Play all audios:
ABSTRACT Alzheimer disease (AD) is characterized by wide heterogeneity in cognitive and behavioural syndromes, risk factors and pathophysiological mechanisms. Addressing this phenotypic
variation will be crucial for the development of precise and effective therapeutics in AD. Sex-related differences in neural anatomy and function are starting to emerge, and sex might
constitute an important factor for AD patient stratification and personalized treatment. Although the effects of sex on AD epidemiology are currently the subject of intense investigation,
the notion of sex-specific clinicopathological AD phenotypes is largely unexplored. In this Review, we critically discuss the evidence for sex-related differences in AD symptomatology,
progression, biomarkers, risk factor profiles and treatment. The cumulative evidence reviewed indicates sex-specific patterns of disease manifestation as well as sex differences in the rates
of cognitive decline and brain atrophy, suggesting that sex is a crucial variable in disease heterogeneity. We discuss critical challenges and knowledge gaps in our current understanding.
Elucidating sex differences in disease phenotypes will be instrumental in the development of a ‘precision medicine’ approach in AD, encompassing individual, multimodal, biomarker-driven and
sex-sensitive strategies for prevention, detection, drug development and treatment. KEY POINTS * Men and women with Alzheimer disease (AD) exhibit different cognitive and psychiatric
symptoms, and women show faster cognitive decline after diagnosis of mild cognitive impairment (MCI) or AD dementia. * Levels of amyloid-β measured with PET-based brain imaging and with
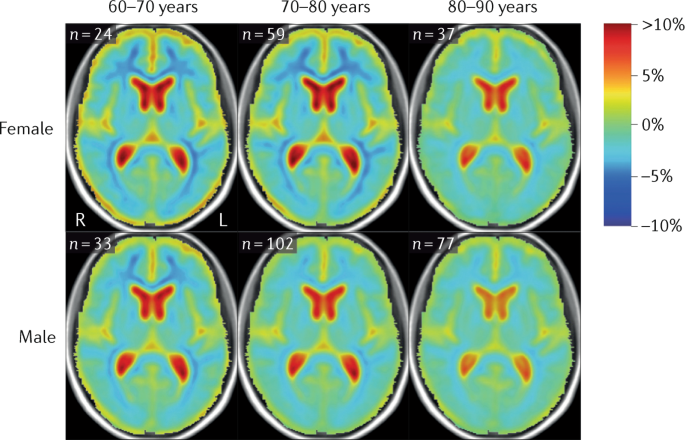
biochemical analysis of cerebrospinal fluid do not differ between the sexes. * Brain atrophy rates and patterns differ along the AD continuum between the sexes; in MCI, brain atrophy is
faster in women than in men. * The prevalence and effects of cerebrovascular, metabolic and socio-economic risk factors for AD are different between men and women. * No data are available on
sex differences in the efficacy and safety of drugs used in recently completed phase III clinical trials for mild to moderate AD. * Systematic studying and reporting of sex differences in
disease symptomatology, biomarkers, progression, risk factors and treatment responses will be crucial for the development and implementation of precision medicine in AD. Access through your
institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio
journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and online access $209.00 per
year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated
during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS ALZHEIMER
DISEASE SEEN THROUGH THE LENS OF SEX AND GENDER Article 14 April 2025 ALZHEIMER’S DISEASE AS A WOMEN’S HEALTH CHALLENGE: A CALL FOR ACTION ON INTEGRATIVE PRECISION MEDICINE APPROACHES
Article Open access 20 May 2024 LEVERAGING RESEARCH INTO SEX DIFFERENCES AND STEROID HORMONES TO IMPROVE BRAIN HEALTH Article 25 November 2024 REFERENCES * World Health Organization and
Alzheimer’s Disease International. _Dementia: a public health priority_. WHO http://www.who.int/mental_health/publications/dementia_report_2012/en/ (2012). * Prince, M. Wimo, A., Guerchet,
M., Ali, G. C., Wu, Y. & Prina, A. M. World Alzheimer Report 2015: the global impact of dementia. _An anlaysis of prevalence, incidence, costs and trends_. Alzheimer’s Disease
International https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf (2015). * Gauthier, S. et al. Why has therapy development for dementia failed in the last two decades? _Alzheimers
Dement._ 12, 60–64 (2016). Article PubMed Google Scholar * Husain, M. Alzheimer’s disease: time to focus on the brain, not just molecules. _Brain_ 140, 251–253 (2017). Article PubMed
Google Scholar * de Bono, J. S. & Ashworth, A. Translating cancer research into targeted therapeutics. _Nature_ 467, 543–549 (2010). Article PubMed CAS Google Scholar * Vargas, A.
J. & Harris, C. C. Biomarker development in the precision medicine era: lung cancer as a case study. _Nat. Rev. Cancer_ 16, 525–537 (2016). Article PubMed CAS Google Scholar * Qian,
J., Hyman, B. T. & Betensky, R. A. Neurofibrillary tangle stage and the rate of progression of Alzheimer symptoms: modeling using an autopsy cohort and application to clinical trial
design. _JAMA Neurol._ 74, 540–548 (2017). Article PubMed PubMed Central Google Scholar * Gamberger, D., Lavrac, N., Srivatsa, S., Tanzi, R. E. & Doraiswamy, P. M. Identification of
clusters of rapid and slow decliners among subjects at risk for Alzheimer’s disease. _Sci. Rep._ 7, 6763 (2017). Article PubMed PubMed Central CAS Google Scholar * Escott-Price, V.,
Myers, A. J., Huentelman, M. & Hardy, J. Polygenic risk score analysis of pathologically confirmed Alzheimer disease. _Ann. Neurol._ 82, 311–314 (2017). Article PubMed PubMed Central
Google Scholar * Ruigrok, A. N. et al. A meta-analysis of sex differences in human brain structure. _Neurosci. Biobehav. Rev._ 39, 34–50 (2014). Article PubMed PubMed Central Google
Scholar * Ingalhalikar, M. et al. Sex differences in the structural connectome of the human brain. _Proc. Natl Acad. Sci. USA_ 111, 823–828 (2014). Article PubMed CAS Google Scholar *
Li, R. & Singh, M. Sex differences in cognitive impairment and Alzheimer’s disease. _Front. Neuroendocrinol._ 35, 385–403 (2014). Article PubMed PubMed Central CAS Google Scholar *
Cordonnier, C. et al. Stroke in women — from evidence to inequalities. _Nat. Rev. Neurol._ 13, 521–532 (2017). THIS PAPER PROVIDES A CLEAR SUMMARY OF THE ROLE OF SEX DIFFERENCES IN CLINICAL
PRACTICE IN THE STROKE FIELD. Article PubMed Google Scholar * Szewczyk-Krolikowski, K. et al. The influence of age and gender on motor and non-motor features of early Parkinson’s disease:
initial findings from the Oxford Parkinson Disease Center (OPDC) discovery cohort. _Parkinsonism Relat. Disord._ 20, 99–105 (2014). Article PubMed Google Scholar * Vetvik, K. G. &
MacGregor, E. A. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. _Lancet Neurol._ 16, 76–87 (2017). Article PubMed CAS Google Scholar * Liu, G.
et al. Prediction of cognition in Parkinson’s disease with a clinical-genetic score: a longitudinal analysis of nine cohorts. _Lancet Neurol._ 16, 620–629 (2017). Article PubMed PubMed
Central Google Scholar * Hampel, H. et al. Precision pharmacology for Alzheimer’s disease. _Pharmacol. Res._ 130, 331–365 (2018). Article PubMed CAS Google Scholar * Snyder, H. M. et
al. Sex biology contributions to vulnerability to Alzheimer’s disease: A think tank convened by the Women’s Alzheimer’s Research Initiative. _Alzheimers Dement._ 12, 1186–1196 (2016).
Article PubMed Google Scholar * Pike, C. J. Sex and the development of Alzheimer’s disease. _J. Neurosci. Res._ 95, 671–680 (2017). Article PubMed PubMed Central CAS Google Scholar *
Mielke, M. M., Vemuri, P. & Rocca, W. A. Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. _Clin. Epidemiol._ 6, 37–48 (2014). Article PubMed PubMed
Central Google Scholar * Rocca, W. A. Time, sex, gender, history, and dementia. _Alzheimer Dis. Assoc. Disord._ 31, 76–79 (2017). THIS PAPER HIGHLIGHTS THE CURRENT DEBATE IN THE FIELD OF
AD EPIDEMIOLOGY AND THE POTENTIAL ROLE OF SECULAR TRENDS IN SOME CONTROVERSIAL RESULTS. Article PubMed PubMed Central Google Scholar * Gale, S. D., Baxter, L. & Thompson, J. Greater
memory impairment in dementing females than males relative to sex-matched healthy controls. _J. Clin. Exp. Neuropsychol._ 38, 527–533 (2016). Article PubMed PubMed Central Google Scholar
* Jack, C. R. Jr. et al. Age, sex, and APOE epsilon4 effects on memory, brain structure, and beta-amyloid across the adult life span. _JAMA Neurol._ 72, 511–519 (2015). Article PubMed
PubMed Central Google Scholar * McCarrey, A. C., An, Y., Kitner-Triolo, M. H., Ferrucci, L. & Resnick, S. M. Sex differences in cognitive trajectories in clinically normal older
adults. _Psychol. Aging_ 31, 166–175 (2016). Article PubMed PubMed Central Google Scholar * Laws, K. R., Irvine, K. & Gale, T. M. Sex differences in cognitive impairment in
Alzheimer’s disease. _World J. Psychiatry_ 6, 54–65 (2016). Article PubMed PubMed Central Google Scholar * Sundermann, E. E. et al. Better verbal memory in women than men in MCI despite
similar levels of hippocampal atrophy. _Neurology_ 86, 1368–1376 (2016). Article PubMed PubMed Central Google Scholar * Sundermann, E. E. et al. Female advantage in verbal memory:
evidence of sex-specific cognitive reserve. _Neurology_ 87, 1916–1924 (2016). Article PubMed PubMed Central Google Scholar * Irvine, K., Laws, K. R., Gale, T. M. & Kondel, T. K.
Greater cognitive deterioration in women than men with Alzheimer’s disease: a meta analysis. _J. Clin. Exp. Neuropsychol_ 34, 989–998 (2012). THIS ARTICLE IS A USEFUL META-ANALYSIS THAT
DEMONSTRATED THE OCCURRENCE OF SEX DIFFERENCES IN COGNITIVE DECLINE IN AD. Article PubMed Google Scholar * Pusswald, G. et al. Gender-specific differences in cognitive profiles of
patients with Alzheimer’s disease: results of the Prospective Dementia Registry Austria (PRODEM-Austria). _J. Alzheimers Dis._ 46, 631–637 (2015). Article PubMed Google Scholar * Benke,
T. et al. Cognition, gender, and functional abilities in Alzheimer’s disease: how are they related? _J. Alzheimers Dis._ 35, 247–252 (2013). Article PubMed Google Scholar * Holland, D.,
Desikan, R. S., Dale, A. M. & McEvoy, L. K., Alzheimer’s Disease Neuroimaging Initiative. Higher rates of decline for women and apolipoprotein E epsilon4 carriers. _AJNR. Am. J.
Neuroradiol._ 34, 2287–2293 (2013). Article PubMed PubMed Central CAS Google Scholar * Lin, K. A. et al. Marked gender differences in progression of mild cognitive impairment over 8
years. _Alzheimers Dement._ 1, 103–110 (2015). THIS ARTICLE PROVIDES STRONG EVIDENCE FROM THE ADNI COHORT OF FASTER COGNITIVE DECLINE IN WOMEN WITH MCI THAN IN MEN WITH MCI. Google Scholar
* Tifratene, K., Robert, P., Metelkina, A., Pradier, C. & Dartigues, J. F. Progression of mild cognitive impairment to dementia due to AD in clinical settings. _Neurology_ 85, 331–338
(2015). Article PubMed Google Scholar * Pradier, C. et al. The mini mental state examination at the time of Alzheimer’s disease and related disorders diagnosis, according to age,
education, gender and place of residence: a cross-sectional study among the French National Alzheimer database. _PLoS ONE_ 9, e103630 (2014). Article PubMed PubMed Central Google Scholar
* Leening, M. J. et al. Sex differences in lifetime risk and first manifestation of cardiovascular disease: prospective population based cohort study. _BMJ_ 349, g5992 (2014). Article
PubMed PubMed Central Google Scholar * Karoglu, E. T. et al. Aging alters the molecular dynamics of synapses in a sexually dimorphic pattern in zebrafish (Danio rerio). _Neurobiol. Aging_
54, 10–21 (2017). Article PubMed CAS Google Scholar * Counts, S. E. et al. Cerebrospinal fluid proNGF: a putative biomarker for early Alzheimer’s disease. _Curr. Alzheimer Res._ 13,
800–808 (2016). Article PubMed PubMed Central CAS Google Scholar * Walker, K. A. et al. Midlife systemic inflammatory markers are associated with late-life brain volume: the ARIC study.
_Neurology_ 89, 2262–2270 (2017). Article PubMed Google Scholar * Schwarz, J. M., Sholar, P. W. & Bilbo, S. D. Sex differences in microglial colonization of the developing rat brain.
_J. Neurochem._ 120, 948–963 (2012). PubMed PubMed Central CAS Google Scholar * Lenz, K. M., Nugent, B. M., Haliyur, R. & McCarthy, M. M. Microglia are essential to masculinization
of brain and behavior. _J. Neurosci._ 33, 2761–2772 (2013). Article PubMed PubMed Central CAS Google Scholar * Villa, A. et al. Sex-specific features of microglia from adult mice. _Cell
Rep._ 23, 3501–3511 (2018). * Ott, B. R., Tate, C. A., Gordon, N. M. & Heindel, W. C. Gender differences in the behavioral manifestations of Alzheimer’s disease. _J. Am. Geriatr. Soc._
44, 583–587 (1996). Article PubMed CAS Google Scholar * Mega, M. S., Cummings, J. L., Fiorello, T. & Gornbein, J. The spectrum of behavioral changes in Alzheimer’s disease.
_Neurology_ 46, 130–135 (1996). Article PubMed CAS Google Scholar * Ott, B. R., Lapane, K. L. & Gambassi, G. Gender differences in the treatment of behavior problems in Alzheimer’s
disease. SAGE Study Group. System. Assess. _Geriatr. Drug Epidemiol. Neurol._ 54, 427–432 (2000). CAS Google Scholar * Kitamura, T., Kitamura, M., Hino, S., Tanaka, N. & Kurata, K.
Gender differences in clinical manifestations and outcomes among hospitalized patients with behavioral and psychological symptoms of dementia. _J. Clin. Psychiatry_ 73, 1548–1554 (2012).
Article PubMed Google Scholar * Teri, L., Borson, S., Kiyak, H. A. & Yamagishi, M. Behavioral disturbance, cognitive dysfunction, and functional skill. Prevalence and relationship in
Alzheimer’s disease. _J. Am. Geriatr. Soc._ 37, 109–116 (1989). Article PubMed CAS Google Scholar * Karttunen, K. et al. Neuropsychiatric symptoms and quality of life in patients with
very mild and mild Alzheimer’s disease. _Int. J. Geriatr. Psychiatry_ 26, 473–482 (2011). Article PubMed Google Scholar * Spalletta, G. et al. Neuropsychiatric symptoms and syndromes in a
large cohort of newly diagnosed, untreated patients with Alzheimer disease. _Am. J. Geriatr. Psychiatry_ 18, 1026–1035 (2010). Article PubMed Google Scholar * Hollingworth, P. et al.
Four components describe behavioral symptoms in 1,120 individuals with late-onset Alzheimer’s disease. _J. Am. Geriatr. Soc._ 54, 1348–1354 (2006). THIS PAPER PRESENTS THE LARGEST STUDY
AVAILABLE DOCUMENTING SEX DIFFERENCES IN PSYCHIATRIC SYMPTOMS OF AD. Article PubMed Google Scholar * Sinforiani, E. et al. Impact of gender differences on the outcome of Alzheimer’s
disease. _Dement. Geriatr. Cogn. Disord._ 30, 147–154 (2010). Article PubMed CAS Google Scholar * Jack, C. R. Jr. et al. Age-specific and sex-specific prevalence of cerebral
beta-amyloidosis, tauopathy, and neurodegeneration in cognitively unimpaired individuals aged 50–95 years: a cross-sectional study. _Lancet Neurol._ 16, 435–444 (2017). THIS LANDMARK STUDY
DOCUMENTS SEX DIFFERENCES IN AD BIOMARKERS ACROSS THE LIFESPAN. Article PubMed PubMed Central CAS Google Scholar * Scheinin, N. M. et al. Cortical (1)(1)C-PIB uptake is associated with
age, APOE genotype, and gender in “healthy aging”. _J. Alzheimers Dis._ 41, 193–202 (2014). Article PubMed CAS Google Scholar * Cavedo, E. et al. Sex differences in functional and
molecular neuroimaging biomarkers of Alzheimer’s disease in a mono-center cohort of cognitively normal older adults with subjective memory complaints. _Alzheimers Dement._ (in the press). *
Gottesman, R. F. et al. The ARIC-PET amyloid imaging study: brain amyloid differences by age, race, sex, and APOE. _Neurology_ 87, 473–480 (2016). Article PubMed PubMed Central Google
Scholar * Vemuri, P. et al. Evaluation of amyloid protective factors and Alzheimer disease neurodegeneration protective factors in elderly individuals. _JAMA Neurol._ 74, 718–726 (2017).
Article PubMed PubMed Central Google Scholar * Barnes, L. L. et al. Sex differences in the clinical manifestations of Alzheimer disease pathology. _Arch. Gen. Psychiatry_ 62, 685–691
(2005). THIS LANDMARK STUDY EXAMINES FOR THE FIRST TIME SEX DIFFERENCES IN THE CLINICAL MANIFESTATION RESULTING FROM THE ACCUMULATION OF AMYLOID PLAQUES AND TANGLES IN THE BRAIN. Article
PubMed Google Scholar * Shinohara, M. et al. Impact of sex and APOE4 on cerebral amyloid angiopathy in Alzheimer’s disease. _Acta Neuropathol._ 132, 225–234 (2016). Article PubMed PubMed
Central CAS Google Scholar * Mattsson, N. et al. Clinical validity of cerebrospinal fluid Abeta42, tau, and phospho-tau as biomarkers for Alzheimer’s disease in the context of a
structured 5-phase development framework. _Neurobiol. Aging_ 52, 196–213 (2017). Article PubMed CAS Google Scholar * Jansen, W. J. et al. Prevalence of cerebral amyloid pathology in
persons without dementia: a meta-nalysis. _JAMA_ 313, 1924–1938 (2015). Article PubMed PubMed Central Google Scholar * Salehi, A., Gonzalez Martinez, V. & Swaab, D. F. A sex
difference and no effect of ApoE type on the amount of cytoskeletal alterations in the nucleus basalis of Meynert in Alzheimer’s disease. _Neurobiol. Aging_ 19, 505–510 (1998). Article
PubMed CAS Google Scholar * Johnson, K. A. et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. _Ann. Neurol._ 79, 110–119 (2016). Article PubMed
Google Scholar * Apostolova, L. G. et al. 3D comparison of hippocampal atrophy in amnestic mild cognitive impairment and Alzheimer’s disease. _Brain_ 129, 2867–2873 (2006). Article PubMed
Google Scholar * Perlaki, G. et al. Are there any gender differences in the hippocampus volume after head-size correction? A volumetric and voxel-based morphometric study. _Neurosci.
Lett._ 570, 119–123 (2014). Article PubMed CAS Google Scholar * Skup, M. et al. Sex differences in grey matter atrophy patterns among AD and aMCI patients: results from ADNI.
_Neuroimage_ 56, 890–906 (2011). Article PubMed PubMed Central Google Scholar * Hua, X. et al. Sex and age differences in atrophic rates: an ADNI study with _n_ = 1368 MRI scans.
_Neurobiol. Aging_ 31, 1463–1480 (2010). THIS LANDMARK PAPER SHOWS THE FASTER ATROPHIC RATE IN WOMEN ENROLLED IN THE ADNI COHORT. Article PubMed PubMed Central Google Scholar * Ardekani,
B. A., Convit, A. & Bachman, A. H. Analysis of the MIRIAD data shows sex differences in hippocampal atrophy progression. _J. Alzheimers Dis._ 50, 847–857 (2016). Article PubMed Google
Scholar * Koran, M. E., Wagener, M. & Hohman, T. J., Alzheimer’s Neuroimaging Initiative. Sex differences in the association between AD biomarkers and cognitive decline. _Brain Imaging
Behav._ 11, 205–213 (2016). Article Google Scholar * Karch, A. et al. Stratification by genetic and demographic characteristics improves diagnostic accuracy of cerebrospinal fluid
biomarkers in rapidly progressive dementia. _J. Alzheimers Dis._ 54, 1385–1393 (2016). Article PubMed CAS Google Scholar * Madsen, T. E. et al. Sex-specific stroke incidence over time in
the Greater Cincinnati/Northern Kentucky Stroke Study. _Neurology_ 89, 990–996 (2017). Article PubMed PubMed Central Google Scholar * Gibson, C. L. Cerebral ischemic stroke: is gender
important? _J. Cereb. Blood Flow Metab._ 33, 1355–1361 (2013). Article PubMed PubMed Central Google Scholar * Longstreth, W. T. Jr. et al. Associations between microinfarcts and other
macroscopic vascular findings on neuropathologic examination in 2 databases. _Alzheimer Dis. Assoc. Disord._ 23, 291–294 (2009). Article PubMed PubMed Central Google Scholar * National
Institute of Mental Health. Major depression. _NIMH_ https://www.nimh.nih.gov/health/statistics/major-depression.shtml (2015). * Mallampalli, M. P. & Carter, C. L. Exploring sex and
gender differences in sleep health: a Society for Women’s Health Research Report. _J. Womens Health (Larchmt)_ 23, 553–562 (2014). Article Google Scholar * Farrer, L. A. et al. Effects of
age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. _JAMA_ 278,
1349–1356 (1997). Article PubMed CAS Google Scholar * Altmann, A., Tian, L., Henderson, V. W. & Greicius, M. D., Alzheimer’s Disease Neuroimaging Initiative Investigators. Sex
modifies the APOE-related risk of developing Alzheimer disease. _Ann. Neurol._ 75, 563–573 (2014). Article PubMed PubMed Central CAS Google Scholar * Kim, S. et al. Gender differences
in risk factors for transition from mild cognitive impairment to Alzheimer’s disease: a CREDOS study. _Compr. Psychiatry_ 62, 114–122 (2015). Article PubMed Google Scholar * Neu, S. C. et
al. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: a meta-analysis. _JAMA Neurol._ 74, 1178–1189 (2017). THIS HIGHLY POWERED META-ANALYSIS REFINES OUR UNDERSTANDING
OF SEX–APOE INTERACTIONS IN AD RISK. Article PubMed PubMed Central Google Scholar * Mosconi, L. et al. Perimenopause and emergence of an Alzheimer’s bioenergetic phenotype in brain and
periphery. _PLoS ONE_ 12, e0185926 (2017). Article PubMed PubMed Central CAS Google Scholar * Ungar, L., Altmann, A. & Greicius, M. D. Apolipoprotein E, gender, and Alzheimer’s
disease: an overlooked, but potent and promising interaction. _Brain Imaging Behav._ 8, 262–273 (2014). Article PubMed PubMed Central Google Scholar * Damoiseaux, J. S. et al. Gender
modulates the APOE epsilon4 effect in healthy older adults: convergent evidence from functional brain connectivity and spinal fluid tau levels. _J. Neurosci._ 32, 8254–8262 (2012). Article
PubMed PubMed Central CAS Google Scholar * Heise, V. et al. Apolipoprotein E genotype, gender and age modulate connectivity of the hippocampus in healthy adults. _Neuroimage_ 98, 23–30
(2014). Article PubMed CAS Google Scholar * Sampedro, F. et al. APOE-by-sex interactions on brain structure and metabolism in healthy elderly controls. _Oncotarget_ 6, 26663–26674
(2015). Article PubMed PubMed Central Google Scholar * Buckley, R. F. et al. Sex, amyloid, and APOE ε4 and risk of cognitive decline in preclinical Alzheimer’s disease: findings from
three well-characterized cohorts. _Alzheimers Dement._ https://doi.org/10.1016/j.jalz.2018.04.010 (2018). * Hohman, T. J. et al. Sex-specific association of Apolipoprotein E with
cerebrospinal fluid levels of tau. _JAMA Neurol._ https://doi.org/10.1001/jamaneurol.2018.0821 (2018). * Snowdon, D. A. et al. Brain infarction and the clinical expression of Alzheimer
disease. The Nun Study. _JAMA_ 277, 813–817 (1997). Article PubMed CAS Google Scholar * Vemuri, P. et al. Vascular and amyloid pathologies are independent predictors of cognitive decline
in normal elderly. _Brain_ 138, 761–771 (2015). Article PubMed PubMed Central Google Scholar * Roberts, R. O. et al. Association of diabetes with amnestic and nonamnestic mild cognitive
impairment. _Alzheimers Dement._ 10, 18–26 (2014). Article PubMed Google Scholar * Gilsanz, P. et al. Female sex, early-onset hypertension, and risk of dementia. _Neurology_ 89,
1886–1893 (2017). Article PubMed Google Scholar * Lorius, N. et al. Vascular disease and risk factors are associated with cognitive decline in the alzheimer disease spectrum. _Alzheimer
Dis. Assoc. Disord._ 29, 18–25 (2015). Article PubMed PubMed Central Google Scholar * Li, J. et al. Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer
disease. _Neurology_ 76, 1485–1491 (2011). Article PubMed CAS Google Scholar * Sachdev, P. S. et al. Risk profiles for mild cognitive impairment vary by age and sex: the Sydney Memory
and Ageing study. _Am. J. Geriatr. Psychiatry_ 20, 854–865 (2012). Article PubMed Google Scholar * Sundermann, E. E., Katz, M. J. & Lipton, R. B. Sex differences in the relationship
between depressive symptoms and risk of amnestic mild cognitive impairment. _Am. J. Geriatr. Psychiatry_ 25, 13–22 (2017). Article PubMed Google Scholar * Pankratz, V. S. et al.
Predicting the risk of mild cognitive impairment in the Mayo Clinic Study of Aging. _Neurology_ 84, 1433–1442 (2015). THIS LANDMARK PAPER SPECIFICALLY EXAMINES SEX DIFFERENCES IN MCI RISK.
Article PubMed PubMed Central Google Scholar * Artero, S. et al. Risk profiles for mild cognitive impairment and progression to dementia are gender specific. _J. Neurol. Neurosurg.
Psychiatry_ 79, 979–984 (2008). Article PubMed CAS Google Scholar * Hayden, K. M. et al. Vascular risk factors for incident Alzheimer disease and vascular dementia: the Cache County
study. _Alzheimer Dis. Assoc. Disord._ 20, 93–100 (2006). Article PubMed Google Scholar * Chene, G. et al. Gender and incidence of dementia in the Framingham Heart Study from mid-adult
life. _Alzheimers Dement._ 11, 310–320 (2015). Article PubMed Google Scholar * Brown, M. C. et al. Cardiovascular disease risk in women with pre-eclampsia: systematic review and
meta-analysis. _Eur. J. Epidemiol._ 28, 1–19 (2013). Article PubMed Google Scholar * Fields, J. A. et al. Preeclampsia and cognitive impairment later in life. _Am J Obstet. Gynecol_ 217,
74.e1–74.e11 (2017). Article Google Scholar * Buhimschi, I. A. et al. Protein misfolding, congophilia, oligomerization, and defective amyloid processing in preeclampsia. _Sci. Transl Med._
6, 245ra292 (2014). Article CAS Google Scholar * Rocca, W. A. et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. _Neurology_
69, 1074–1083 (2007). Article PubMed CAS Google Scholar * Bove, R. et al. Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. _Neurology_ 82,
222–229 (2014). Article PubMed PubMed Central Google Scholar * Norton, S., Matthews, F. E., Barnes, D. E., Yaffe, K. & Brayne, C. Potential for primary prevention of Alzheimer’s
disease: an analysis of population-based data. _Lancet Neurol._ 13, 788–794 (2014). Article PubMed Google Scholar * American Association of University Women. The simple truth about the
gender pay gap. AAUW https://www.aauw.org/aauw_check/pdf_download/show_pdf.php?file=The-Simple-Truth (2018). * Brown, J. E., Rhee, A., Saad-Lessler, J. & Oakley, D. Shortchanged in
retirement, the continuing challenges to women’s financial future. _National Institute on Retirement Security_
https://www.nirsonline.org/wp-content/uploads/2017/06/final_shortchanged_retirement_report_2016.pdf (2016). * Prince, M. et al. Dementia incidence and mortality in middle-income countries,
and associations with indicators of cognitive reserve: a 10/66 Dementia Research Group population-based cohort study. _Lancet_ 380, 50–58 (2012). Article PubMed PubMed Central Google
Scholar * Swinkels, J., Tilburg, T. V., Verbakel, E. & Broese van Groenou, M. Explaining the gender gap in the caregiving burden of partner caregivers. _J. Gerontol. B Psychol. Sci.
Soc. Sci_. https://doi.org/10.1093/geronb/gbx036 (2017). * Alzheimer’s Association. 2014 Alzheimer’s disease facts and figures. _Alzheimers Dement._ 10, e47–e92 (2014). Article Google
Scholar * Sharma, N., Chakrabarti, S. & Grover, S. Gender differences in caregiving among family — caregivers of people with mental illnesses. _World J. Psychiatry_ 6, 7–17 (2016).
Article PubMed PubMed Central Google Scholar * Stahl, S. T., Beach, S. R., Musa, D. & Schulz, R. Living alone and depression: the modifying role of the perceived neighborhood
environment. _Aging Ment. Health_ 21, 1065–1071 (2017). Article PubMed Google Scholar * Doody, R. S. et al. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. _N. Engl.
J. Med._ 369, 341–350 (2013). Article PubMed CAS Google Scholar * Egan, M. F. et al. Randomized trial of verubecestat for mild-to-moderate Alzheimer’s disease. _N. Engl. J. Med._ 378,
1691–1703 (2018). Article PubMed CAS Google Scholar * Doody, R. S. et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. _N. Engl. J. Med._ 370, 311–321 (2014).
Article PubMed CAS Google Scholar * Salloway, S. et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. _N. Engl. J. Med._ 370, 322–333 (2014). Article
PubMed PubMed Central CAS Google Scholar * Religa, D. et al. Dementia diagnosis differs in men and women and depends on age and dementia severity: data from SveDem, the Swedish Dementia
Quality Registry. _Dement. Geriatr. Cogn. Disord._ 33, 90–95 (2012). Article PubMed Google Scholar * Ferris, S. et al. Effects of gender on response to treatment with rivastigmine in mild
cognitive impairment: a post hoc statistical modeling approach. _Gend. Med._ 6, 345–355 (2009). Article PubMed Google Scholar * MacGowan, S. H., Wilcock, G. K. & Scott, M. Effect of
gender and apolipoprotein E genotype on response to anticholinesterase therapy in Alzheimer’s disease. _Int. J. Geriatr. Psychiatry_ 13, 625–630 (1998). Article PubMed CAS Google Scholar
* Canevelli, M. et al. Sex and gender differences in the treatment of Alzheimer’s disease: a systematic review of randomized controlled trials. _Pharmacol. Res._ 115, 218–223 (2017). THIS
SYSTEMATIC REVIEW OF RANDOMIZED, DOUBLE-BLIND TRIALS WITH CHOLINESTERASE INHIBITORS AND MEMANTINE REVEALS THAT ONLY 4% OF STUDIES EXAMINED SEX EFFECTS IN THEIR DATA SETS. Article PubMed
CAS Google Scholar * Haywood, W. M. & Mukaetova-Ladinska, E. B. Sex influences on cholinesterase inhibitor treatment in elderly individuals with Alzheimer’s disease. _Am. J. Geriatr.
Pharmacother._ 4, 273–286 (2006). Article PubMed CAS Google Scholar * Davis, M. L. & Barrett, A. M. Selective benefit of donepezil on oral naming in Alzheimer’s disease in men
compared to women. _CNS Spectr._ 14, 175–176 (2009). Article PubMed PubMed Central Google Scholar * Buccafusco, J. J., Jackson, W. J., Stone, J. D. & Terry, A. V. Sex dimorphisms in
the cognitive-enhancing action of the Alzheimer’s drug donepezil in aged Rhesus monkeys. _Neuropharmacology_ 44, 381–389 (2003). Article PubMed CAS Google Scholar * Scacchi, R., Gambina,
G., Broggio, E. & Corbo, R. M. Sex and ESR1 genotype may influence the response to treatment with donepezil and rivastigmine in patients with Alzheimer’s disease. _Int. J. Geriatr.
Psychiatry_ 29, 610–615 (2014). Article PubMed Google Scholar * Wattmo, C., Londos, E. & Minthon, L. Risk factors that affect life expectancy in Alzheimer’s disease: a 15-year
follow-up. _Dement. Geriatr. Cogn. Disord._ 38, 286–299 (2014). Article PubMed Google Scholar * Rhodes, M. E. & Rubin, R. T. Functional sex differences (‘sexual diergism’) of central
nervous system cholinergic systems, vasopressin, and hypothalamic-pituitary-adrenal axis activity in mammals: a selective review. _Brain Res. Brain Res. Rev._ 30, 135–152 (1999). Article
PubMed CAS Google Scholar * Counts, S. E., Che, S., Ginsberg, S. D. & Mufson, E. J. Gender differences in neurotrophin and glutamate receptor expression in cholinergic nucleus basalis
neurons during the progression of Alzheimer’s disease. _J. Chem. Neuroanat._ 42, 111–117 (2011). Article PubMed PubMed Central CAS Google Scholar * Wang, R. H., Bejar, C. &
Weinstock, M. Gender differences in the effect of rivastigmine on brain cholinesterase activity and cognitive function in rats. _Neuropharmacology_ 39, 497–506 (2000). Article PubMed CAS
Google Scholar * Smith, C. D., Wright, L. K., Garcia, G. E., Lee, R. B. & Lumley, L. A. Hormone-dependence of sarin lethality in rats: sex differences and stage of the estrous cycle.
_Toxicol. Appl. Pharmacol._ 287, 253–257 (2015). Article PubMed PubMed Central CAS Google Scholar * Venerosi, A., Ricceri, L., Tait, S. & Calamandrei, G. Sex dimorphic behaviors as
markers of neuroendocrine disruption by environmental chemicals: the case of chlorpyrifos. _Neurotoxicology_ 33, 1420–1426 (2012). Article PubMed CAS Google Scholar * Alves-Amaral, G.,
Pires-Oliveira, M., Andrade-Lopes, A. L., Chiavegatti, T. & Godinho, R. O. Gender-related differences in circadian rhythm of rat plasma acetyl- and butyrylcholinesterase: effects of sex
hormone withdrawal. _Chem. Biol. Interact._ 186, 9–15 (2010). Article PubMed CAS Google Scholar * Mehta, N. et al. Systematic review of sex-specific reporting of data: cholinesterase
inhibitor example. _J. Am. Geriatr. Soc._ 65, 2213–2219 (2017). Article PubMed Google Scholar * Hampel, H. et al. The cholinergic system in the pathophysiology and treatment of
Alzheimer’s disease. _Brain_ 141, 1917–1933 (2018). Article PubMed Google Scholar * Ezio, G. & Giancarlo P. Sex and gender differences in the brain cholinergic system and in the
response to therapy of Alzheimer disease with cholinesterase inhibitors. _Curr. Alzheimer Res._ https://doi.org/10.2174/1567205015666180613111504 (2018). Article PubMed Google Scholar *
Moga, D. C. et al. A comparison of sex differences in psychotropic medication use in older people with Alzheimer’s disease in the US and Finland. _Drugs Aging_ 34, 55–65 (2017). Article
PubMed PubMed Central CAS Google Scholar * Cooper, C. et al. Inequalities in receipt of mental and physical healthcare in people with dementia in the UK. _Age Ageing_ 46, 393–400 (2017).
Article PubMed Google Scholar * Cojutti, P., Arnoldo, L., Cattani, G., Brusaferro, S. & Pea, F. Polytherapy and the risk of potentially inappropriate prescriptions (PIPs) among
elderly and very elderly patients in three different settings (hospital, community, long-term care facilities) of the Friuli Venezia Giulia region, Italy: are the very elderly at higher risk
of PIPs? _Pharmacoepidemiol. Drug Saf._ 25, (1070–1078 (2016). Google Scholar * Henley, D. B., May, P. C., Dean, R. A. & Siemers, E. R. Development of semagacestat (LY450139), a
functional gamma-secretase inhibitor, for the treatment of Alzheimer’s disease. _Expert Opin. Pharmacother._ 10, 1657–16642 (2009). Article PubMed CAS Google Scholar * Farlow, M. et al.
Safety and biomarker effects of solanezumab in patients with Alzheimer’s disease. _Alzheimers Dement._ 8, 261–271 (2012). Article PubMed CAS Google Scholar * Legato, M. J., Johnson, P.
A. & Manson, J. E. Consideration of sex differences in medicine to improve health care and patient outcomes. _JAMA_ 316, 1865–1866 (2016). Article PubMed Google Scholar * Berger, J.
S. et al. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. _JAMA_ 295, 306–313 (2006). Article
PubMed CAS Google Scholar * Claxton, A. et al. Sex and ApoE genotype differences in treatment response to two doses of intranasal insulin in adults with mild cognitive impairment or
Alzheimer’s disease. _J. Alzheimers Dis._ 35, 789–797 (2013). Article PubMed PubMed Central CAS Google Scholar * Ngandu, T. et al. A 2 year multidomain intervention of diet, exercise,
cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. _Lancet_ 385, 2255–2263
(2015). Article PubMed Google Scholar * Andrieu, S. et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive
function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. _Lancet Neurol._ 16, 377–389 (2017). Article PubMed CAS Google Scholar * Farlow, M. R.
et al. Treatment outcome of tacrine therapy depends on apolipoprotein genotype and gender of the subjects with Alzheimer’s disease. _Neurology_ 50, 669–677 (1998). THIS LANDMARK PAPER
SUGGESTED FOR THE FIRST TIME A SEX–GENOTYPE INTERACTION IN THE EFFECT OF TACRINE. Article PubMed CAS Google Scholar * Depypere, H., Vierin, A., Weyers, S. & Sieben, A. Alzheimer’s
disease, apolipoprotein E and hormone replacement therapy. _Maturitas_ 94, 98–105 (2016). THIS COMPREHENSIVE REVIEW SUMMARIZES THE CURRENT UNDERSTANDING OF HRT AND THE FUTURE CHALLENGES.
Article PubMed CAS Google Scholar * Burkhardt, M. S. et al. Oestrogen replacement therapy may improve memory functioning in the absence of APOE epsilon4. _J. Alzheimers Dis._ 6, 221–228
(2004). Article PubMed CAS Google Scholar * Yaffe, K., Haan, M., Byers, A., Tangen, C. & Kuller, L. Estrogen use, APOE, and cognitive decline: evidence of gene-environment
interaction. _Neurology_ 54, 1949–1954 (2000). Article PubMed CAS Google Scholar * Ryan, J. et al. Characteristics of hormone therapy, cognitive function, and dementia: the prospective
3C Study. _Neurology_ 73, 1729–1737 (2009). Article PubMed PubMed Central CAS Google Scholar * Kang, J. H. & Grodstein, F. Postmenopausal hormone therapy, timing of initiation, APOE
and cognitive decline. _Neurobiol. Aging_ 33, 1129–1137 (2012). Article PubMed CAS Google Scholar * Zandi, P. P. et al. Hormone replacement therapy and incidence of Alzheimer disease in
older women: the Cache County Study. _JAMA_ 288, 2123–2129 (2002). Article PubMed CAS Google Scholar * Gleason, C. E. et al. Effects of hormone therapy on cognition and mood in recently
postmenopausal women: findings from the randomized, controlled KEEPS-Cognitive and Affective Study. _PLoS Med._ 12, e1001833 (2015). Article PubMed PubMed Central CAS Google Scholar *
Lista, S. et al. Application of systems theory in longitudinal studies on the origin and progression of Alzheimer’s disease. _Methods Mol. Biol._ 1303, 49–67 (2016). Article PubMed Google
Scholar * Kosik, K. S. Personalized medicine for effective Alzheimer disease treatment. _JAMA Neurol._ 72, 497–498 (2015). Article PubMed Google Scholar * Hampel, H. et al. A precision
medicine initiative for Alzheimer’s disease: the road ahead to biomarker-guided integrative disease modeling. _Climacteric_ 20, 107–118 (2017). THIS LANDMARK PAPER DESCRIBES THE INCEPTION OF
THE APMI. Article PubMed CAS Google Scholar * Hampel, H. et al. PRECISION MEDICINE — the golden gate for detection, treatment and prevention of Alzheimer’s disease. _J. Prev. Alzheimers
Dis._ 3, 243–259 (2016). PubMed PubMed Central CAS Google Scholar * Hebert, L. E., Weuve, J., Scherr, P. A. & Evans, D. A. Alzheimer disease in the United States (2010–2050)
estimated using the 2010 census. _Neurology_ 80, 1778–1783 (2013). Article PubMed PubMed Central Google Scholar * Katz, M. J. et al. Age-specific and sex-specific prevalence and
incidence of mild cognitive impairment, dementia, and Alzheimer dementia in blacks and whites: a report from the Einstein Aging Study. _Alzheimer Dis. Assoc. Disord._ 26, 335–343 (2012).
Article PubMed PubMed Central Google Scholar * Roberts, R. O. et al. The incidence of MCI differs by subtype and is higher in men: the Mayo Clinic Study of Aging. _Neurology_ 78, 342–351
(2012). Article PubMed PubMed Central CAS Google Scholar * Lin, K. A. & Doraiswamy, P. M. When Mars versus Venus is not a cliche: gender differences in the neurobiology of
Alzheimer’s disease. _Front. Neurol._ 5, 288 (2014). Article PubMed Google Scholar * Dubois, B. et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria.
_Lancet Neurol._ 13, 614–629 (2014). Article PubMed Google Scholar * Lyketsos, C. G. et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from
the cardiovascular health study. _JAMA_ 288, 1475–1483 (2002). Article PubMed Google Scholar * Steinberg, M. et al. Vascular risk factors and neuropsychiatric symptoms in Alzheimer’s
disease: the Cache County Study. _Int. J. Geriatr. Psychiatry_ 29, 153–159 (2014). Article PubMed Google Scholar * Petersen, R. C. Mild cognitive impairment as a diagnostic entity. _J.
Intern. Med._ 256, 183–194 (2004). Article PubMed CAS Google Scholar * Wang, L. et al. Evaluation of tau imaging in staging Alzheimer disease and revealing interactions between
beta-amyloid and tauopathy. _JAMA Neurol._ 73, 1070–1077 (2016). Article PubMed PubMed Central Google Scholar * US Department of Health and Human Services, Food and Drug Administration,
Center for Drug Evaluation and Research & Center for Biologics Evaluation and Research. Early Alzheimer’s disease: developing drugs for treatment. Guidance for industry. Draft guidance.
_FDA_ https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM596728.pdf (2018). * European Medicines Agency. Guideline on the clinical investigation of
medicines for the treatment of Alzheimer’s disease. _EMA_ http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2018/02/WC500244609.pdf (2018). * Standford University.
Gendered innovations terminology. _Gendered Innovations_ http://genderedinnovations.stanford.edu/terms.html (2018). * Jary, D. & Jary, A. Collins Dictionary of Sociology 3rd edn.
(Collins, Glasgow, 2005). * World Health Organization. Gender. _WHO_ http://www.who.int/en/news-room/fact-sheets/detail/gender (2015). * Dubal, D. B., Broestl, L. & Worden, K. Sex and
gonadal hormones in mouse models of Alzheimer’s disease: what is relevant to the human condition? _Biol. Sex. Differ._ 3, 24 (2012). THIS PAPER IS A COMPREHENSIVE OVERVIEW OF SEX DIFFERENCES
IN THE MOST WIDELY USED TRANSGENIC MODELS OF AD-LIKE AMYLOIDOSIS. Article PubMed PubMed Central Google Scholar * Middeldorp, J. et al. Preclinical assessment of young blood plasma for
Alzheimer disease. _JAMA Neurol._ 73, 1325–1333 (2016). Article PubMed PubMed Central Google Scholar * LaClair, K. D. et al. Treatment with bexarotene, a compound that increases
apolipoprotein-E, provides no cognitive benefit in mutant APP/PS1 mice. _Mol. Neurodegener._ 8, 18 (2013). Article PubMed PubMed Central CAS Google Scholar * Melnikova, T. et al.
Sex-related dimorphism in dentate gyrus atrophy and behavioral phenotypes in an inducible tTa:APPsi transgenic model of Alzheimer’s disease. _Neurobiol. Dis._ 96, 171–185 (2016). Article
PubMed CAS Google Scholar * Granger, M. W. et al. A TgCRND8 mouse model of Alzheimer’s disease exhibits sexual dimorphisms in behavioral indices of cognitive reserve. _J. Alzheimers Dis._
51, 757–773 (2016). Article PubMed CAS Google Scholar * Lewis, J. et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. _Science_ 293, 1487–1491
(2001). Article PubMed CAS Google Scholar * Oikawa, N., Ogino, K., Masumoto, T., Yamaguchi, H. & Yanagisawa, K. Gender effect on the accumulation of hyperphosphorylated tau in the
brain of locus-ceruleus-injured APP-transgenic mouse. _Neurosci. Lett._ 468, 243–247 (2010). Article PubMed CAS Google Scholar * Dumont, M. et al. Behavioral deficit, oxidative stress,
and mitochondrial dysfunction precede tau pathology in P301S transgenic mice. _FASEB J._ 25, 4063–4072 (2011). Article PubMed PubMed Central CAS Google Scholar * Yue, M., Hanna, A.,
Wilson, J., Roder, H. & Janus, C. Sex difference in pathology and memory decline in rTg4510 mouse model of tauopathy. _Neurobiol. Aging_ 32, 590–603 (2011). Article PubMed CAS Google
Scholar * Bour, A. et al. Middle-aged human apoE4 targeted-replacement mice show retention deficits on a wide range of spatial memory tasks. _Behav. Brain Res._ 193, 174–182 (2008). Article
PubMed CAS Google Scholar * Reverte, I., Klein, A. B., Ratner, C., Domingo, J. L. & Colomina, M. T. Behavioral phenotype and BDNF differences related to apoE isoforms and sex in
young transgenic mice. _Exp. Neurol._ 237, 116–125 (2012). Article PubMed CAS Google Scholar * Rijpma, A. et al. Sex differences in presynaptic density and neurogenesis in middle-aged
ApoE4 and ApoE knockout mice. _J. Neurodegener Dis._ 2013, 531326 (2013). PubMed PubMed Central CAS Google Scholar * Cacciottolo, M. et al. The APOE4 allele shows opposite sex bias in
microbleeds and Alzheimer’s disease of humans and mice. _Neurobiol. Aging_ 37, 47–57 (2016). Article PubMed CAS Google Scholar * Hampel, H. et al. Revolution of Alzheimer precision
neurology. Passageway of systems biology and neurophysiology. _J. Alzheimers Dis._ 64, S47–S105 (2018). * Hampel, H. et al. Precision medicine and drug development in Alzheimer’s disease:
the importance of sexual dimorphism and patient stratification. _Front. Neuroendocrinol._ https://doi.org/10.1016/j.yfrne.2018.06.001 (2018). Download references ACKNOWLEDGEMENTS H.H. was
supported by the AXA Research Fund, the Fondation Partenariale Sorbonne Université, the Fondation pour la Recherche sur Alzheimer, Paris, France and the programme ‘Investissements d’avenir’
(ANR-10-IAIHU-06; Agence Nationale de la Recherche-10-IA, Agence Institut Hospitalo-Universitaire-6; awarded to H.H.). Further support was provided by the Colam Initiatives and the Fondation
pour la Recherche sur Alzheimer, Paris, France (awarded to H.H. and P.A.C.) and the programme ‘PHOENIX’, led by the Sorbonne University Foundation and sponsored by the Fondation pour la
Recherche sur Alzheimer (awarded to H.H. and E.C.). H.G. acknowledges support from the Heart and Stroke Foundation of Canada, the Canadian Institutes of Health Research and the Canadian
Foundation for Innovation. H.G. is also the holder of an investigator award from the Fonds de Recherche du Québec-Santé. M.T.F. is supported by a research fellowship by the Synapsis
Foundation–Alzheimer Research Switzerland (ARS). M.F.I. acknowledges support from the Fonds de Recherche du Québec-Santé and from the Herbert H. Jasper Postdoctoral Research Fellowship from
the Groupe de Recherche sur le Système Nerveux Central (GRSNC), Université de Montréal. The authors thank A. Kato (Department of Basic Neuroscience, University of Geneva, Geneva,
Switzerland) and L. Kulic (Institute for Regenerative Medicine, University of Zurich, Schlieren, Switzerland) for encouragement and help with the first draft of the manuscript and A.
Herrmann (Cambridge Stem Cell Institute, University of Cambridge, Cambridge, UK) for continuous support, insightful discussions and editorial work. The authors thank the contributors to the
Alzheimer Precision Medicine Initiative Working Group (Supplementary Box 1). The initial idea and draft of this Review was conceived by the Women’s Brain Project (a non-profit organization
advocating for women’s brain and mental health; www.womensbrainproject.com) as part of its advocacy and scientific activity. REVIEW CRITERIA We searched PubMed and Google Scholar for
articles published in English without time limitations with the search terms “Alzheimer AND gender (or sex or women or female)”, “Amyloid AND gender (or sex or women or female)”, “plaques
AND gender (or sex or women or female)”, “tau AND gender (or sex or women or female)”, “atrophy AND Alzheimer AND gender (or sex or women or female)”, “cognitive decline AND gender (or sex
or women or female)”, “risk AND Alzheimer AND gender (or sex or women or female)”, “stroke AND Alzheimer AND gender (or sex or women or female)”, “cardiovascular AND Alzheimer AND gender (or
sex or women or female)”, “cerebrovascular AND gender (or sex or women or female)”, “diabetes AND Alzheimer AND gender (or sex or women or female)”, “depression AND Alzheimer AND gender (or
sex or women or female)” and “APOE AND Alzheimer AND gender (or sex or women or female)”. We also searched in the reference lists of identified articles for additional relevant
publications. The final reference list was generated by choosing only papers published since 2012. Papers preceding 2012 were included only if considered by the authors to be landmark
studies. Papers were selected on the basis of their perceived relevance to the topics covered in this Review. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Institute for Regenerative
Medicine, University of Zurich, Schlieren, Switzerland Maria Teresa Ferretti * Neuroscience Center Zurich, University of Zurich, Zurich, Switzerland Maria Teresa Ferretti * Département de
Neurosciences, Université de Montréal, Montreal, Quebec, Canada Maria Florencia Iulita * Groupe de Recherche sur le Système Nerveux Central (GRSNC), Université de Montréal, Montreal, Quebec,
Canada Maria Florencia Iulita & Hélène Girouard * AXA Research Fund and Sorbonne University, Paris, France Enrica Cavedo, Patrizia Andrea Chiesa & Harald Hampel * Sorbonne
University, GRC n° 21, Alzheimer Precision Medicine, Pitié-Salpêtrière Hospital, Paris, France Enrica Cavedo, Patrizia Andrea Chiesa & Harald Hampel * Brain and Spine Institute, INSERM
U, 1127, Paris, France Enrica Cavedo, Patrizia Andrea Chiesa & Harald Hampel * Institute of Memory and Alzheimer’s Disease, Department of Neurology, Pitié-Salpêtrière Hospital, Paris,
France Enrica Cavedo, Patrizia Andrea Chiesa & Harald Hampel * IRCCS Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy Enrica Cavedo * Interdisciplinary Competence Centre for
Ageing IKOA-FHS, University of Applied Sciences, St. Gallen, Switzerland Annemarie Schumacher Dimech & Sabina Misoch * Swiss Agency for Therapeutic Products, Bern, Switzerland Antonella
Santuccione Chadha * Pulmonary Clinic, Division of Pulmonology, University Hospital Zurich, Zurich, Switzerland Francesca Baracchi * Départment de Pharmacologie et Physiologie, Université de
Montréal, Montreal, Quebec, Canada Hélène Girouard * Institut Universitaire de Gériatrie de Montréal, Montreal, Quebec, Canada Hélène Girouard * Department of Internal Medicine,
Rehabilitation and Geriatrics, University of Geneva Hospitals, Geneva, Switzerland Ezio Giacobini * Department of Obstetrics and Gynaecology, Ghent University Hospital, Ghent, Belgium Herman
Depypere Authors * Maria Teresa Ferretti View author publications You can also search for this author inPubMed Google Scholar * Maria Florencia Iulita View author publications You can also
search for this author inPubMed Google Scholar * Enrica Cavedo View author publications You can also search for this author inPubMed Google Scholar * Patrizia Andrea Chiesa View author
publications You can also search for this author inPubMed Google Scholar * Annemarie Schumacher Dimech View author publications You can also search for this author inPubMed Google Scholar *
Antonella Santuccione Chadha View author publications You can also search for this author inPubMed Google Scholar * Francesca Baracchi View author publications You can also search for this
author inPubMed Google Scholar * Hélène Girouard View author publications You can also search for this author inPubMed Google Scholar * Sabina Misoch View author publications You can also
search for this author inPubMed Google Scholar * Ezio Giacobini View author publications You can also search for this author inPubMed Google Scholar * Herman Depypere View author
publications You can also search for this author inPubMed Google Scholar * Harald Hampel View author publications You can also search for this author inPubMed Google Scholar CONSORTIA FOR
THE WOMEN’S BRAIN PROJECT AND THE ALZHEIMER PRECISION MEDICINE INITIATIVE CONTRIBUTIONS M.T.F., E.G. and H.H. conceived the paper. All authors contributed to the literature search and to the
writing. M.T.F., E.C. and P.A.C. designed the figures. E.G., H.H., H.D., H.G. and S.M. provided guidance for specific areas of competence and overall paper design. A.C.S. contributed to the
paper with her own expertise and points of view; the views and opinions expressed herein are those of the author and do not reflect the view of the Swiss Agency for Therapeutic Products
(Swissmedic). CORRESPONDING AUTHOR Correspondence to Maria Teresa Ferretti. ETHICS DECLARATIONS COMPETING INTERESTS H.H. is a Senior Associate Editor for the journal _Alzheimer’s &
Dementia_. He has received fees for lecturing from Biogen and Roche; research grants from Pfizer, Avid, and MSD Avenir (all three paid to his institution); travel funding from Axovant, Eli
Lilly, Functional Neuromodulation, GE Healthcare, Oryzon Genomics and Takeda and Zinfandel; and consultancy fees from Anavex, Axovant, Cytox, Functiona Neuromoduation, GE Healthcare, Jung
Diagnostics, Oryzon Genomics and Takeda and Zinfandel. He participated in scientific advisory boards of Axovant, Cytox, Eli Lilly, Functional Neuromodulation, GE Healthcare, Oryzon Genomics,
Roche Diagnostics and Takeda and Zinfandel. He is a co-inventor on several patents related to markers and the diagnosis of neurodegenerative disease (numbers 8916388, 8298784, 20120196300,
20100062463, 20100035286, 20090263822, 7547553, 20080206797, 20080199966 and 20080131921) but has received no royalties. All other authors declare no conflicts of interest. ADDITIONAL
INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RELATED LINKS THE WOMEN’S BRAIN PROJECT:
www.womensbrainproject.com ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY BOX 1 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ferretti, M., Iulita,
M.F., Cavedo, E. _et al._ Sex differences in Alzheimer disease — the gateway to precision medicine. _Nat Rev Neurol_ 14, 457–469 (2018). https://doi.org/10.1038/s41582-018-0032-9 Download
citation * Published: 09 July 2018 * Issue Date: August 2018 * DOI: https://doi.org/10.1038/s41582-018-0032-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative