Centre, Norwich Research Park, Norwich, UK Caroline Dean * Division of Innate Immunity, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, MA, USA
Katherine A. Fitzgerald * Cold Spring Harbour Laboratory, Cold Spring Harbour, NY, USA Thomas R. Gingeras & David L. Spector * Division of Biology and Biological Engineering, California
Institute of Technology, Pasadena, CA, USA Mitchell Guttman * Graduate School of Frontier Biosciences, Osaka University, Osaka, Japan Tetsuro Hirose * Department of Gene Therapy and
Regulation of Gene Expression, Center for Applied Medical Research, University of Navarra, Pamplona, Spain Maite Huarte * Institute of Health Research of Navarra, Pamplona, Spain Maite
Huarte * School of Biology and Environmental Science, University College Dublin, Dublin, Ireland Rory Johnson * Conway Institute for Biomolecular and Biomedical Research, University College
Dublin, Dublin, Ireland Rory Johnson * Department of Medical Biochemistry and Cell Biology, Institute of Biomedicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
Chandrasekhar Kanduri * Institute of Genomics, School of Medicine, Huaqiao University, Xiamen, China Philipp Kapranov * Department of Neurology, University of Massachusetts Chan Medical
School, Worcester, MA, USA Jeanne B. Lawrence * Department of Molecular Biology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA Jeannie T. Lee * Department of
Genetics, Harvard Medical School, Boston, MA, USA Jeannie T. Lee * Howard Hughes Medical Institute, UT Southwestern Medical Center, Dallas, TX, USA Joshua T. Mendell * Department of
Molecular Biology, UT Southwestern Medical Center, Dallas, TX, USA Joshua T. Mendell * Australian Institute for Bioengineering and Nanotechnology, University of Queensland, Brisbane, QLD,
Australia Timothy R. Mercer * Department of Medicine, New York University Grossman School of Medicine, New York, NY, USA Kathryn J. Moore * RNA Biology Laboratory, Faculty of Pharmaceutical
Sciences, Hokkaido University, Sapporo, Japan Shinichi Nakagawa * Department of Biochemistry, University of Colorado Boulder, Boulder, CO, USA John L. Rinn * BioFrontiers Institute,
University of Colorado Boulder, Boulder, CO, USA John L. Rinn * Howard Hughes Medical Institute, University of Colorado Boulder, Boulder, CO, USA John L. Rinn * Department of Biological
Regulation, Weizmann Institute of Science, Rehovot, Israel Igor Ulitsky * Laboratory of RNA Genomics and Structure, Genome Institute of Singapore, A*STAR, Singapore, Singapore Yue Wan *
Department of Biochemistry, National University of Singapore, Singapore, Singapore Yue Wan * Verna and Marrs McLean Department of Biochemistry and Molecular Biology, Therapeutic Innovation
Center, Baylor College of Medicine, Houston, TX, USA Jeremy E. Wilusz * Translational Research Institute, Henan Provincial People’s Hospital, Academy of Medical Science, Zhengzhou
University, Zhengzhou, China Mian Wu Authors * John S. Mattick View author publications You can also search for this author inPubMed Google Scholar * Paulo P. Amaral View author publications
You can also search for this author inPubMed Google Scholar * Piero Carninci View author publications You can also search for this author inPubMed Google Scholar * Susan Carpenter View
author publications You can also search for this author inPubMed Google Scholar * Howard Y. Chang View author publications You can also search for this author inPubMed Google Scholar *
Ling-Ling Chen View author publications You can also search for this author inPubMed Google Scholar * Runsheng Chen View author publications You can also search for this author inPubMed
Google Scholar * Caroline Dean View author publications You can also search for this author inPubMed Google Scholar * Marcel E. Dinger View author publications You can also search for this
author inPubMed Google Scholar * Katherine A. Fitzgerald View author publications You can also search for this author inPubMed Google Scholar * Thomas R. Gingeras View author publications
You can also search for this author inPubMed Google Scholar * Mitchell Guttman View author publications You can also search for this author inPubMed Google Scholar * Tetsuro Hirose View
author publications You can also search for this author inPubMed Google Scholar * Maite Huarte View author publications You can also search for this author inPubMed Google Scholar * Rory
Johnson View author publications You can also search for this author inPubMed Google Scholar * Chandrasekhar Kanduri View author publications You can also search for this author inPubMed
Google Scholar * Philipp Kapranov View author publications You can also search for this author inPubMed Google Scholar * Jeanne B. Lawrence View author publications You can also search for
this author inPubMed Google Scholar * Jeannie T. Lee View author publications You can also search for this author inPubMed Google Scholar * Joshua T. Mendell View author publications You can
also search for this author inPubMed Google Scholar * Timothy R. Mercer View author publications You can also search for this author inPubMed Google Scholar * Kathryn J. Moore View author
publications You can also search for this author inPubMed Google Scholar * Shinichi Nakagawa View author publications You can also search for this author inPubMed Google Scholar * John L.
Rinn View author publications You can also search for this author inPubMed Google Scholar * David L. Spector View author publications You can also search for this author inPubMed Google
Scholar * Igor Ulitsky View author publications You can also search for this author inPubMed Google Scholar * Yue Wan View author publications You can also search for this author inPubMed
Google Scholar * Jeremy E. Wilusz View author publications You can also search for this author inPubMed Google Scholar * Mian Wu View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS All authors researched data for the article, made substantial contributions to discussion of the content and reviewed and/or edited the manuscript
before submission. J.S.M. drafted the manuscript. CORRESPONDING AUTHOR Correspondence to John S. Mattick. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests.
PEER REVIEW PEER REVIEW INFORMATION _Nature Reviews Molecular Cell Biology_ and the authors thank Thomas Cech, Xiangting Wang and the other, anonymous, reviewer(s) for their contribution to
the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
RELATED LINKS FANTOM: http://fantom.gsc.riken.jp/cat/ GENCODE: https://www.gencodegenes.org HUGO GENE NOMENCLATURE COMMITTEE: https://www.genenames.org/about/guidelines/#!/#tocAnchor-1-4
GLOSSARY * Cajal bodies Nuclear structures often associated with the nucleolus that have important roles in RNA metabolism and the formation of ribonucleoproteins involved in transcription,
splicing, ribosome biogenesis and telomere maintenance. * Coacervates Condensed liquid-like droplets formed by oppositely charged macromolecules, in vivo involving the interaction of
positively charged amino acids in the intrinsically disordered regions of proteins with the negatively charged backbone of RNAs. * GC–AG splice sites A non-canonical variant of the major
U2-type GT–AG splice junctions. * Intrinsically disordered region A polypeptide segment containing a high proportion of polar or charged residues and insufficient hydrophobic residues to
form a stable tertiary structure. * Modified RNA mimics Chemically synthesized RNA molecules containing chemical modifications to increase their stability or target affinity, facilitate
their action and/or bypass detection by innate immunity. * Nuclear speckles Irregularly shaped nuclear domains enriched in pre-mRNA splicing factors, located in the interchromatin regions of
the nucleoplasm of mammalian cells. * Pol V transcripts Short RNAs transcribed by a specialized plant-specific RNA polymerase (Pol) which maintain the repression of transposons and genomic
repeats. * Polycomb bodies Nuclear foci of Polycomb group proteins, within which Polycomb group protein-bound regions of DNA are localized and contact each other. * Pioneer transcription
factors Proteins that have the unique ability to bind target sites in closed chromatin and open closed chromatin to activate gene expression and implement new cell fates. * Primary
transcription units The long precursor RNAs transcribed from chromosomal regions (‘genes’) before splicing and assembly of the exons into an mRNA or long non-coding RNA and before processing
of the intronic RNAs into _trans_-acting smaller RNAs. * Quantitative traits Phenotypic characteristics that vary continuously in natural populations, including many aspects of morphology,
physiology, behaviour and disease susceptibility. * Small interspersed nuclear elements Repetitive non-coding sequences of 100–600 bp that are common in animal and plant genomes. They are
derived from retrotransposons and are propagated through an RNA intermediate * Super-enhancers Enhancer-dense genomic regions found near genes that have key roles in determining cellular
identity. * Transcription factor hubs Complex topological assemblies, in which multiple genes and regulatory factors interact with each other. * X inactivation centre A complex locus in the
X chromosome that is required for the near-global inactivation of one of the two X chromosomes in female mammals, a spreading process initiated by the expression of _XIST_. RIGHTS AND
PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s);
author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Mattick, J.S., Amaral, P.P., Carninci, P. _et al._ Long non-coding RNAs: definitions, functions, challenges and recommendations. _Nat Rev Mol Cell Biol_ 24,
430–447 (2023). https://doi.org/10.1038/s41580-022-00566-8 Download citation * Accepted: 16 November 2022 * Published: 03 January 2023 * Issue Date: June 2023 * DOI:
https://doi.org/10.1038/s41580-022-00566-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative