Play all audios:
ABSTRACT Bariatric surgery is an effective therapy for obesity, hypertension and type 2 diabetes mellitus that is refractory to maximal medical therapy. Results of long-term cohort studies
and emerging evidence from randomized clinical trials have revealed that, in addition to its beneficial effects on weight reduction, blood pressure and metabolic control, bariatric surgery
might reduce the incidence and long-term progression of chronic kidney disease (CKD). Preclinical studies have provided experimental verification that bariatric surgery improves key
parameters of kidney injury at the functional, structural and ultrastructural levels, and effects a programme of transcriptomic change in the kidney that is coherent with injury resolution.
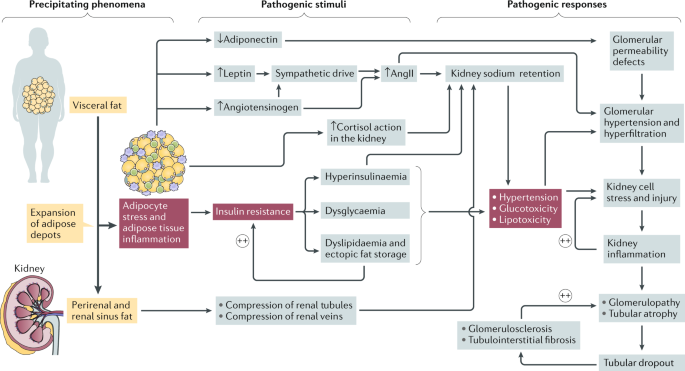
Multiple mechanisms explain these observations, ranging from predictable aspects of risk-factor reduction to some novel and unforeseen renoprotective benefits of surgery. Current evidence
therefore supports the judicious use of bariatric surgery to treat patients with obesity, diabetes and CKD. Optimizing the benefits of surgery requires careful patient selection and
consideration of how to identify and mitigate some of the challenges associated with these surgical procedures. KEY POINTS * A causal link between elevated BMI and the incidence and
progression of chronic kidney disease (CKD) is now well substantiated. * Bariatric surgery reduces risk factors implicated in the progression of kidney injury in obesity and type 2 diabetes
mellitus. * Long-term outcomes of bariatric surgery confirm the role of obesity as a modifiable risk factor for advanced CKD. * Preclinical studies demonstrate that bariatric surgery
improves biochemical, structural and ultrastructural measures of experimental diabetic kidney disease and interrupts the transcriptional programme characteristic of progressive CKD. *
Definitive randomized clinical trial studies comparing kidney outcomes and safety following best medical therapy alone or best medical therapy in combination with bariatric surgery at
various stages of disease progression are required. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS
Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $32.99 / 30 days cancel any time Learn more
Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full
article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs *
Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS REMISSION AND PROGRESSION OF PRE-EXISTING MICRO- AND MACROALBUMINURIA OVER 15 YEARS AFTER BARIATRIC SURGERY IN SWEDISH OBESE
SUBJECTS STUDY Article 07 November 2020 OBESITY AND THE KIDNEY: MECHANISTIC LINKS AND THERAPEUTIC ADVANCES Article 13 February 2024 SLEEVE GASTRECTOMY AMELIORATES RENAL INJURY IN
OBESITY-COMBINED HYPERURICEMIC NEPHROPATHY MICE BY MODULATING THE AMPK/NRF2/ABCG2 PATHWAY Article Open access 01 October 2024 REFERENCES * Kyle, T. K., Dhurandhar, E. J. & Allison, D. B.
Regarding obesity as a disease: evolving policies and their implications. _Endocrinol. Metab. Clin. North. Am._ 45, 511–520 (2016). PubMed PubMed Central Google Scholar * NCD Risk Factor
Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. _Lancet_
386, 1377–1396 (2016). Google Scholar * Lim, C. C. et al. Elevated serum leptin, adiponectin and leptin to adiponectin ratio is associated with chronic kidney disease in Asian adults. _PloS
ONE_ 10, e0122009 (2015). PubMed PubMed Central Google Scholar * Foster, M. C. et al. Overweight, obesity, and the development of stage 3 CKD: the Framingham heart study. _Am. J. Kidney
Dis._ 52, 39–48 (2008). PubMed PubMed Central Google Scholar * Sinclair, P., Brennan, D. J. & le Roux, C. W. Gut adaptation after metabolic surgery and its influences on the brain,
liver and cancer. _Nat. Rev. Gastroenterol. Hepatol._ 15, 606–624 (2018). CAS PubMed Google Scholar * Sinclair, P., Docherty, N. & le Roux, C. W. Metabolic effects of bariatric
surgery. _Clin. Chem._ 64, 72–81 (2018). CAS PubMed Google Scholar * D’Agati, V. D. et al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. _Nat.
Rev. Nephrol._ 12, 453–471 (2016). PubMed Google Scholar * Garofalo, C. et al. A systematic review and meta-analysis suggests obesity predicts onset of chronic kidney disease in the
general population. _Kidney Int._ 91, 1224–1235 (2017). PubMed Google Scholar * Vivante, A. et al. Body mass index in 1.2 million adolescents and risk for end-stage renal disease. _Arch.
Intern. Med._ 172, 1644–1650 (2012). PubMed PubMed Central Google Scholar * Hsu, C. Y., McCulloch, C. E., Iribarren, C., Darbinian, J. & Go, A. S. Body mass index and risk for
end-stage renal disease. _Ann. Intern. Med._ 144, 21–28 (2006). PubMed Google Scholar * Ntuk, U. E., Gill, J. M., Mackay, D. F., Sattar, N. & Pell, J. P. Ethnic-specific obesity
cutoffs for diabetes risk: cross-sectional study of 490,288 UK biobank participants. _Diabetes Care_ 37, 2500–2507 (2014). PubMed Google Scholar * Xu, H. et al. Higher body mass index is
associated with incident diabetes and chronic kidney disease independent of genetic confounding. _Kidney Int._ 95, 1225–1233 (2019). PubMed Google Scholar * Hall, J. E., do Carmo, J. M.,
da Silva, A. A., Wang, Z. & Hall, M. E. Obesity, kidney dysfunction and hypertension: mechanistic links. _Nat. Rev. Nephrol._ 15, 367–385 (2019). PubMed PubMed Central Google Scholar
* Freedland, E. S. Role of a critical visceral adipose tissue threshold (CVATT) in metabolic syndrome: implications for controlling dietary carbohydrates: a review. _Nutr. Metab._ 1, 12
(2004). Google Scholar * Zhu, Q. & Scherer, P. E. Immunologic and endocrine functions of adipose tissue: implications for kidney disease. _Nat. Rev. Nephrol._ 14, 105–120 (2018). CAS
PubMed Google Scholar * Thomas, M. C. et al. Diabetic kidney disease. _Nat. Rev. Dis. Prim._ 1, 15018 (2015). PubMed Google Scholar * Vallon, V. & Docherty, N. G. Intestinal
regulation of urinary sodium excretion and the pathophysiology of diabetic kidney disease: a focus on glucagon-like peptide 1 and dipeptidyl peptidase 4. _Exp. Physiol._ 99, 1140–1145
(2014). CAS PubMed PubMed Central Google Scholar * de Vries, A. P. et al. Fatty kidney: emerging role of ectopic lipid in obesity-related renal disease. _Lancet Diabetes Endocrinol._ 2,
417–426 (2014). PubMed Google Scholar * Choi, S. R. et al. Adiponectin receptor agonist AdipoRon decreased ceramide, and lipotoxicity, and ameliorated diabetic nephropathy. _Metab. Clin.
Exp._ 85, 348–360 (2018). CAS PubMed Google Scholar * Lennon, R. et al. Saturated fatty acids induce insulin resistance in human podocytes: implications for diabetic nephropathy.
_Nephrol. Dial. Transplant._ 24, 3288–3296 (2009). CAS PubMed Google Scholar * Chandran, M., Phillips, S. A., Ciaraldi, T. & Henry, R. R. Adiponectin: more than just another fat cell
hormone? _Diabetes Care_ 26, 2442–2450 (2003). CAS PubMed Google Scholar * Yamauchi, T. et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating
AMP-activated protein kinase. _Nat. Med._ 8, 1288–1295 (2002). CAS PubMed Google Scholar * Sharma, K. et al. Adiponectin regulates albuminuria and podocyte function in mice. _J. Clin.
Invest._ 118, 1645–1656 (2008). CAS PubMed PubMed Central Google Scholar * Kim, Y. & Park, C. W. Mechanisms of adiponectin action: implication of adiponectin receptor agonism in
diabetic kidney disease. _Int. J. Mol. Sci._ 20, 1782 (2019). CAS PubMed Central Google Scholar * Briffa, J. F., McAinch, A. J., Poronnik, P. & Hryciw, D. H. Adipokines as a link
between obesity and chronic kidney disease. _Am. J. Physiol. Renal Physiol._ 305, F1629–F1636 (2013). CAS PubMed Google Scholar * Cnop, M. et al. Relationship of adiponectin to body fat
distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. _Diabetologia_ 46, 459–469 (2003). CAS PubMed Google Scholar * Kern, P. A., Di
Gregorio, G. B., Lu, T., Rassouli, N. & Ranganathan, G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha
expression. _Diabetes_ 52, 1779–1785 (2003). CAS PubMed Google Scholar * Scheja, L. & Heeren, J. The endocrine function of adipose tissues in health and cardiometabolic disease. _Nat.
Rev. Endocrinol._ 15, 507–524 (2019). CAS PubMed Google Scholar * La Cava, A. Leptin in inflammation and autoimmunity. _Cytokine_ 98, 51–58 (2017). PubMed PubMed Central Google Scholar
* Caron, A., Lee, S., Elmquist, J. K. & Gautron, L. Leptin and brain-adipose crosstalks. _Nat. Rev. Neurosci._ 19, 153–165 (2018). CAS PubMed PubMed Central Google Scholar * Shand,
B. I., Scott, R. S., Elder, P. A. & George, P. M. Plasma adiponectin in overweight, nondiabetic individuals with or without insulin resistance. _Diabetes Obes. Metab._ 5, 349–353
(2003). CAS PubMed Google Scholar * Oosterhuis, N. R. et al. Extravascular renal denervation ameliorates juvenile hypertension and renal damage resulting from experimental hyperleptinemia
in rats. _J. Hypertens._ 35, 2537–2547 (2017). CAS PubMed Google Scholar * Shi, Z., Li, B. & Brooks, V. L. Role of the paraventricular nucleus of the hypothalamus in the
sympathoexcitatory effects of leptin. _Hypertension_ 66, 1034–1041 (2015). CAS PubMed PubMed Central Google Scholar * Faulkner, J. L. & Belin de Chantemele, E. J. Leptin and
aldosterone. _Vitam. Horm._ 109, 265–284 (2019). CAS PubMed Google Scholar * Yiannikouris, F. et al. Adipocyte deficiency of angiotensinogen prevents obesity-induced hypertension in male
mice. _Hypertension_ 60, 1524–1530 (2012). CAS PubMed PubMed Central Google Scholar * Nakamura, M. et al. Stimulatory effect of insulin on renal proximal tubule sodium transport is
preserved in type 2 diabetes with nephropathy. _Biochem. Biophys. Res. Commun._ 461, 154–158 (2015). CAS PubMed Google Scholar * Artunc, F. et al. The impact of insulin resistance on the
kidney and vasculature. _Nat. Rev. Nephrol._ 12, 721–737 (2016). CAS PubMed Google Scholar * Lay, A. C. et al. Prolonged exposure of mouse and human podocytes to insulin induces insulin
resistance through lysosomal and proteasomal degradation of the insulin receptor. _Diabetologia_ 60, 2299–2311 (2017). CAS PubMed PubMed Central Google Scholar * Bailey, M. A.
11beta-hydroxysteroid dehydrogenases and hypertension in the metabolic syndrome. _Curr. Hypertens. Rep._ 19, 100 (2017). PubMed PubMed Central Google Scholar * Gant, C. M. et al. Lower
renal function is associated with derangement of 11-beta hydroxysteroid dehydrogenase in type 2 diabetes. _J. Endocr. Soc._ 2, 609–620 (2018). CAS PubMed PubMed Central Google Scholar *
Standeven, K. F. et al. Neprilysin, obesity and the metabolic syndrome. _Int. J. Obes._ 35, 1031–1040 (2011). CAS Google Scholar * Lamacchia, O. et al. Para- and perirenal fat thickness is
an independent predictor of chronic kidney disease, increased renal resistance index and hyperuricaemia in type-2 diabetic patients. _Nephrol. Dial. Transplant._ 26, 892–898 (2011). PubMed
Google Scholar * Foster, M. C. et al. Fatty kidney, hypertension, and chronic kidney disease: the Framingham Heart Study. _Hypertension_ 58, 784–790 (2011). CAS PubMed PubMed Central
Google Scholar * Welbourn, R. et al. Bariatric surgery worldwide: baseline demographic description and one-year outcomes from the second IFSO global registry report 2013–2015. _Obes. Surg._
28, 313–322 (2018). PubMed Google Scholar * DeMaria, E. J., Pate, V., Warthen, M. & Winegar, D. A. Baseline data from American society for metabolic and bariatric surgery-designated
bariatric surgery centers of excellence using the bariatric outcomes longitudinal database. _Surg. Obes. Relat. Dis._ 6, 347–355 (2010). PubMed Google Scholar * Fried, M. et al.
Interdisciplinary European guidelines on metabolic and bariatric surgery. _Obes. Surg._ 24, 42–55 (2014). CAS PubMed Google Scholar * Rubino, F. et al. Metabolic surgery in the treatment
algorithm for type 2 diabetes: a joint statement by international diabetes organizations. _Diabetes Care_ 39, 861–877 (2016). CAS PubMed Google Scholar * Peterli, R. et al. Laparoscopic
sleeve gastrectomy versus Roux-Y-Gastric bypass for morbid obesity-3-year outcomes of the prospective randomized swiss multicenter bypass or sleeve study (SM-BOSS). _Ann. Surg._ 265, 466–473
(2017). PubMed Google Scholar * Salminen, P. et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y Gastric bypass on weight loss at 5 years among patients with morbid
obesity: the SLEEVEPASS randomized clinical trial. _JAMA_ 319, 241–254 (2018). PubMed PubMed Central Google Scholar * Kissler, H. J. & Settmacher, U. Bariatric surgery to treat
obesity. _Semin. Nephrol._ 33, 75–89 (2013). PubMed Google Scholar * Wolfe, B. M., Kvach, E. & Eckel, R. H. Treatment of obesity: weight loss and bariatric surgery. _Circ. Res._ 118,
1844–1855 (2016). CAS PubMed PubMed Central Google Scholar * Thereaux, J. et al. Long-term adverse events after sleeve gastrectomy or gastric bypass: a 7-year nationwide, observational,
population-based, cohort study. _Lancet Diabetes Endocrinol._ 7, 786–795 (2019). PubMed Google Scholar * Hofso, D. et al. Gastric bypass versus sleeve gastrectomy in patients with type 2
diabetes (Oseberg): a single-centre, triple-blind, randomised controlled trial. _Lancet Diabetes Endocrinol._ 7, 912–924 (2019). PubMed Google Scholar * Sheetz, K. H. et al. Trends in
bariatric surgery procedures among patients with ESKD in the United States. _Clin. J. Am. Soc. Nephrol._ 14, 1193–1199 (2019). PubMed PubMed Central Google Scholar * Sjostrom, L. Review
of the key results from the Swedish Obese Subjects (SOS) trial — a prospective controlled intervention study of bariatric surgery. _J. Intern. Med._ 273, 219–234 (2013). CAS PubMed Google
Scholar * Sjöström, L. et al. Effects of bariatric surgery on mortality in Swedish obese subjects. _N. Engl. J. Med._ 357, 741–752 (2007). PubMed Google Scholar * Eliasson, B. et al.
Cardiovascular disease and mortality in patients with type 2 diabetes after bariatric surgery in Sweden: a nationwide, matched, observational cohort study. _Lancet Diabetes Endocrinol._ 3,
847–854 (2015). PubMed Google Scholar * Adams, T. D. et al. Long-term mortality after gastric bypass surgery. _N. Engl. J. Med._ 357, 753–761 (2007). CAS PubMed Google Scholar *
Sjostrom, L. et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. _N. Engl. J. Med._ 351, 2683–2693 (2004). PubMed Google Scholar * le Roux, C. W.
et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. _Ann. Surg._ 243, 108–114 (2006). PubMed PubMed
Central Google Scholar * Docherty, N. G. & le Roux, C. W. Reconfiguration of the small intestine and diabetes remitting effects of Roux-en-Y gastric bypass surgery. _Curr. Opin.
Gastroenterol._ 32, 61–66 (2016). PubMed Google Scholar * Saeidi, N. et al. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. _Science_ 341,
406–410 (2013). CAS PubMed PubMed Central Google Scholar * Quercia, I., Dutia, R., Kotler, D. P., Belsley, S. & Laferrere, B. Gastrointestinal changes after bariatric surgery.
_Diabetes Metab._ 40, 87–94 (2014). CAS PubMed Google Scholar * Bojsen-Møller, K. N. et al. Early enhancements of hepatic and later of peripheral insulin sensitivity combined with
increased postprandial insulin secretion contribute to improved glycemic control after Roux-en-Y gastric bypass. _Diabetes_ 63, 1725–1737 (2014). PubMed Google Scholar * Cummings, D. E. et
al. Gastric bypass surgery vs intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomised controlled trial. _Diabetologia_ 59, 945–953 (2016). CAS PubMed
PubMed Central Google Scholar * Ikramuddin, S. et al. Roux-en-Y gastric bypass for diabetes (the diabetes surgery study): 2-year outcomes of a 5-year, randomised, controlled trial. _Lancet
Diabetes Endocrinol._ 3, 413–422 (2015). PubMed PubMed Central Google Scholar * Mingrone, G. et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. _N. Engl.
J. Med._ 366, 1577–1585 (2012). CAS PubMed Google Scholar * Courcoulas, A. P. et al. Three-year outcomes of bariatric surgery vs lifestyle intervention for type 2 diabetes mellitus
treatment: a randomized clinical trial. _JAMA Surg._ 150, 931–940 (2015). PubMed PubMed Central Google Scholar * Dixon, J. B. et al. Adjustable gastric banding and conventional therapy
for type 2 diabetes: a randomized controlled trial. _JAMA_ 299, 316–323 (2008). CAS PubMed Google Scholar * Gloy, V. L. et al. Bariatric surgery versus non-surgical treatment for obesity:
a systematic review and meta-analysis of randomised controlled trials. _BMJ_ 347, f5934 (2013). PubMed PubMed Central Google Scholar * Carlsson, L. M. et al. Bariatric surgery and
prevention of type 2 diabetes in Swedish obese subjects. _N. Engl. J. Med._ 367, 695–704 (2012). CAS PubMed Google Scholar * Hallersund, P. et al. Gastric bypass surgery is followed by
lowered blood pressure and increased diuresis - long term results from the Swedish obese subjects (SOS) study. _PLoS ONE_ 7, e49696 (2012). CAS PubMed PubMed Central Google Scholar *
Schiavon, C. A. et al. Effects of bariatric surgery in obese patients with hypertension: the GATEWAY randomized trial (gastric bypass to treat obese patients with steady hypertension).
_Circulation_ 137, 1132–1142 (2018). PubMed Google Scholar * Rosenstock, J. L., Pommier, M., Stoffels, G., Patel, S. & Michelis, M. F. Prevalence of proteinuria and albuminuria in an
obese population and associated risk factors. _Front. Med._ 5, 122 (2018). Google Scholar * Hallan, S. I. et al. Combining GFR and albuminuria to classify CKD improves prediction of ESRD.
_J. Am. Soc. Nephrol._ 20, 1069–1077 (2009). CAS PubMed PubMed Central Google Scholar * Gaede, P., Tarnow, L., Vedel, P., Parving, H. H. & Pedersen, O. Remission to normoalbuminuria
during multifactorial treatment preserves kidney function in patients with type 2 diabetes and microalbuminuria. _Nephrol. Dial. Transplant._ 19, 2784–2788 (2004). PubMed Google Scholar *
Heerspink, H. J., Kropelin, T. F., Hoekman, J. & de Zeeuw, D. Drug-induced reduction in albuminuria is associated with subsequent renoprotection: a meta-analysis. _J. Am. Soc. Nephrol._
26, 2055–2064 (2015). CAS PubMed Google Scholar * Bilha, S. C. et al. The effects of bariatric surgery on renal outcomes: a systematic review and meta-analysis. _Obes. Surg._ 28,
3815–3833 (2018). PubMed Google Scholar * Li, K. et al. Effects of bariatric surgery on renal function in obese patients: a systematic review and meta analysis. _PLoS ONE_ 11, e0163907
(2016). PubMed PubMed Central Google Scholar * Scheurlen, K. M. et al. Metabolic surgery improves renal injury independent of weight loss: a meta-analysis. _Surg. Obes. Relat. Dis._ 15,
1006–1020 (2019). PubMed Google Scholar * Herder, C. et al. Adiponectin and bariatric surgery: associations with diabetes and cardiovascular disease in the Swedish Obese Subjects study.
_Diabetes Care_ 37, 1401–1409 (2014). CAS PubMed Google Scholar * Stephens, J. W. et al. Temporal effects of laparoscopic sleeve gastrectomy on adipokines, inflammation, and oxidative
stress in patients with impaired glucose homeostasis. _Surg. Obes. Relat. Dis._ 15, 2011–2017 (2019). PubMed Google Scholar * Unamuno, X. et al. Increase of the adiponectin/leptin ratio in
patients with obesity and type 2 diabetes after Roux-en-Y gastric bypass. _Nutrients_ 11, 2069 (2019). CAS PubMed Central Google Scholar * Billeter, A. T. et al. Meta-analysis of
metabolic surgery versus medical treatment for microvascular complications in patients with type 2 diabetes mellitus. _Br. J. Surg._ 105, 168–181 (2018). CAS PubMed Google Scholar *
Bjornstad, P. et al. Effect of surgical versus medical therapy on diabetic kidney disease over 5 years in severely obese adolescents with type 2 diabetes. _Diabetes Care_ 43, 187–195 (2020).
PubMed Google Scholar * Cohen, R. V. et al. Microvascular outcomes after metabolic surgery (MOMS) in patients with type 2 diabetes mellitus and class I obesity: rationale and design for a
randomised controlled trial. _BMJ Open_ 7, e013574 (2017). PubMed PubMed Central Google Scholar * Cohen, R. V. et al. Effect of gastric bypass vs best medical treatment on early-stage
chronic kidney disease in patients with type 2 diabetes and obesity a randomized clinical trial. _JAMA Surg._ https://doi.org/10.1001/jamasurg.2020.0420 (2020). Article PubMed Google
Scholar * Carlsson, L. M. et al. The incidence of albuminuria after bariatric surgery and usual care in Swedish obese subjects (SOS): a prospective controlled intervention trial. _Int. J.
Obes._ 39, 169–175 (2015). CAS Google Scholar * Belle, S. H. et al. Baseline characteristics of participants in the longitudinal assessment of bariatric surgery-2 (LABS-2) study. _Surg.
Obes. Relat. Dis._ 9, 926–935 (2013). PubMed PubMed Central Google Scholar * Friedman, A. N. et al. Effect of bariatric surgery on CKD risk. _J. Am. Soc. Nephrol._ 29, 1289–1300 (2018).
PubMed PubMed Central Google Scholar * Funes, D. R. et al. Metabolic surgery reduces the risk of progression from chronic kidney disease to kidney failure. _Ann. Surg._ 270, 511–518
(2019). PubMed Google Scholar * Grams, M. E. et al. Predicting timing of clinical outcomes in patients with chronic kidney disease and severely decreased glomerular filtration rate.
_Kidney Int._ 93, 1442–1451 (2018). PubMed PubMed Central Google Scholar * Neff, K. J. et al. Effect of Roux-en-Y gastric bypass and diet-induced weight loss on diabetic kidney disease in
the Zucker diabetic fatty rat. _Surg. Obes. Relat. Dis._ 13, 21–27 (2017). PubMed Google Scholar * Canney, A. L. et al. Improvements in diabetic albuminuria and podocyte differentiation
following Roux-en-Y gastric bypass surgery. _Diab. Vasc. Dis. Res._ 17, 1479164119879039 (2019). PubMed Google Scholar * Nair, M. et al. Characterisation of the renal cortical
transcriptome following roux-en-y gastric bypass surgery in experimental diabetic kidney disease. Preprint at bioRxiv https://doi.org/10.1101/2020.06.01.120980v1 (2020). * Wang, C., He, B.,
Piao, D. & Han, P. Roux-en-Y esophagojejunostomy ameliorates renal function through reduction of renal inflammatory and fibrotic markers in diabetic nephropathy. _Obes. Surg._ 26,
1402–1413 (2016). PubMed Google Scholar * Zhiqing, W. et al. Renal function is ameliorated in a diabetic nephropathy rat model through a duodenal-jejunal bypass. _Diabetes Res. Clin.
Pract._ 103, 26–34 (2014). PubMed Google Scholar * Wu, D. et al. Downregulation of lncRNA MALAT1 contributes to renal functional improvement after duodenal-jejunal bypass in a diabetic rat
model. _J. Physiol. Biochem._ 74, 431–439 (2018). PubMed Google Scholar * Carrara, F. et al. Simplified method to measure glomerular filtration rate by iohexol plasma clearance in
conscious rats. _Nephron_ 133, 62–70 (2016). CAS PubMed Google Scholar * Schock-Kusch, D. et al. Transcutaneous assessment of renal function in conscious rats with a device for measuring
FITC-sinistrin disappearance curves. _Kidney Int._ 79, 1254–1258 (2011). CAS PubMed Google Scholar * Mangan, A., Le Roux, C. W., Miller, N. G. & Docherty, N. G. Iron and vitamin
D/calcium deficiency after gastric bypass: mechanisms involved and strategies to improve oral supplement disposition. _Curr. Drug Metab._ 20, 244–252 (2019). CAS PubMed Google Scholar *
Stein, J., Stier, C., Raab, H. & Weiner, R. Review article: the nutritional and pharmacological consequences of obesity surgery. _Aliment. Pharmacol. Ther._ 40, 582–609 (2014). CAS
PubMed Google Scholar * Milone, M. et al. Incidence of successful pregnancy after weight loss interventions in infertile women: a systematic review and meta-analysis of the literature.
_Obes. Surg._ 26, 443–451 (2016). PubMed Google Scholar * Kwong, W., Tomlinson, G. & Feig, D. S. Maternal and neonatal outcomes after bariatric surgery; a systematic review and
meta-analysis: do the benefits outweigh the risks? _Am. J. Obstetr. Gynecol._ 218, 573–580 (2018). Google Scholar * Stephansson, O., Johansson, K., Söderling, J., Näslund, I. & Neovius,
M. Delivery outcomes in term births after bariatric surgery: population-based matched cohort study. _PLoS Med._ 15, e1002656 (2018). PubMed PubMed Central Google Scholar * Luyckx, V. A.
& Brenner, B. M. Birth weight, malnutrition and kidney-associated outcomes — a global concern. _Nat. Rev. Nephrol._ 11, 135–149 (2015). PubMed Google Scholar * Lee, Y. Q. et al.
Relationship between maternal global nutrient restriction during pregnancy and offspring kidney structure and function: a systematic review of animal studies. _Am. J. Physiol. Renal
Physiol._ 316, F1227–F1235 (2019). CAS PubMed Google Scholar * Lieske, J. C. et al. Kidney stones are common after bariatric surgery. _Kidney Int._ 87, 839–845 (2015). PubMed Google
Scholar * Nazzal, L., Puri, S. & Goldfarb, D. S. Enteric hyperoxaluria: an important cause of end-stage kidney disease. _Nephrol. Dial. Transplant._ 31, 375–382 (2016). PubMed Google
Scholar * Asplin, J. R. The management of patients with enteric hyperoxaluria. _Urolithiasis_ 44, 33–43 (2016). CAS PubMed Google Scholar * Nor Hanipah, Z. et al. Impact of early
postbariatric surgery acute kidney injury on long-term renal function. _Obes. Surg._ 28, 3580–3585 (2018). PubMed Google Scholar * Montgomery, J. R., Waits, S. A., Dimick, J. B. &
Telem, D. A. Perioperative risks of sleeve gastrectomy versus Roux-en-Y gastric bypass among patients with chronic kidney disease: a review of the MBSAQIP database. _Ann. Surg_.
https://doi.org/10.1097/SLA.0000000000003627 (2019). Article PubMed Google Scholar * Lee, J. E. et al. Risk of ESRD and all cause mortality in type 2 diabetes according to circulating
levels of FGF-23 and TNFR1. _PLoS ONE_ 8, e58007 (2013). CAS PubMed PubMed Central Google Scholar * Niewczas, M. A. et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2
diabetes. _J. Am. Soc. Nephrol._ 23, 507–515 (2012). CAS PubMed PubMed Central Google Scholar * Pavkov, M. E. et al. Tumor necrosis factor receptors 1 and 2 are associated with early
glomerular lesions in type 2 diabetes. _Kidney Int._ 89, 226–234 (2016). CAS PubMed PubMed Central Google Scholar * Saulnier, P. J. et al. Association of serum concentration of TNFR1
with all-cause mortality in patients with type 2 diabetes and chronic kidney disease: follow-up of the SURDIAGENE Cohort. _Diabetes Care_ 37, 1425–1431 (2014). CAS PubMed Google Scholar *
Doody, A. et al. Validating the association between plasma tumour necrosis factor receptor 1 levels and the presence of renal injury and functional decline in patients with type 2 diabetes.
_J. Diabetes Complicat._ 32, 95–99 (2018). Google Scholar * Ahlqvist, E. et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis
of six variables. _Lancet Diabetes Endocrinol._ 6, 361–369 (2018). PubMed Google Scholar * Sjostrom, L. et al. Association of bariatric surgery with long-term remission of type 2 diabetes
and with microvascular and macrovascular complications. _JAMA_ 311, 2297–2304 (2014). PubMed Google Scholar * Ahren, B. et al. Semaglutide induces weight loss in subjects with type 2
diabetes regardless of baseline BMI or gastrointestinal adverse events in the SUSTAIN 1 to 5 trials. _Diabetes, Obes. Metab._ 20, 2210–2219 (2018). CAS Google Scholar * Neuen, B. L. et al.
SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. _Lancet Diabetes Endocrinol._ 7, 845–854 (2019). CAS PubMed
Google Scholar * Potluri, K. & Hou, S. Obesity in kidney transplant recipients and candidates. _Am. J. Kidney Dis._ 56, 143–156 (2010). PubMed Google Scholar * Sheetz, K. H.,
Gerhardinger, L., Dimick, J. B. & Waits, S. A. Bariatric surgery and long-term survival in patients with obesity and end-stage kidney disease. _JAMA Surg._
https://doi.org/10.1001/jamasurg.2020.0829 (2020). Article PubMed Google Scholar * Hansel, B. et al. Severe chronic kidney disease is associated with a lower efficiency of bariatric
surgery. _Obes. Surg._ 29, 1514–1520 (2019). PubMed Google Scholar * Al-Bahri, S., Fakhry, T. K., Gonzalvo, J. P. & Murr, M. M. Bariatric surgery as a bridge to renal transplantation
in patients with end-stage renal disease. _Obes. Surg._ 27, 2951–2955 (2017). PubMed Google Scholar * Salehi, M., Vella, A., McLaughlin, T. & Patti, M. E. Hypoglycemia after gastric
bypass surgery: current concepts and controversies. _J. Clin. Endocrinol. Metab._ 103, 2815–2826 (2018). PubMed PubMed Central Google Scholar * Abrahamsson, N., Engstrom, B. E., Sundbom,
M. & Karlsson, F. A. Gastric bypass surgery elevates NT-ProBNP levels. _Obes. Surg._ 23, 1421–1426 (2013). PubMed Google Scholar * Bueter, M. et al. Sodium and water handling after
gastric bypass surgery in a rat model. _Surg. Obes. Relat. Dis._ 7, 68–73 (2011). PubMed Google Scholar * Docherty, N. G., Fandriks, L., le Roux, C. W., Hallersund, P. & Werling, M.
Urinary sodium excretion after gastric bypass surgery. _Surg. Obes. Relat. Dis._ 13, 1506–1514 (2017). PubMed Google Scholar * Arapis, K., Kadouch, D., Caillieret, O., Roussel, R. &
Hansel, B. Bariatric surgery and chronic kidney disease: much hope, but proof is still awaited. _Int. J. Obes._ 42, 1532–1533 (2018). Google Scholar * US National Library of Medicine.
_ClinicalTrials.gov_ https://clinicaltrials.gov/ct2/show/NCT02612831 (2018). * Perakakis, N. et al. Circulating levels of gastrointestinal hormones in response to the most common types of
bariatric surgery and predictive value for weight loss over one year: evidence from two independent trials. _Metab. Clin. Exp._ 101, 153997 (2019). CAS PubMed Google Scholar * Elliott, J.
A., Reynolds, J. V., le Roux, C. W. & Docherty, N. G. Physiology, pathophysiology and therapeutic implications of enteroendocrine control of food intake. _Expert Rev. Endocrinol.
Metab._ 11, 475–499 (2016). CAS PubMed Google Scholar * Werling, M. et al. Biliopancreatic diversion is associated with greater increases in energy expenditure than Roux-en-Y gastric
bypass. _PLoS ONE_ 13, e0194538 (2018). PubMed PubMed Central Google Scholar * Werling, M. et al. Roux-en-Y gastric bypass surgery increases respiratory quotient and energy expenditure
during food intake. _PLoS ONE_ 10, e0129784 (2015). PubMed PubMed Central Google Scholar * Sondergaard Nielsen, M. et al. Bariatric surgery does not affect food preferences, but
individual changes in food preferences may predict weight loss. _Obesity_ 26, 1879–1887 (2018). CAS PubMed Google Scholar * Ghanim, H. et al. Decreases in neprilysin and vasoconstrictors
and increases in vasodilators following bariatric surgery. _Diabetes Obes. Metab._ 20, 2029–2033 (2018). CAS PubMed Google Scholar * Sharma, A. M. & Kushner, R. F. A proposed clinical
staging system for obesity. _Int. J. Obes._ 33, 289–295 (2009). CAS Google Scholar * Yan, W., Bai, R., Yan, M. & Song, M. Preoperative fasting plasma C-peptide levels as predictors of
remission of type 2 diabetes mellitus after bariatric surgery: a systematic review and meta-analysis. _J. Investig. Surg._ 30, 383–393 (2017). Google Scholar * Scheurlen, K. M. et al.
Serum uromodulin and Roux-en-Y gastric bypass: improvement of a marker reflecting nephron mass. _Surg. Obes. Relat. Dis._ 15, 1319–1325 (2019). PubMed Google Scholar Download references
ACKNOWLEDGEMENTS N.G.D. is also a visiting researcher at the Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Sweden. C.W.l.R. also holds an adjunct Professor
position in Investigative Science at Imperial College London, UK. The authors acknowledge funding support from the following agencies: Swedish Medical Research Council (2015-02733) and
European Foundation for the Study of Diabetes/Boehringer Ingelheim European Diabetes Research Programme (BI 2017_3) to C.W.l.R. and N.G.D., and Science Foundation Ireland (12/YI/B2480) to
C.W.l.R. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Diabetes Complications Research Centre, Conway Institute, School of Medicine and Medical Sciences, University College, Dublin, Ireland
Neil G. Docherty & Carel W. le Roux Authors * Neil G. Docherty View author publications You can also search for this author inPubMed Google Scholar * Carel W. le Roux View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Both authors researched data for the article, wrote the manuscript, made substantial contributions to
discussions of the content, and reviewed or edited the manuscript before submission. CORRESPONDING AUTHOR Correspondence to Neil G. Docherty. ETHICS DECLARATIONS COMPETING INTERESTS C.W.l.R.
is an advisory board member for Novo Nordisk, Herbalife, Johnson & Johnson, Keyron and GI Dynamics, and has received honoraria for speaking from Novo Nordisk, Herbalife, Johnson &
Johnson, GI Dynamics, Lilly, MSD and Consilient Health. N.G.D. declares no competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Reviews Nephrology_ thanks A.
Courcoulas, T. Diwan, R. Roussel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. GLOSSARY * Critical adipose threshold A concept that postulates that adipose storage capacity varies among
individuals owing to a threshold or upper limit in the triglyceride storage capacity of individual adipocytes. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Docherty, N.G., le Roux, C.W. Bariatric surgery for the treatment of chronic kidney disease in obesity and type 2 diabetes mellitus. _Nat Rev Nephrol_ 16, 709–720 (2020).
https://doi.org/10.1038/s41581-020-0323-4 Download citation * Accepted: 30 June 2020 * Published: 10 August 2020 * Issue Date: December 2020 * DOI: https://doi.org/10.1038/s41581-020-0323-4
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative