Play all audios:
ABSTRACT The coronavirus disease 2019 (COVID-19) pandemic is concerning for patients with neuroimmunological diseases who are receiving immunotherapy. Uncertainty remains about whether
immunotherapies increase the risk of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or increase the risk of severe disease and death upon infection. National and
international societies have developed guidelines and statements, but consensus does not exist in several areas. In this Review, we attempt to clarify where consensus exists and where
uncertainty remains to inform management approaches based on the first principles of neuroimmunology. We identified key questions that have been addressed in the literature and collated the
recommendations to generate a consensus calculation in a Delphi-like approach to summarize the information. We summarize the international recommendations, discuss them in light of the first
available data from patients with COVID-19 receiving immunotherapy and provide an overview of management approaches in the COVID-19 era. We stress the principles of medicine in general and
neuroimmunology in particular because, although the risk of viral infection has become more relevant, most of the considerations apply to the general management of neurological
immunotherapy. We also give special consideration to immunosuppressive treatment and cell-depleting therapies that might increase susceptibility to SARS-CoV-2 infection but reduce the risk
of severe COVID-19. KEY POINTS * The risk that the coronavirus disease 2019 (COVID-19) pandemic poses for people who are receiving immunotherapy for neuroimmunological disease remains
unclear. * Guidelines and statements have been published by societies and individuals, but the level of consensus differs for different aspects; we use a Delphi-like process to clarify where
consensus exists. * Without evidence, management of neuroimmunological diseases in the context of COVID-19 requires application of the first principles of immunotherapy, taking into account
disease-related, patient-related, physician-related, environment-related and COVID-19-related factors. * In general, corticosteroids, intravenous immunoglobulin and/or plasma exchange for
the treatment of acute neuroimmunological deteriorations can be administered with low risk in the COVID-19 pandemic. * In general, ongoing immunotherapy should not be stopped because of the
COVID-19 pandemic; treatment initiation and optimization are also recommended. * For some aspects of immunotherapy in the context of COVID-19, consensus in the literature is low, and
collection of data in patient registries is important for resolving these uncertainties. SIMILAR CONTENT BEING VIEWED BY OTHERS LIFTING THE MASK ON NEUROLOGICAL MANIFESTATIONS OF COVID-19
Article 24 August 2020 COVID-19: IMMUNOPATHOGENESIS AND IMMUNOTHERAPEUTICS Article Open access 25 July 2020 RECOMMENDATIONS FOR THE MANAGEMENT OF COVID-19 IN PATIENTS WITH HAEMATOLOGICAL
MALIGNANCIES OR HAEMATOPOIETIC CELL TRANSPLANTATION, FROM THE 2021 EUROPEAN CONFERENCE ON INFECTIONS IN LEUKAEMIA (ECIL 9) Article 29 April 2022 INTRODUCTION Coronavirus disease 2019
(COVID-19) is a novel illness caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)1,2. The first infections were reported in China in late 2019, and the WHO
declared a global pandemic on 11 March 2020. The disease course is highly heterogeneous, ranging from infectious but asymptomatic to severe disease and death, often due to respiratory
failure3. The host reaction to the virus can include antibody-mediated inflammation4 and a cytokine storm5 that are thought to have a major impact on outcome. Most people recover with only
supportive care, indicating that the infection induces short-term immunity, but whether long-term immunity develops or recurrent circulation of SARS-CoV-2 can be expected — as seen with
other coronaviruses — is unknown6. The COVID-19 pandemic raises important questions about the risk to patients with neuroimmunological diseases who are treated with immunotherapies. These
diseases — a key example of which is multiple sclerosis (MS) — are thought to result from immune tolerance dysfunction that affects immune regulatory networks7 and often necessitates
continuous immunosuppressive or immunomodulatory therapy. Immunosuppressive therapies can limit immune competence, whereas immunomodulatory therapies adjust the immune system without
affecting immune competence8. Immunotherapies can affect the risk of infections9 and some therapies are associated with an increased risk from particular types of pathogens10. Several
elements of the risk of COVID-19 in patients with neuroimmunological disease are unclear; for example, whether these patients have an increased risk of being infected by SARS-CoV-2 after
exposure, whether they have an increased risk of developing a clinical rather than asymptomatic infection, and whether they have an increased risk of a clinical infection becoming severe
enough to require intensive care or to lead to persistent disability or death. We refer to the sum of these risks as an ‘increased risk of severe COVID-19’. A further complication is that
there is a rationale for immunotherapy being beneficial in COVID-19, especially in the management of complications such as acute respiratory distress syndrome (ARDS)11,12, though caution is
needed given the negative history of immunotherapy for infection, including negative trials of corticosteroids for ARDS13. Initial reports of patients with immunosuppression and COVID-19 are
complicated but suggest no general deleterious effect14 and a potentially helpful role of immunosuppression, especially in patients who are at risk of serious complications in the second
phase of the disease15,16. Several national and international guidelines, recommendations and statements and individual opinion pieces have addressed the management of patients with
neuroimmunological disease, especially MS, during the COVID-19 pandemic. However, these publications are heterogeneous in their level of detail and their findings. In this Review, we bring
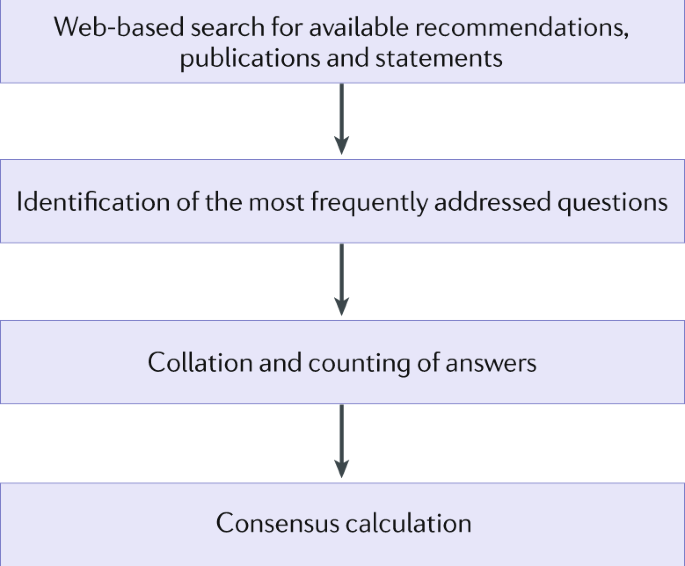
together the existing information and develop an overview of the current consensus in the literature based on a ‘Delphi-like’ process17 (Box 1). We searched the available literature,
including publications of national and international societies and statements related to COVID-19 and neurology (Supplementary Table 1). We identified key questions addressed within these
publications and compiled the answers, indicating the level of consensus in the literature (Table 1). On this basis, we propose an algorithm that encompasses the factors that influence
decision-making for patients with neuroimmunological diseases in the COVID-19 era. BOX 1 DEVELOPMENT OF A DELPHI-LIKE PROCESS The homepages of national and international neurological
societies and patient organizations were screened for relevant recommendations, statements and agreements (Supplementary Table 1). The formats and content of the search results were
heterogeneous, ranging from short, general advice to very detailed disease-specific and, in some instances, product-specific recommendations. On the basis of the frequency with which topics
were addressed in the relevant literature — probably a reflection of the most urgent real-world needs — key questions were developed. Answers to these questions were drawn out of the
literature and an approval rate was calculated (see the figure). A summary of each question, the relevant answer/recommendation and level of consensus are presented in Table 1. THE
CHALLENGES OF COVID-19 In the modern medical era of randomized controlled trials, Cochrane analyses and, at the very least, detailed series, we are habituated to making decisions supported
by an evidence base. In the unusual situation of the COVID-19 pandemic, a potentially lethal novel construct has been superimposed on the existing complexities of our patients and their
management, and little specific evidence is available, making management decisions difficult and complex. In multiple series published to date, a total of several hundreds of patients
receiving immunosuppressive therapy for various diseases have recovered from COVID-19, thereby reassuring us that the infection is not necessarily catastrophic. Nevertheless, we must keep in
mind that very large numbers of patients must be studied before small effects can be excluded. For example, in a trial in which population X receives intervention Y that increases the
likelihood of outcome Z from 2% to 4%, a sample size of 1,141 per group is needed for an α of 0.05, a β of 0.2 and a power of 0.8. In this scenario, X represents patients with
neuroimmunological disease, Y represents the patients’ immunotherapy and Z is the likelihood of requiring intensive care or of death if the drug doubles the risk of catastrophic COVID-19. We
simply do not have that amount of data yet — until we do, we should work from first principles. In this scenario an explicit reiteration of the first principles of medicine is warranted in
balancing neuroimmune treatments with the risk of COVID-19 (Box 2), including pertinent factors that influence decision making in addition to the recommendations (Box 3; Table 1). The
following points and factors are critical for making decisions about the initiation, continuation and optimization of a patient’s neuroimmunological treatment: * First, do no harm —
inactivity that leads to permanent damage is a form of harm by neglect, but caution should be exercised if the situation allows (for example, if the patients have highly relevant
comorbidities, or if the burden of the underlying disease is low). * In general, immunosuppressive strategies produce a substantial benefit in relation to the disease being treated, but the
trade-off is often a modest increase in the risk of infection that is dependent on dose, duration and intensity of therapy. * Disease-related factors; for example, how serious and reversible
the patient’s disease is (for example, myasthenia is reversible, so undertreatment is more acceptable than for aquaporin 4-related neuromyelitis optica, which is not), the individual’s
clinical course and the current disease activity. * Patient-related factors; for example, the factors that influence the patient’s risk of serious or lethal COVID-19 (including age, sex,
comorbidities and perception of the situation, such as risk aversion). * The patient should be asked whether the disease they have or the one they could get matters more to them — the
doctor’s risk prioritization does not always match the patient’s. * Physician-related factors, such as experience, understanding of the disease and its treatment, treatments available and
monitoring capabilities. * Environmental factors; for example, the local likelihood of exposure to COVID-19 now and in the future, and local logistical issues, such as limitations in patient
support, infusion units or intensive care units. * Treatment-related factors, such as the extent to which treatment could affect the response to COVID-19, whether the treatment is
reversible and whether the dose can be reduced or deferred. BOX 2 FACTORS IN TREATMENT DECISIONS FOR NEUROIMMUNOLOGICAL DISEASES FIRST DIMENSION — GENERAL FACTORS * Patient-related factors
(including age, comorbidities, gender, personal perspective) * Disease-related factors (including diagnosis, prognosis, disease activity, reversibility) * Physician-related factors
(including experience, personal evaluation) * Treatment-associated factors (including immunosuppressive effects, cell depletion, reversibility) SECOND DIMENSION — PHASE OF TREATMENT * Acute
deterioration (corticosteroids, plasma exchange or intravenous immunoglobulin) * Treatment initiation (time point of start, mode of action) * Treatment continuation (dosage, possibility of
delaying, safety controls, cessation) * Treatment optimization (dosage, time point, mode of action, reversibility) THIRD DIMENSION — SARS-COV-2-ASSOCIATED FACTORS * No infection * Acute mild
infection * Acute severe infection * Recovered from infection or COVID-19 * Local infection rate * Local strategy for pandemic management COVID-19, coronavirus disease 2019; SARS-CoV-2,
severe acute respiratory syndrome coronavirus 2. BOX 3 SUMMARY OF THE MAIN RESULTS OF THE DELPHI-LIKE PROCESS ASPECTS FOR WHICH CONSENSUS IS STRONG * Patients without infection should not
stop ongoing immunotherapy. * In acute deterioration of neuroimmunological conditions, corticosteroids, intravenous immunoglobulin or plasma exchange can be administered. * In MS, glatiramer
acetate and IFNβ do not have a negative effect in relation to SARS-CoV-2 infection or the course of COVID-19. * In active MS, natalizumab is considered to be associated with a lower risk of
COVID-19 than other high-efficacy disease-modifying therapies. * In MS, sphingosine 1-phosphate receptor modulators and cell-depleting therapies increase the risk of SARS-CoV-2 infection. *
In MS, postponement of cell-depleting therapy can be considered in patients continuing on therapy. ASPECTS FOR WHICH CONSENSUS IS LOW * Whether teriflunomide and dimethyl fumarate increase
the risk of SARS-CoV-2 infection * Whether patients with mild or asymptomatic COVID-19 and those with a high risk of exposure to SARS-CoV-2 should stop immunotherapy during the COVID-19
pandemic * How disease-modifying therapies influence the course of severe COVID-19 in which overwhelming inflammation occurs COVID-19, coronavirus disease 2019; MS, multiple sclerosis;
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2. TREATMENT OF ACUTE DETERIORATIONS Acute deteriorations or relapses of neuroimmunological diseases are usually treated with
corticosteroid pulse therapy, plasma exchange or intravenous immunoglobulin (IVIg) before initiation of or changes to long-term disease-modifying therapies (DMTs). In this section, we
discuss the use of these acute treatments in the context of COVID-19. CORTICOSTEROIDS Corticosteroids dampen cytokine responses and the recruitment of leukocytes, and suppress T cell
activation and differentiation18; for these reasons, they can increase the risk of infection as a result of short-term lymphopenia19. However, short-term therapy is not usually associated
with a marked increase in the risk of infection20. Chronic, high doses of steroids increase the rate of infections and are associated with other long-term adverse effects, such as
hypertension, osteoporosis and muscle weakness. The question of short-term corticosteroid therapy for acute relapses of neuroimmunological diseases in the COVID-19 era was not addressed in
all sources included in our Delphi-like analysis. Where this question was addressed, it related to MS, and we identified agreement that steroid pulse therapy should be administered for a
relapse with a relevant neurological deficit (Table 1, Question 2). For relapses with very minor symptoms (for example, dysaesthesias) or where no evidence indicates a benefit of treatment
(for example, repetitive pulses in progressive disease), corticosteroids should currently be avoided. Steroid tapering — which is sometimes done to prolong and consolidate the pulse effect
when pulse treatment has not produced substantial clinical improvement — is not recommended. Overall, short-term treatment with corticosteroids carries a low risk in relation to COVID-19 and
should be administered whenever necessary. Corticosteroid therapy has been used in the treatment of SARS-associated and Middle East respiratory syndrome (MERS)-associated pneumonia21, but
analysis has revealed no beneficial effects and, in some cases, harmful effects22. Evidence in relation to the use of corticosteroids for the treatment of COVID-19 is lacking and expert
opinions are contradictory — their use has been recommended for severe COVID-19 when the criteria for ARDS is fulfilled, but not for severe COVID-19 without ARDS23. Preliminary results of an
ongoing trial suggest a survival benefit of low dose dexamethasone in patients requiring at least oxygen treatment24. Ongoing clinical trials of corticosteroids in COVID-19 might provide
further insights. PLASMA EXCHANGE AND IVIG Plasma exchange or IVIg are used for acute therapy in many neuroimmunological diseases. In some diseases, such as chronic inflammatory
demyelinating polyneuropathy (CIDP) and myasthenia gravis, these therapies are used repeatedly with or without other DMTs. Plasma exchange usually requires the patient to attend hospital,
sometimes requires a central venous catheter with an associated risk of infection and reduces levels of the patient’s own immunoglobulins and complement factors for several weeks, which
could affect COVID-19 response and outcome. IVIg usually requires the patient to attend hospital or an infusion unit, although home administration is possible in some cases (depending also
on the regulatory environment). This treatment does not lower immunoglobulin levels and arguably has no compromising effects related to COVID-19. Discussion in the literature about the use
of plasma exchange and IVIg in the context of COVID-19 is limited, but overall, the risk of increasing the likelihood of infection or of severe disease is considered mild (Table 1, Question
14) and is likely to offset risks associated with the neuroimmunological disease. Use of plasma exchange or IVIg can reduce the need for concurrent corticosteroid use and accelerate recovery
and discharge from hospital, thereby reducing time in hospital and the related risks. Subcutaneous immunoglobulin can be used for CIDP and enables home administration that avoids the risk
of additional exposure to SARS-CoV-2. Beneficial effects of IVIg in COVID-19 are highly unlikely if standard, SARS-CoV-2-naive immunoglobulin preparations are used, although the
immunomodulatory effects and a consequent reduction in antibody-dependent hyperinflammation have the potential to be beneficial25,26. By contrast, some evidence suggests that IVIg might
increase the risk of thrombosis and substantial thrombotic complications, including multifocal stroke, in COVID-19 (ref.27). SERIOUS EXACERBATIONS In neuromyelitis optica spectrum disorders
(NMOSD), disability is generally relapse-related28 and early treatment of relapses improves outcomes29. Consequently, any risk of COVID-19 that is associated with corticosteroid pulse
therapy or plasma exchange is likely to be outweighed by the risk of long-term disability if not treated30. In myasthenia gravis, acute deterioration can require plasma exchange or IVIg31
and the first reports on myasthenic crisis as a complication of COVID-19 and on the course of COVID-19 in patients with myasthenia gravis are heterogeneous32,33. Dysphagia or respiratory
insufficiency can be life threatening, so IVIg or plasma exchange should be administered to patients with these symptoms34. Results are currently collected in various registries, with
increasing numbers providing a better repository for evaluations; these registries include: Coronavirus and MS reporting database (COVIMS), Lean European Open Survey on SARS-CoV-2 Infected
Patients (LEOSS) and COVID-19 Associated Risks and Effects in Myasthenia Gravis (CARE-MG). GENERAL CHRONIC IMMUNOSUPPRESSION Approved therapies are available for few neuroimmunological
diseases other than MS, so most are likely to be treated with general immunosuppressive medications, such as corticosteroids, azathioprine, mycophenolate, methotrexate and rituximab, with
adjunctive IVIg and plasma exchange. Exceptions include neurosarcoidosis, giant cell arteritis (GCA) and NMOSD, for which infliximab, tocilizumab and eculizumab, respectively, can be used.
In our opinion, these drugs should not substantially increase the risk of serious COVID-19, but data from registries are needed. Similarly, life-threatening conditions such as vasculitis and
autoimmune encephalitis can necessitate use of potent immunosuppression with cyclophosphamide, sometimes in combination with rituximab and other drugs. In these conditions, the severity and
potentially irreversible nature of the underlying diseases should take priority over probable increases in the risk of severe COVID-19. Long-term oral corticosteroid treatment for several
diseases, including rheumatoid arthritis, systemic lupus erythematosus (SLE) and GCA, clearly increases the risk of serious infections for which the patient requires hospitalization,
although data in most neuroimmunological diseases are less cohesive35,36,37. Higher doses, longer durations of therapy and older age are all additional risk factors for infection during
chronic treatment with corticosteroids20. In a study of patients with immune-mediated inflammatory diseases and COVID-19 in New York, USA, patients treated with chronic corticosteroids or
any small-molecule drug were more likely to require hospitalization for COVID-19 than patients who were not receiving corticosteroids (see the supplementary files in the original study)16,
even though the rate of hospitalization among patients with immune-mediated inflammatory disease was not higher than among the general population. In patients with newly diagnosed CIDP, IVIg
or plasma exchange are suggested in preference to corticosteroids38. The immunosuppressive drugs azathioprine, mycophenolate and methotrexate are clearly associated with occasional atypical
infections, such as progressive multifocal leukoencephalopathy (PML) and _Pneumocystis jirovecii_ pneumonia39. These infections can occur with these drugs when used alone but are more
common when the drugs are used as combined immunotherapies, such as after organ transplantation. These drugs are also associated with a small increase in the relative risk of all serious
infections in conditions such as SLE, but the risk is smaller than that with corticosteroids35. Whether azathioprine, mycophenolate and methotrexate should be stopped or their dose reduced
in the event of an active SARS-COV-2 infection or a high-risk exposure is disputed not only in the recommendations made so far (Table 1, Question 22) but even in the approved product
information documents40,41. Authors of opinion pieces have recommended that treatment decisions with these drugs in the context of COVID-19 are decided on the basis of the immunological
disease, latency of response to specific immune therapies, other patient risks and the strength of evidence that treatment will be beneficial26,38. Long-term corticosteroid treatment should
not be stopped abruptly and weaning should be done under physician supervision. In our opinion, minimizing the dosage of chronic corticosteroids is a reasonable way to reduce the risk of
infection without compromising immune competence. The available evidence supports steroid-sparing immunotherapy in general practice, meaning that the introduction of an immunosuppressive
agent such as mycophenolate should not be delayed if the alternative is continuation of high-dose corticosteroids, even in the context of COVID-19. If an individual who is receiving chronic
immunosuppressive therapy is diagnosed with COVID-19 or is considered to be at high risk of infection, a patient-focused individual decision should be made on whether to stop or reduce the
dosage of their immunosuppressive drug. This decision should take into account factors such as the severity of the two diseases, the patient’s age, the presence of risk factors for COVID-19,
the likelihood that short-term drug withdrawal will precipitate a relapse (negligible in myasthenia gravis but high for some drugs in MS), current and recent lymphocyte counts (which can
also be low in acute COVID-19), and the pharmacokinetics of the immunosuppressive drug. THERAPIES FOR MULTIPLE SCLEROSIS TREATMENT Most therapeutic approaches to MS involve long-term
immunotherapy. The general consensus among the available recommendations is that patients with MS should not stop their DMT during the COVID-19 pandemic without advice from their neurologist
(Table 1, Question 3). Discontinuation carries the risk of deterioration or relapse that could lead to an increase in disability and hospital admission. The risks of each therapy are
discussed in more detail below. IFNΒ1A AND IFNΒ1B IFNβ1a and IFNβ1b are recombinant cytokines that increase the cytotoxicity of natural killer cells, the phagocytic activity of macrophages
and the antibody-dependent cytotoxicity of leukocytes42. National and international neurological societies agree that treatment with IFNβ does not increase the risk for patients during the
COVID-19 pandemic. Interferons are not considered to be immunosuppressive and could therefore be a safe option for initiation of DMT in patients with mild-to-moderate MS disease activity
during the COVID-19 pandemic (Table 1, Questions 4 and 15). In general, interferons impair viral replication43 and previous evidence suggests that IFNβ is mildly effective in animal models
of MERS when used in combination with lopinavir and ritonavir44, so the potential for IFNβ in the treatment of COVID-19 could be worth investigating. GLATIRAMER ACETATE Glatiramer acetate is
a synthetic amino acid copolymer that contains the key amino acids from myelin basic protein45. Its mechanism of action includes attenuation and rebalancing of T cell responses (a shift
towards the type 2 T helper cell phenotype), cytokine secretion and B cell function46,47. Glatiramer acetate is not immunosuppressive and is not associated with increased risk of infection.
The consensus in the literature is that treatment with glatiramer acetate should not increase the risk of severe COVID-19 in patients with MS (Table 1, Question 4 and 10), so is another good
option for initiation of DMT in patients with mild-to-moderate disease during the COVID-19 pandemic. TERIFLUNOMIDE Teriflunomide selectively and reversibly inhibits dihydro-orotate
dehydrogenase (DHODH), a key mitochondrial enzyme in the de novo pyrimidine synthesis pathway48. By influencing mitochondrial metabolism, teriflunomide causes selective immune suppression by
inhibiting growth and division of rapidly dividing cells, including activated T cells and B cells49. Owing to the mechanism of action, mild decreases in lymphocyte counts have been observed
during teriflunomide treatment but rates of grade 2 lymphopenia and serious infections are low, even after long-term therapy50. Expert opinions on whether teriflunomide can increase the
risk of severe COVID-19 are heterogeneous, probably owing to the known risk of lymphopenia. Decreases in lymphocyte counts occur within 6 months of teriflunomide initiation but are mostly
mild when the dosing scheme approved for MS is used. On this basis, most, but not all, recommendations suggest that teriflunomide should not increase the risk of severe COVID-19 (Table 1,
Questions 20 and 10). Importantly, teriflunomide has a long half-life (~16 days in the plasma) and elimination takes time because the drug persists in the enterohepatic cycle, so health-care
providers and patients should keep these factors in mind if stopping teriflunomide is considered upon SARS-CoV-2 infection. Teriflunomide can inhibit replication of Epstein–Barr virus,
cytomegalovirus (CMV), herpes simplex virus (HSV) 1 and 2, and poliovirus51,52,53,54. These observations suggest that DHODH inhibition could be a therapeutic strategy for COVID-19 (ref.55).
DIMETHYL FUMARATE Dimethyl fumarate reduces the absolute lymphocyte count, although the mechanisms of action are not fully understood. Memory CD8+ T cells are most profoundly affected but
levels of pro-inflammatory CD4+ T cell subsets, and B cell subsets are also decreased, creating a bias towards an anti-inflammatory state56,57. Lymphocytes normally decrease within the first
6 months of treatment but moderate or severe prolonged lymphopenia has been observed in 2–9% of patients58. Lymphopenia is considered to be a risk factor for dimethyl fumarate-associated
PML (caused by JC virus)59, so dimethyl fumarate therapy must be stopped if lymphocyte counts are ≤500 cells/µl. In addition, in rare cases, low lymphocyte counts in patients receiving
dimethyl fumarate have been associated with HSV encephalitis60. Though the risk of lymphopenia after initiation of dimethyl fumarate treatment is a potential risk for COVID-19, most national
and international recommendations suggest that dimethyl fumarate treatment should not increase the risk of severe COVID-19 (Table 1, Question 21). NATALIZUMAB Natalizumab was the first
monoclonal antibody to be approved for the treatment of relapsing–remitting MS. The target of the antibody is integrin α4, which mediates immune cell adhesion and migration via interactions
with vascular cell adhesion molecule 1 and fibronectin61. In general, infection rates among patients receiving natalizumab are low10,62 besides a slight increase in the risk of HSV
infection. The initial trial of natalizumab did suggest an increased risk of pneumonia63 but this observation does not seem to have been replicated in real-world series62,64. The most
concerning adverse effect of natalizumab is a low risk of PML, which is influenced by the JC virus antibody index, the duration of therapy and prior exposure to immune-suppressive agents65.
Most, but not all, international and national recommendations suggest that natalizumab therapy should not increase the risk of severe COVID-19 (Table 1, Question 18). On this basis
natalizumab is a candidate for patients who need to start on a high-efficacy therapy during the COVID-19 pandemic, and natalizumab should be continued for all patients who are already
receiving the therapy (Table 1, Question 11). Indeed, cessation or interruption of natalizumab treatment carries a considerable risk of MS disease exacerbation or rebound activity in
patients with highly active MS66, the consequences of which are likely to outweigh the risk of COVID-19. Natalizumab has been suggested as a bridging therapy (a drug that can be used for an
immediate effect owing to its fast onset of activity and high efficacy but that is not necessarily considered a long-term option) during the COVID-19 pandemic, and the first reports of
SARS-CoV-2 infection in patients receiving natalizumab therapy have suggested no increase in risk67,68. Furthermore, extended dosing intervals can be used to limit infusions and,
consequently, limit the exposure of patients to hospitals69. Extended dosing intervals could also decrease the risk of COVID-19-related encephalitis by allowing some CNS immunosurveillance.
The CNS is not thought to be the primary site or a major site of SARS-CoV-2 infection, although CNS manifestations, including dizziness, headache, impaired consciousness, acute
cerebrovascular disease, ataxia and seizures, have been reported in patients with severe COVID-19 (refs70,71). Some evidence indicates that integrins have a role in host cell entry by
SARS-CoV-2 (refs72,73), suggesting that natalizumab could even have beneficial effects in cases where CNS infection does occur. FINGOLIMOD, SIPONIMOD AND OZANIMOD Fingolimod, siponimod and
ozanimod are sphingosine 1-phosphate receptor (S1PR) modulators that result in downregulation of S1PR, which prevents egress of lymphocytes from lymphoid tissue. All three drugs are known to
decrease blood lymphocyte counts, but importantly this effect does not reflect the death or loss of lymphocytes, especially not those in the bone marrow — it means that these cells might
still be recruitable and functional but entrapped. Fingolimod treatment has been associated with increased rates of varicella zoster virus (VZV) activation or reactivation74, and other viral
infections, such as PML, have been described in rare cases75,76,77. Siponimod and ozanimod are more selective for the S1PR than is fingolimod but they have similar effects on lymphocyte
counts and have been associated with similar infections78,79,80,81. The effects of these drugs are reversible and their half-lives are short but the effects of S1PR modulators on lymphocyte
count can persist for several weeks to months82. National and international experts foresee a mild-to-moderate increase in the risk of SARS-CoV-2 infection among patients who are treated
with fingolimod, siponimod or ozanimod (Table 1, Question 6). However, cessation or interruption of therapy with S1PR modulators can trigger a severe disease rebound with a high risk of
relapse, so patients who are already receiving fingolimod, siponimod or ozanimod should continue their therapy. For patients who need to switch therapy or start a new therapy, other
treatment options should be considered. Fingolimod also has the potential to be of benefit in COVID-19 on the basis that secondary invasion of alveoli by lymphocytes is thought to be harmful
in this disease83 and could be prevented by fingolimod. A clinical trial of this approach is currently recruiting patients84. The effect of S1PR modulation on the outcome of the second
phase of COVID-19 is the question under investigation. IMMUNE-DEPLETING AND REPOPULATING THERAPIES B CELL-DEPLETING THERAPIES Ocrelizumab, ofatumumab and rituximab are monoclonal CD20
antibodies that deplete CD20+ B cells and small populations of CD20+ T cells. Inebilizumab is a CD19 antibody that depletes B cells expressing CD19. Ocrelizumab is approved for the treatment
of relapsing–remitting and primary progressive MS, ofatumumab is expected to be approved soon, and rituximab is used off-label in several neuroimmunological diseases, including NMOSD,
myasthenia gravis, immune neuropathies and MS. Inebilizumab has recently been approved for the treatment of NMOSD. B cell depletion reduces humoral and cellular immunity. Immunoglobulin
levels can decrease as the duration of treatment increases, but broad cytopenia rarely occurs85. In phase III studies, ocrelizumab treatment was associated with a slight increase in the risk
of infections (generally mild to moderate and none unusual, such as opportunistic infections)86. Ofatumumab was not associated with an increased risk of infections in the phase II study
programme, but longer term observations are currently lacking87. Similarly, although serious infections were not associated with rituximab treatment in phase III studies, real-world use has
revealed some risks — long-term use of rituximab is associated with an increased risk of severe infection, and rituximab and ocrelizumab have been associated with reactivation of hepatitis B
and C viruses10,88,89. In addition, PML has been reported in several patients receiving rituximab for various diseases and PML was associated with ocrelizumab in one patient (as of April
2020), although multiple influencing factors have to be considered in this case90. The first reports on the impact of B cell-depleting therapy on COVID-19 have been published14,67,91. In 232
patients with MS and suspected or proven COVID-19, the severity of COVID-19 was classified as mild (no or mild pneumonia) in 222 (96%), severe (shortness of breath, respiratory rate ≥30
breaths/min, blood oxygen saturation ≤93%, PaO2:FiO2 <300 mmHg/%, and an increase in lung infiltrates of >50% within 24–48 h) in 4 (2%) and critical (respiratory failure, septic shock
and multiple organ dysfunction or failure) in 6 (3%). Of the 6 patients with critical illness, 1 recovered and 5 died. Of the 28 patients receiving a B cell-depleting therapy, 3 (10%)
developed a severe or critical disease course14. In addition, 100 patients with confirmed or suspected SARS-CoV-2 infection have been studied within pharmacovigilance reporting conditions by
Roche92; 26 of these patients were admitted to hospital and 13 remained in hospital at the time of writing. Five patients were classified as critically ill and needed intensive care. Among
the limited data currently available, outcomes of COVID-19 during B cell-depleting therapy range from mild disease to death but — although the number of patients is low — rates of critical
illness and death do not seem to be increased dramatically relative to the wider population93. This observation could be explained by the fact that, although B cell-depleting therapies are
thought to increase susceptibility to acute respiratory infections94, antibody-mediated inflammation and a cytokine storm are thought to mediate severe COVID-19 outcomes, so B cell depletion
might not necessarily be associated with more severe disease95. International and national recommendations suggest that B cell-depleting therapy is likely to increase the risk of severe
COVID-19 (Table 1, Question 12) but that the risk level depends on the duration of therapy and the time since the last dose because immunoglobulin deficiency occurs more frequently after 2–3
years of treatment and circulating drug levels are likely to decline during the treatment interval. Depletion of B cells in the peripheral circulation lasts for at least 5–6 months after
the last dose and full recovery of B cells after a course of treatment with ocrelizumab takes up to 72 weeks. On the basis of current knowledge, postponement of the next dose of B
cell-depleting therapy is advisable for some patients with MS (Table 1, Question 13), especially those with additional risk factors and progressive disease, or unsafe infusion environments.
However, the pandemic is ongoing and therapy cannot be delayed indefinitely — waiting until disease activity returns is not advisable. Levels of peripheral B cells and the recovery of these
levels is one possible indicator that can be monitored to assess the urgency of the next infusion. In principle, CD20 antibody therapy could be initiated during the COVID-19 pandemic in
suitable patients (for example, those with high disease activity, no comorbidities and a younger age), but the risk to benefit ratio should be taken into account, especially in older
patients and those with comorbidities. B cell depletion in older patients with progressive MS and in patients with mild MS and no activity should be viewed with particular caution because
these patients are least likely to benefit from anti-CD20 therapies. Another issue that must be considered with B cell-depleting therapy is the nature of the immune response to either a
primary infection with SARS-CoV-2 or a primary vaccination, should a vaccine be developed. Rituximab treatment is known to inhibit the primary antibody response to a neoantigen to a greater
extent than the secondary response to a known antigen96, whereas in one patient undergoing ocrelizumab therapy, B cell and T cell responses to primary VZV infection were not impaired97. In
68 patients receiving ocrelizumab, pre-existing humoral immunity was not affected by B cell depletion and even if the humoral response to a neoantigen or vaccine was attenuated, patients
were able to mount humoral responses98. However, the primary immune response and the associated class shifting of immunoglobulins to IgG and IgA might be affected more than the response to a
secondary immunization (contact with the antigen for a second time)98, which could result in a cohort of patients who have little immunity to SARS-CoV-2 infection despite vaccination.
ALEMTUZUMAB Alemtuzumab is a monoclonal antibody to CD52 that leads to depletion of T cells and B cells99,100. Treatment impairs cellular and humoral immunity; the extent of impairment
depends on the time since the last dose and the number of treatment cycles101,102. Alemtuzumab changes the immune system from a pro-inflammatory state to a regulatory state, and the effect
is long-lasting; low lymphocyte counts — specifically low CD4+ T cell counts — can last for months and neutropenia occurs in some patients103. In the first few months after dosing, the risk
of HSV re-activation is increased104. Frequent CMV infection and VZV activation have also been reported and immunity against intracellular bacteria (for example, listeria) seems to be
compromised during and shortly after infusion periods104. Analysis of long-term data has shown that the incidence of infections decreases substantially 2 years after the last dose of
alemtuzumab and that most infections after this time were mild104. Secondary autoimmunity after CD52 depletion can occur up to 4 years after the last infusion cycle but long-term extension
studies do not indicate altered immune competence or high infection rates. The consensus among national and international recommendations is that patients receiving alemtuzumab have an
increased risk of severe COVID-19 (moderate to high confidence) owing to the profound effects of the drug on the immune system (Table 1, Question 8). The absolute risk of infection clearly
depends on the time since the last dose — the highest risk is in the weeks that follow immune depletion102. Therefore, alemtuzumab should only be used with caution in the COVID-19 pandemic;
careful evaluation of the risks and benefits in a particular patient is needed. CLADRIBINE TABLETS Cladribine is a synthetic purine nucleoside analogue that inhibits DNA synthesis
selectively, mainly in circulating T cells and B cells. This action leads to an extended decrease in lymphocyte counts (depletion) with minimal effect on innate immunity, followed by gradual
repopulation of the lymphocytes105,106. Depletion of CD19+ B cells occurs earlier and to a greater extent than that of CD4+ and CD8+ T cells107,108. However, depletion is less profound than
that seen with alemtuzumab107,109,110,111, which might account for the fact that the rates and severity of infection are generally lower with cladribine and usually occur within the first
few months after dosing. Cladribine might affect immune competence against intracellular bacteria (for example, _Mycobacterium tuberculosis_)112. No data are yet available on the impact of
cladribine tablets on COVID-19 but consensus among national and international societies is that cladribine can increase the risk of severe COVID-19 (moderate to high confidence), depending
on the time since the last dose — a shorter time since the last dose is associated with a higher risk (Table 1, Question 9). Given that cladribine has long-lasting effects and the interval
between treatment cycles is 1 year, a second cycle of treatment can be delayed if the patient is stable, but this option depends on the local infection rate (Table 1, Question 12) — if the
local infection rate is high, delay should be considered. Cladribine tablets can be initiated in suitable patients (for example, patients with high disease activity, younger age and no
comorbidities) during the COVID-19 pandemic, but the risk-to-benefit ratio should be considered, especially for older patients and those with comorbidities. HAEMATOPOIETIC STEM CELL THERAPY
Haematopoietic stem cell therapy (HSCT) is an off-label treatment used for MS113 and, in rare cases, other neuroimmunological conditions100,114. Preparation for the transplantation involves
profound immunosuppression or immunoablation, which carries a high risk of infection. The consensus is that patients who underwent HSCT within the past 6–12 months are at risk of severe
COVID-19 (Table 1, Question 16). ASYMPTOMATIC, MILD AND SEVERE COVID-19 ASYMPTOMATIC OR MILD COVID-19 Worldwide SARS-CoV-2 infection rates are increasing, and evidence indicates that a high
number of people who are infected have no symptoms115. For example, in a study conducted in Iceland, 43% of people who tested positive for SARS-CoV-2 exhibited no symptoms116. On this basis,
there are undoubtedly patients who are receiving immunotherapy and are unknowingly infected with SARS-CoV-2. No consensus exists about how to manage ongoing immunotherapy in patients who
are at high risk of exposure to SARS-CoV-2, have asymptomatic infection or who develop mild symptoms of COVID-19 (Table 1, Question 22). In making the decision to stop or continue
immunotherapy, health-care providers should consider the duration of the therapeutic effects, the risk of relapse, the postulated duration of COVID-19 and patient-related factors (Box 2). In
the literature, general agreement exists that local recommendations for disease control, such as social distancing, should be followed by individuals receiving immunotherapy (Table 1,
Question 1). However, there is no clarity about regular laboratory tests for safety monitoring, which might require the patient to travel to a collection facility or hospital, thereby
increasing the risk of exposure to SARS-CoV-2. The risk of infection in local infusion centres and hospitals depends strongly on local infection rates and the precautions taken; in high-risk
areas, postponing treatment should be considered whenever possible. If collection and infusion are possible at home, this approach would be preferable. The risks of complying with
monitoring procedures versus those of failing to do so depend heavily on local circumstances. Another important consideration is the effect of immunotherapy on SARS-CoV-2 viral shedding. The
time for which viral shedding continues can vary widely, and a high viral load can persist in otherwise healthy people without comorbidities for >2 months117, and evidence from previous
studies suggests that immunosuppression can prolong viral shedding further. For example, viral shedding of MERS-CoV was prolonged in immunocompromised macaques even though the disease course
was not more severe in these animals than in immunocompetent animals118. In humans, prolonged viral shedding of various respiratory viruses has been described in patients receiving
immunotherapy after transplantation119. The possibility of prolonged shedding of SARS-CoV-2 could, therefore, influence recommended isolation periods and re-testing strategies during
convalescence for people receiving immunotherapy. Although no vaccination against SARS-CoV-2 is yet available, viral co-infection has been found in 13.1% of individuals who are positive for
SARS-CoV-2 (ref.120) and bacterial and fungal co-infections have been observed in at least 8%121. Therefore, vaccinations against influenza and/or pneumococcus might be helpful in patients
receiving immunotherapy to reduce the risk, but few recommendations have been made on this topic (Table 1, Question 7). An important question for future management is whether and how the
efficacy of a SARS-CoV-2 vaccine might be influenced by immunotherapy. Responses to vaccinations can be altered by immunotherapy, depending on the particular mechanism of action of the drug.
For example, response rates to seasonal influenza vaccination were low among patients with MS who were being treated with natalizumab or fingolimod122,123. In patients who are receiving
immune-depleting therapies, vaccination response rates are attenuated but some extent of immune response is seen even after B cell depletion98. The possibility of altered responses to
certain types of vaccination with some immunotherapies is already included in the ‘checklist’ for de-risking immunotherapy124. Ideally, vaccinations are performed before initiation of an
immune therapy, particularly high-efficacy immunotherapy. In the case of immune-depleting, pulsed therapies, vaccinations should be administered during phases of immune system recovery (≥6
months after a course of immune-depleting therapy with alemtuzumab; ≥2–4 months after a course of CD20-depleting therapy). This approach holds true for seasonal vaccines (for example
influenza). To date, the potential for altered vaccination responses has not been a major factor in choosing immunotherapies, as the problem can be mitigated by vaccinating before therapy
initiation or some immunotherapies still allow sufficient immune responses. Whether the same will hold true in the context of a SARS-CoV-2 vaccine remains to be determined when the
properties of any vaccine are known. SEVERE COVID-19 Neuroimmunological disease alone is probably not a risk factor for symptomatic infection with SARS-CoV-2 or for severe COVID-19 (Table 1,
Question 19). However, some immunotherapies seem to increase susceptibility to SARS-CoV-2 infection, so stopping therapy might seem reasonable based on the assumption that immunotherapy and
associated dampening of primary infection control (the innate immune system) could negatively impact the disease course. However, the influence of immunotherapy on the risk of SARS-CoV-2
infection might differ from that on the risk of a severe disease course. Innate immunity, natural killer cells and type I interferons are considered to be the first line of defence in viral
infection125. Adaptive immune responses to viral infections consist of humoral immunity involving antibodies and cytotoxic T cells. In severe COVID-19, however, the host response to
SARS-CoV-2 infection leads to fulminant inflammation that includes peripheral lymphopenia, sequestration of mononuclear cells in the infected tissues and reduction of CD8+ lymphocytes in the
blood126. In addition, upregulation of cytokines can lead to a cytokine storm127. In this second phase of severe COVID-19, some immunotherapies might have the potential to attenuate or even
prevent critical illness93,128, although this potential is largely theoretical at present and there is a long history of similar hypotheses that have rarely come to fruition. Low levels of
immunoglobulins are associated with B cell-depleting therapies and anti-CD52 therapy101. These low levels are unlikely to reduce primary responses to SARS-CoV-2 infection because these
responses involve CD8+ T cells and innate immune components. However, low levels of immunoglobulins could attenuate antibody-dependent cellular cytotoxicity, which is thought to contribute
to severe secondary hyperinflammation in COVID-19. This hypothesis has been proposed as an explanation as to why higher IgG levels are a risk factor for severe COVID-19 (ref.129). Individual
cytokines can be blocked, with the potential to attenuate the cytokine storm in COVID-19. IL-6 receptor antagonists (for example, tocilizumab), IL-1 blockers (for example, anakinra) and
Janus kinase (JAK) inhibitors (for example, baricitinib) are either being tested in clinical trials or have been proposed as therapeutic agents to target the excessive inflammation130,131.
Another target of therapies that are currently being trialled in COVID-19 is the complement system, activation of which might contribute to the secondary inflammatory response and
microvascular injury in patients with severe COVID-19 (ref.132). Reports of patients with COVID-19 being successfully treated with the compstatin-based complement inhibitor AMY-101 (ref.133)
and the anti-C5 therapy eculizumab have been published134. Early reports indicate that patients who are receiving immunotherapy after solid organ transplantation or for inflammatory bowel
disease do not have an increased risk of developing severe COVID-19 (refs135,136). On this basis, one possibility is that an increased risk of infection due to the immunotherapy is
outweighed by the anti-inflammatory effects of immunosuppression137. FACTORS TO CONSIDER IN DECISION MAKING The COVID-19 pandemic presents a new situation that challenges the field of
neuroimmunology. Owing to the rapid and novel evolution of the pandemic, decision-making processes remain largely non-evidence-based but are assisted by several national and international
society recommendations and opinion papers. Nevertheless, several relevant questions are not addressed and conflicting advice has been given about some aspects. The decision-making process
involves several dimensions and key contributing factors (Box 2). The complexities of the existing dimensions — dimensions one and two — are now complicated further by a third dimension,
which comprises SARS-Cov-2-related factors. Importantly, however, the COVID-19 pandemic has not led to a complete modification of how the initiation, continuation and optimization of
neurological immunotherapy should be managed. The novelty comes from what is not known about SARS-CoV-2 and from the different conditions that individual health-care systems face.
Nevertheless, it is unlikely that all patients receiving immunotherapy have a substantially increased risk of severe COVID-19, except for patients who are receiving potent immunosuppression
or who have confounding factors that further increase the risk of poor outcomes (such as old age, smoking or cardiovascular comorbidities)138. CONCLUSIONS During the COVID-19 pandemic,
initiation, continuation and optimization of treatment for neuroimmunological disease that requires disease-modifying immunotherapy should be based on the features of the underlying disease,
the characteristics of the patient, and caregiver considerations. In general, delaying treatment could risk a poor long-term prognosis and persistent disability, especially with very active
neuroimmunological disease or when the pathology is irreversible. Some questions about the use of disease-modifying immunotherapies in the context of the COVID-19 pandemic cannot currently
be answered on the basis of good evidence or even on the basis of consensus, although a strong consensus exists for some questions (Box 3). Collection of data in registries of all patients
with neuroimmunological disease and SARS-CoV-2 infection should be an important goal to gain additional information on outcomes and inform future management approaches. In addition, most of
the available information in this field — demonstrated by the sources included in our Delphi-like analysis — relates to MS, leaving many unanswered questions and/or limited consensus in
relation to other areas of neuroimmunology. These questions include whether people with neuroimmunological disease are at increased risk of severe COVID-19; which immunotherapies and which
comorbidities and confounding factors increase the risk of severe COVID-19; and which therapies should be discontinued in those with confirmed SARS-CoV-2 infection if possible. Until we know
the answers to these questions, the practice of neuroimmunology during the COVID-19 pandemic requires a thorough understanding of first principles and a careful balancing of the risks that
are particular to each individual patient. CHANGE HISTORY * _ 22 JULY 2020 A Correction to this paper has been published: https://doi.org/10.1038/s41582-020-0392-9 _ REFERENCES * Zhu, N. et
al. A novel coronavirus from patients with pneumonia in China, 2019. _N. Engl. J. Med._ 382, 727–733 (2020). CAS PubMed PubMed Central Google Scholar * Zhou, P. et al. A pneumonia
outbreak associated with a new coronavirus of probable bat origin. _Nature_ 579, 270–273 (2020). CAS PubMed PubMed Central Google Scholar * Siordia, J. A. Epidemiology and clinical
features of COVID-19: a review of current literature. _J. Clin. Virol._ 127, 104357 (2020). CAS PubMed PubMed Central Google Scholar * Iwasaki, A. & Yang, Y. The potential danger of
suboptimal antibody responses in COVID-19. _Nat. Rev. Immunol._ 20, 339–341 (2020). PubMed PubMed Central Google Scholar * Herold, T. et al. Elevated levels of IL-6 and CRP predict the
need for mechanical ventilation in COVID-19. _J. Allergy Clin. Immunol._ https://doi.org/10.1016/j.jaci.2020.05.008 (2020). Article PubMed PubMed Central Google Scholar * Gorse, G. J.,
Donovan, M. M. & Patel, G. B. Antibodies to coronaviruses are higher in older compared with younger adults and binding antibodies are more sensitive than neutralizing antibodies in
identifying coronavirus-associated illnesses. _J. Med. Virol._ 92, 512–517 (2020). CAS PubMed PubMed Central Google Scholar * Wang, L., Wang, F.-S. & Gershwin, M. E. Human autoimmune
diseases: a comprehensive update. _J. Intern. Med._ 278, 369–395 (2015). CAS PubMed Google Scholar * Kovarik, J. From immunosuppression to immunomodulation: current principles and future
strategies. _Pathobiology_ 80, 275–281 (2013). CAS PubMed Google Scholar * Willis, M. D. & Robertson, N. P. Multiple sclerosis and the risk of infection: considerations in the threat
of the novel coronavirus, COVID-19/SARS-CoV-2. _J. Neurol._ 267, 1567–1569 (2020). CAS PubMed PubMed Central Google Scholar * Luna, G. et al. Infection risks among patients with
multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. _JAMA Neurol._ 77, 184–191 (2020). PubMed Google Scholar * Fu, Y., Cheng, Y. & Wu, Y.
Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. _Virol. Sin._ https://doi.org/10.1007/s12250-020-00207-4 (2020). Article PubMed
PubMed Central Google Scholar * Jamilloux, Y. et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. _Autoimmun. Rev._ 19, 102567
(2020). CAS PubMed PubMed Central Google Scholar * Steinberg, K. P. et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. _N. Engl. J. Med._
354, 1671–1684 (2006). CAS PubMed Google Scholar * Sormani, M. P. Italian study group on COVID-19 infection in multiple sclerosis. An Italian programme for COVID-19 infection in multiple
sclerosis. _Lancet Neurol._ 19, 481–482 (2020). CAS PubMed PubMed Central Google Scholar * Minotti, C., Tirelli, F., Barbieri, E., Giaquinto, C. & Donà, D. How is immunosuppressive
status affecting children and adults in SARS-CoV-2 infection? A systematic review. _J. Infect._ 81, e61–e66 (2020). CAS PubMed PubMed Central Google Scholar * Haberman, R. et al.
Covid-19 in immune-mediated inflammatory diseases – case series from New York. _N. Engl. J. Med._ https://doi.org/10.1056/NEJMc2009567 (2020). Article PubMed PubMed Central Google Scholar
* Boulkedid, R., Abdoul, H., Loustau, M., Sibony, O. & Alberti, C. Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. _PLoS One_ 6,
e20476 (2011). CAS PubMed PubMed Central Google Scholar * Cain, D. W. & Cidlowski, J. A. Immune regulation by glucocorticoids. _Nat. Rev. Immunol._ 17, 233–247 (2017). CAS PubMed
Google Scholar * Fan, P. T. et al. Effect of corticosteroids on the human immune response: comparison of one and three daily 1 gm intravenous pulses of methylprednisolone. _J. Lab. Clin.
Med._ 91, 625–634 (1978). CAS PubMed Google Scholar * Youssef, J., Novosad, S. A. & Winthrop, K. L. Infection risk and safety of corticosteroid use. _Rheum. Dis. Clin. North. Am._ 42,
157–176 (2016). PubMed Google Scholar * Arabi, Y. M. et al. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. _Am. J. Respir. Crit. Care Med._ 197,
757–767 (2018). PubMed Google Scholar * Russell, C. D., Millar, J. E. & Baillie, J. K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. _Lancet_
395, 473–475 (2020). CAS PubMed PubMed Central Google Scholar * Alhazzani, W. et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus
disease 2019 (COVID-19). _Crit. Care Med._ https://doi.org/10.1097/CCM.0000000000004363 (2020). Article PubMed PubMed Central Google Scholar * Horby, P et al. Effect of dexamethasone in
hospitalized patients with COVID-19: preliminary report. Preprint at _medRxiv_ https://doi.org/10.1101/2020.06.22.20137273 (2020). Article Google Scholar * Nguyen, A. A. et al.
Immunoglobulins in the treatment of COVID-19 infection: proceed with caution! _Clin. Immunol._ 216, 108459 (2020). CAS PubMed PubMed Central Google Scholar * Guidon, A. C. & Amato,
A. A. COVID-19 and neuromuscular disorders. _Neurology_ https://doi.org/10.1212/WNL.0000000000009566 (2020). Article PubMed Google Scholar * Klok, F. A. et al. Confirmation of the high
cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. _Thromb. Res._ https://doi.org/10.1016/j.thromres.2020.04.041 (2020).
Article PubMed PubMed Central Google Scholar * Palace, J. et al. Outcome prediction models in AQP4-IgG positive neuromyelitis optica spectrum disorders. _Brain_ 142, 1310–1323 (2019).
PubMed PubMed Central Google Scholar * Kleiter, I. et al. Apheresis therapies for NMOSD attacks: a retrospective study of 207 therapeutic interventions. _Neurol. Neuroimmunol.
Neuroinflamm_ 5, e504 (2018). PubMed PubMed Central Google Scholar * Carnero Contentti, E. & Correa, J. Immunosuppression during the COVID-19 pandemic in neuromyelitis optica spectrum
disorders patients: a new challenge. _Mult. Scler. Relat. Disord._ 41, 102097 (2020). PubMed PubMed Central Google Scholar * Sanders, D. B. et al. International consensus guidance for
management of myasthenia gravis: Executive summary. _Neurology_ 87, 419–425 (2016). PubMed PubMed Central Google Scholar * Delly, F., Syed, M. J., Lisak, R. P. & Zutshi, D. Myasthenic
crisis in COVID-19. _J. Neurol. Sci._ 414, 116888 (2020). CAS PubMed PubMed Central Google Scholar * Anand, P. et al. COVID-19 in patients with myasthenia gravis. _Muscle Nerve_ 19, 1
(2020). Google Scholar * International MG/COVID-19 Working Group. et al. Guidance for the management of myasthenia gravis (MG) and Lambert-Eaton myasthenic syndrome (LEMS) during the
COVID-19 pandemic. _J. Neurol. Sci._ 412, 116803 (2020). PubMed Central Google Scholar * Feldman, C. H. et al. Serious infections among adult Medicaid beneficiaries with systemic lupus
erythematosus and lupus nephritis. _Arthritis Rheumatol._ 67, 1577–1585 (2015). PubMed PubMed Central Google Scholar * Wilson, J. C. et al. Serious adverse effects associated with
glucocorticoid therapy in patients with giant cell arteritis (GCA): a nested case-control analysis. _Semin. Arthritis Rheum._ 46, 819–827 (2017). CAS PubMed Google Scholar * Wilson, J. C.
et al. Incidence and risk of glucocorticoid-associated adverse effects in patients with rheumatoid arthritis. _Arthritis Care Res._ 71, 498–511 (2019). Google Scholar * Rajabally, Y. A.,
Goedee, H. S., Attarian, S. & Hartung, H.-P. Management challenges for chronic dysimmune neuropathies during the COVID-19 pandemic. _Muscle Nerve_ 62, 34–40 (2020). CAS PubMed Google
Scholar * Schmedt, N., Andersohn, F. & Garbe, E. Signals of progressive multifocal leukoencephalopathy for immunosuppressants: a disproportionality analysis of spontaneous reports
within the US Adverse Event Reporting System (AERS). _Pharmacoepidemiol. Drug. Saf._ 21, 1216–1220 (2012). CAS PubMed Google Scholar * Sebela Ireland Ltd. _IMURAN (azathioprine): Product
information._ https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/016324s039lbl.pdf (2018). * Aspen. _Australian Product Information: Azathioprine (Imuran)_
https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2010-PI-06832-3 (2019). * Goodkin, D. E. Interferon beta-1b. _Lancet_ 344, 1057–1060 (1994). CAS PubMed
Google Scholar * Mark, D. F., Lu, S. D., Creasey, A. A., Yamamoto, R. & Lin, L. S. Site-specific mutagenesis of the human fibroblast interferon gene. _Proc. Natl Acad. Sci. USA_ 81,
5662–5666 (1984). CAS PubMed PubMed Central Google Scholar * Sheahan, T. P. et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon
beta against MERS-CoV. _Nat. Commun._ 11, 222 (2020). CAS PubMed PubMed Central Google Scholar * Weinstock-Guttman, B., Nair, K. V., Glajch, J. L., Ganguly, T. C. & Kantor, D. Two
decades of glatiramer acetate: from initial discovery to the current development of generics. _J. Neurol. Sci._ 376, 255–259 (2017). CAS PubMed Google Scholar * Häusler, D. et al.
Glatiramer acetate immune modulates B-cell antigen presentation in treatment of MS. _Neurol. Neuroimmunol. Neuroinflamm_ 7, e698 (2020). PubMed PubMed Central Google Scholar * Ziemssen,
T. & Schrempf, W. Glatiramer acetate: mechanisms of action in multiple sclerosis. _Int. Rev. Neurobiol._ 79, 537–570 (2007). CAS PubMed Google Scholar * Bar-Or, A. Teriflunomide
(Aubagio®) for the treatment of multiple sclerosis. _Exp. Neurol._ 262, 57–65 (2014). CAS PubMed Google Scholar * Klotz, L. et al. Teriflunomide treatment for multiple sclerosis modulates
T cell mitochondrial respiration with affinity-dependent effects. _Sci. Transl. Med._ 11, eaao5563 (2019). CAS PubMed Google Scholar * Comi, G. et al. Characterizing lymphocyte counts
and infection rates with long-term teriflunomide treatment: pooled analysis of clinical trials. _Mult. Scler._ https://doi.org/10.1177/1352458519851981 (2019). Article PubMed PubMed
Central Google Scholar * Bilger, A. et al. Leflunomide/teriflunomide inhibit Epstein-Barr virus (EBV)-induced lymphoproliferative disease and lytic viral replication. _Oncotarget_ 8,
44266–44280 (2017). PubMed PubMed Central Google Scholar * Chon, W. J. et al. Use of leflunomide in renal transplant recipients with ganciclovir-resistant/refractory cytomegalovirus
infection: a case series from the University of Chicago. _Case Rep. Nephrol. Dial._ 5, 96–105 (2015). PubMed PubMed Central Google Scholar * Henao-Martínez, A. F., Weinberg, A., Waldman,
W. J. & Levi, M. E. Successful treatment of acyclovir-resistant herpes simplex virus type 2 proctitis with leflunomide in an HIV-infected man. _J. Clin. Virol._ 54, 276–278 (2012).
PubMed Google Scholar * Lamarche, C. et al. BK polyomavirus and the transplanted kidney: immunopathology and therapeutic approaches. _Transplantation_ 100, 2276–2287 (2016). CAS PubMed
PubMed Central Google Scholar * Xiong, R. et al. Novel and potent inhibitors targeting DHODH, a rate-limiting enzyme in de novo pyrimidine biosynthesis, are broad-spectrum antiviral
against RNA viruses including newly emerged coronavirus SARS-CoV-2. Preprint at _b__ioRxiv_ https://doi.org/10.1101/2020.03.11.983056 (2020). Article PubMed PubMed Central Google Scholar
* Ghadiri, M. et al. Dimethyl fumarate-induced lymphopenia in MS due to differential T-cell subset apoptosis. _Neurol. Neuroimmunol. Neuroinflamm_ 4, e340 (2017). PubMed PubMed Central
Google Scholar * Li, R. et al. Dimethyl fumarate treatment mediates an anti-inflammatory shift in B cell subsets of patients with multiple sclerosis. _J. Immunol._ 198, 691–698 (2017). CAS
PubMed Google Scholar * Mehta, D. et al. Effect of dimethyl fumarate on lymphocytes in RRMS: implications for clinical practice. _Neurology_ 92, e1724–e1738 (2019). CAS PubMed PubMed
Central Google Scholar * Rosenkranz, T., Novas, M. & Terborg, C. PML in a patient with lymphocytopenia treated with dimethyl fumarate. _N. Engl. J. Med._ 372, 1476–1478 (2015). CAS
PubMed Google Scholar * Perini, P. et al. Herpes simplex virus encephalitis temporally associated with dimethyl fumarate-induced lymphopenia in a multiple sclerosis patient. _Multiple
Scler. Relat. Disord._ 26, 68–70 (2018). Google Scholar * Niino, M. et al. Natalizumab effects on immune cell responses in multiple sclerosis. _Ann. Neurol._ 59, 748–754 (2006). CAS PubMed
Google Scholar * Butzkueven, H. et al. Long-term safety and effectiveness of natalizumab treatment in clinical practice: 10 years of real-world data from the Tysabri Observational Program
(TOP). _J. Neurol. Neurosurg. Psychiatr._ 91, 660–668 (2020). Google Scholar * Polman, C. H. et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis.
_N. Engl. J. Med._ 354, 899–910 (2006). CAS PubMed Google Scholar * Foley, J. et al. The 5-year Tysabri Global Observational Program in Safety (TYGRIS) study confirms the long-term safety
profile of natalizumab treatment in multiple sclerosis. _Multiple Scler. Relat. Disord._ 39, 101863 (2019). Google Scholar * Schwab, N., Schneider-Hohendorf, T., Melzer, N., Cutter, G.
& Wiendl, H. Natalizumab-associated PML: challenges with incidence, resulting risk, and risk stratification. _Neurology_ 88, 1197–1205 (2017). CAS PubMed Google Scholar * Prosperini,
L. et al. Post-natalizumab disease reactivation in multiple sclerosis: systematic review and meta-analysis. _Ther. Adv. Neurol. Disord._ https://doi.org/10.1177/1756286419837809 (2019).
Article PubMed PubMed Central Google Scholar * Louapre, C. et al. Patients with MS treated with immunosuppressive agents: across the COVID-19 spectrum. _Rev. Neurol._ 176, 523–525
(2020). CAS PubMed Google Scholar * Borriello, G. & Ianniello, A. COVID-19 occurring during natalizumab treatment: a case report in a patient with extended interval dosing approach.
_Mult. Scler. Relat. Disord._ 41, 102165 (2020). PubMed PubMed Central Google Scholar * Ryerson, L. Z. et al. Risk of natalizumab-associated PML in patients with MS is reduced with
extended interval dosing. _Neurology_ 93, e1452–e1462 (2019). CAS PubMed PubMed Central Google Scholar * Paniz-Mondolfi, A. et al. Central nervous system involvement by severe acute
respiratory syndrome coronavirus-2 (SARS-CoV-2). _J. Med. Virol._ 92, 699–702 (2020). CAS PubMed Google Scholar * Asadi-Pooya, A. A. & Simani, L. Central nervous system manifestations
of COVID-19: a systematic review. _J. Neurol. Sci._ 413, 116832 (2020). CAS PubMed PubMed Central Google Scholar * Sigrist, C. J., Bridge, A. & Le Mercier, P. A potential role for
integrins in host cell entry by SARS-CoV-2. _Antivir. Res._ 177, 104759 (2020). CAS PubMed Google Scholar * Tresoldi, I., Sangiuolo, C. F., Manzari, V. & Modesti, A. SARS-COV-2 and
infectivity: possible increase in infectivity associated to integrin motif expression. _J. Med. Virol._ 177, 104759 (2020). Google Scholar * Arvin, A. M. et al. Varicella-zoster virus
infections in patients treated with fingolimod: risk assessment and consensus recommendations for management. _JAMA Neurol._ 72, 31–39 (2015). PubMed PubMed Central Google Scholar *
Tagawa, A. et al. Hepatitis C virus (HCV) reactivation during fingolimod treatment for relapsing and remitting multiple sclerosis. _Mult. Scler. Relat. Disord._ 9, 155–157 (2016). PubMed
Google Scholar * Beadnall, H. N., Gill, A. J., Riminton, S. & Barnett, M. H. Virus-related Merkel cell carcinoma complicating fingolimod treatment for multiple sclerosis. _Neurology_
87, 2595–2597 (2016). PubMed Google Scholar * Benedetti, M. D. et al. HPV-related papillary squamous cell carcinoma of the tonsil during treatment with fingolimod. _Mult. Scler. Relat.
Disord._ 23, 24–26 (2018). PubMed Google Scholar * Cohen, J. A. et al. Safety and efficacy of the selective sphingosine 1-phosphate receptor modulator ozanimod in relapsing multiple
sclerosis (RADIANCE): a randomised, placebo-controlled, phase 2 trial. _Lancet Neurol._ 15, 373–381 (2016). CAS PubMed Google Scholar * Cohen, J. A. et al. Efficacy and safety of ozanimod
in multiple sclerosis: dose-blinded extension of a randomized phase II study. _Mult. Scler._ 25, 1255–1262 (2019). CAS PubMed Google Scholar * Comi, G. et al. Safety and efficacy of
ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): a multicentre, randomised, minimum 12-month, phase 3 trial. _Lancet Neurol._ 18, 1009–1020 (2019). CAS PubMed
Google Scholar * Kappos, L. et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. _Lancet_ 391, 1263–1273 (2018).
CAS PubMed Google Scholar * Ghadiri, M. et al. Reconstitution of the peripheral immune repertoire following withdrawal of fingolimod. _Mult. Scler._ 23, 1225–1232 (2017). CAS PubMed
Google Scholar * Tay, M. Z., Poh, C. M., Rénia, L., MacAry, P. A. & Ng, L. F. P. The trinity of COVID-19: immunity, inflammation and intervention. _Nat. Rev. Immunol._ 20, 363–374
(2020). CAS PubMed Google Scholar * US National Library of Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/ct2/show/NCT04280588 (2020). * Cooper, N. & Arnold, D. M. The
effect of rituximab on humoral and cell mediated immunity and infection in the treatment of autoimmune diseases. _Br. J. Haematol._ 149, 3–13 (2010). CAS PubMed Google Scholar * Hauser,
S. L. et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. _N. Engl. J. Med._ 376, 221–234 (2017). CAS PubMed Google Scholar * Bar-Or, A. et al. Subcutaneous
ofatumumab in patients with relapsing-remitting multiple sclerosis: The MIRROR study. _Neurology_ 90, e1805–e1814 (2018). CAS PubMed PubMed Central Google Scholar * Ciardi, M. R. et al.
Reactivation of hepatitis B virus with immune-escape mutations after ocrelizumab treatment for multiple sclerosis. _Open. Forum Infect. Dis._ 6, ofy356 (2019). PubMed Google Scholar * Lin,
K.-M., Lin, J.-C., Tseng, W.-Y. & Cheng, T.-T. Rituximab-induced hepatitis C virus reactivation in rheumatoid arthritis. _J. Microbiol. Immunol. Infect._ 46, 65–67 (2013). PubMed
Google Scholar * Sul, J. et al. Progressive multifocal leukoencephalopathy in a patient on ocrelizumab monotherapy. _Neurology_ 94, 4875 (2020). * Novi, G. et al. COVID-19 in a MS patient
treated with ocrelizumab: does immunosuppression have a protective role? _Mult. Scler. Relat. Disord._ 42, 102120 (2020). PubMed PubMed Central Google Scholar * Hughes, R., Pedotti, R.
& Koendgen, H. COVID-19 in persons with multiple sclerosis treated with ocrelizumab – a pharmacovigilance case series. _Mult. Scler. Relat. Disord._ 42, 102192 (2020). PubMed PubMed
Central Google Scholar * Amor, S., Baker, D., Khoury, S. J., Schmierer, K. & Giovanonni, G. SARS-CoV-2 and multiple sclerosis: not all immune depleting DMTs are equal or bad. _Ann.
Neurol._ 87, 794–797 (2020). CAS PubMed PubMed Central Google Scholar * Safavi, F., Nourbakhsh, B. & Azimi, A. R. B-cell depleting therapies may affect susceptibility to acute
respiratory illness among patients with multiple sclerosis during the early COVID-19 epidemic in Iran. _Mult. Scler. Relat. Disord._ 43, 102195 (2020). PubMed PubMed Central Google Scholar
* Quinti, I. et al. A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. _J. Allergy Clin. Immunol._ https://doi.org/10.1016/j.jaci.2020.04.013 (2020).
Article PubMed PubMed Central Google Scholar * Bearden, C. M. et al. Rituximab inhibits the in vivo primary and secondary antibody response to a neoantigen, bacteriophage phiX174. _Am.
J. Transplant._ 5, 50–57 (2005). CAS PubMed Google Scholar * Novi, G. et al. Ocrelizumab does not impair B- and T-cell responses to primary VZV infection in a patient with MS. _Neurol.
Neuroimmunol. Neuroinflamm._ 7, e695 (2020). PubMed PubMed Central Google Scholar * Stokmeier, D. et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis
[abstract]. _Neurology_ 90 (Suppl. 15), S36.002 (2018). Google Scholar * Wiendl, H. & Kieseier, B. Reprogramming the immune repertoire with alemtuzumab in MS. _Nat. Rev. Neurol._ 9,
125–126 (2013). CAS PubMed Google Scholar * Lünemann, J. D., Ruck, T., Muraro, P. A., Bar-Or, A. & Wiendl, H. Immune reconstitution therapies: concepts for durable remission in
multiple sclerosis. _Nat. Rev. Neurol._ 16, 56–62 (2020). PubMed Google Scholar * Möhn, N. et al. Alemtuzumab therapy changes immunoglobulin levels in peripheral blood and CSF. _Neurol.
Neuroimmunol. Neuroinflamm._ 7, e654 (2020). PubMed Google Scholar * Wray, S. et al. Infection risk with alemtuzumab decreases over time: pooled analysis of 6-year data from the CAMMS223,
CARE-MS I, and CARE-MS II studies and the CAMMS03409 extension study. _Mult. Scler._ 25, 1605–1617 (2019). CAS PubMed Google Scholar * Wiendl, H. et al. Lymphocyte pharmacodynamics are
not associated with autoimmunity or efficacy after alemtuzumab. _Neurol. Neuroimmunol. Neuroinflamm._ 7, e635 (2020). PubMed Google Scholar * Coles, A. J. et al. Alemtuzumab CARE-MS II
5-year follow-up: efficacy and safety findings. _Neurology_ 89, 1117–1126 (2017). CAS PubMed PubMed Central Google Scholar * Giovannoni, G. Cladribine to treat relapsing forms of
multiple sclerosis. _Neurotherapeutics_ 14, 874–887 (2017). CAS PubMed PubMed Central Google Scholar * Wiendl, H. Cladribine – an old newcomer for pulsed immune reconstitution in MS.
_Nat. Rev. Neurol._ 13, 573–574 (2017). CAS PubMed Google Scholar * Baker, D. et al. Both cladribine and alemtuzumab may effect MS via B-cell depletion. _Neurol. Neuroimmunol.
Neuroinflamm._ 4, e360 (2017). PubMed PubMed Central Google Scholar * Ceronie, B. et al. Cladribine treatment of multiple sclerosis is associated with depletion of memory B cells. _J.
Neurol._ 265, 1199–1209 (2018). CAS PubMed PubMed Central Google Scholar * Havrdova, E. et al. Alemtuzumab CARE-MS I 5-year follow-up: durable efficacy in the absence of continuous MS
therapy. _Neurology_ 89, 1107–1116 (2017). CAS PubMed PubMed Central Google Scholar * Willis, M. D. & Robertson, N. P. Alemtuzumab for the treatment of multiple sclerosis. _Ther.
Clin. Risk Manag._ 11, 525–534 (2015). PubMed PubMed Central Google Scholar * Thomas, K., Eisele, J., Rodriguez-Leal, F. A., Hainke, U. & Ziemssen, T. Acute effects of alemtuzumab
infusion in patients with active relapsing-remitting MS. _Neurol. Neuroimmunol. Neuroinflamm._ 3, e228 (2016). PubMed PubMed Central Google Scholar * Jacobs, B. M. et al. Cladribine:
mechanisms and mysteries in multiple sclerosis. _J. Neurol. Neurosurg. Psychiatry_ 89, 1266–1271 (2018). PubMed Google Scholar * Burt, R. K. et al. Effect of nonmyeloablative hematopoietic
stem cell transplantation vs continued disease-modifying therapy on disease progression in patients with relapsing-remitting multiple sclerosis: a randomized clinical trial. _JAMA_ 321,
165–174 (2019). PubMed PubMed Central Google Scholar * Muraro, P. A. et al. Autologous haematopoietic stem cell transplantation for treatment of multiple sclerosis. _Nat. Rev. Neurol._
13, 391–405 (2017). CAS PubMed Google Scholar * Zhou, F. et al. SARS-CoV-2 shedding and infectivity – authors’ reply. _Lancet_ 395, 1340 (2020). CAS PubMed PubMed Central Google
Scholar * Gudbjartsson, D. F. et al. Spread of SARS-CoV-2 in the Icelandic population. _N. Engl. J. Med._ 382, 2302–2315 (2020). CAS PubMed Google Scholar * Yang, J.-R. et al. Persistent
viral RNA positivity during recovery period of a patient with SARS-CoV-2 infection. _J. Med. Virol._ https://doi.org/10.1002/jmv.25940 (2020). Article PubMed PubMed Central Google
Scholar * Prescott, J. et al. Pathogenicity and viral shedding of MERS-CoV in immunocompromised rhesus macaques. _Front. Immunol._ 9, 205 (2018). PubMed PubMed Central Google Scholar *
de Lima, C. R. A. et al. Prolonged respiratory viral shedding in transplant patients. _Transpl. Infect. Dis._ 16, 165–169 (2014). PubMed Google Scholar * Nowak, M. D., Sordillo, E. M.,
Gitman, M. R. & Paniz Mondolfi, A. E. Co-infection in SARS-CoV-2 infected patients: where are influenza virus and rhinovirus/enterovirus? _J. Med. Virol._
https://doi.org/10.1002/jmv.25953 (2020). Article PubMed PubMed Central Google Scholar * Rawson, T. M. et al. Bacterial and fungal co-infection in individuals with coronavirus: a rapid
review to support COVID-19 antimicrobial prescribing. _Clin. Infect. Dis._ https://doi.org/10.1093/cid/ciaa530 (2020). Article PubMed Google Scholar * Olberg, H. K. et al. Antibody
response to seasonal influenza vaccination in patients with multiple sclerosis receiving immunomodulatory therapy. _Eur. J. Neurol._ 25, 527–534 (2018). CAS PubMed Google Scholar * Metze,
C. et al. Immunogenicity and predictors of response to a single dose trivalent seasonal influenza vaccine in multiple sclerosis patients receiving disease-modifying therapies. _CNS
Neurosci. Ther._ 25, 245–254 (2019). CAS PubMed Google Scholar * Klotz, L. et al. Risks and risk management in modern multiple sclerosis immunotherapeutic treatment. _Ther. Adv. Neurol.
Disord._ 12, 1756286419836571 (2019). PubMed PubMed Central Google Scholar * Koyama, S., Ishii, K. J., Coban, C. & Akira, S. Innate immune response to viral infection. _Cytokine_ 43,
336–341 (2008). CAS PubMed Google Scholar * Cao, X. COVID-19: immunopathology and its implications for therapy. _Nat. Rev. Immunol._ 20, 269–270 (2020). CAS PubMed PubMed Central
Google Scholar * Mehta, P. et al. COVID-19: consider cytokine storm syndromes and immunosuppression. _Lancet_ 395, 1033–1034 (2020). CAS PubMed PubMed Central Google Scholar * Berger,
J. R., Brandstadter, R. & Bar-Or, A. COVID-19 and MS disease-modifying therapies. _Neurol. Neuroimmunol. Neuroinflamm._ 7, e761 (2020). PubMed PubMed Central Google Scholar * Zhang,
B. et al. Immune phenotyping based on neutrophil-to-lymphocyte ratio and IgG predicts disease severity and outcome for patients with COVID-19. Preprint at _medrxiv.org_
https://doi.org/10.1101/2020.03.12.20035048 (2020). * Sarzi-Puttini, P. et al. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? _Clin.
Exp. Rheumatol._ 38, 337–342 (2020). PubMed Google Scholar * Napolitano, M., Fabbrocini, G. & Patruno, C. Potential role of Janus kinase inhibitors in COVID-19. _J. Am. Acad.
Dermatol._ 83, e65 (2020). CAS PubMed PubMed Central Google Scholar * Risitano, A. M. et al. Complement as a target in COVID-19? _Nat. Rev. Immunol._ 20, 343–344 (2020). CAS PubMed
Google Scholar * Mastaglio, S. et al. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. _Clin. Immunol._ 215, 108450 (2020). CAS PubMed PubMed Central Google
Scholar * Diurno, F. et al. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. _Eur. Rev. Med. Pharmacol. Sci._ 24, 4040–4047
(2020). CAS PubMed Google Scholar * Seminari, E. et al. SARS Cov2 infection in a renal transplanted patient: a case report. _Am. J. Transplant._ https://doi.org/10.1111/ajt.15902 (2020).
Article PubMed Google Scholar * Norsa, L. et al. Uneventful course in IBD patients during severe acute respiratory syndrome coronovirus 2 outbreak in northern Italy. _Gastroenterology_
https://doi.org/10.1053/j.gastro.2020.03.062 (2020). Article PubMed Google Scholar * Romanelli, A. & Mascolo, S. Immunosuppression drug-related and clinical manifestation of
coronavirus disease 2019: a therapeutical hypothesis. _Am. J. Transplant._ https://doi.org/10.1111/ajt.15905 (2020). Article PubMed PubMed Central Google Scholar * Guan, W.-J. et al.
Clinical characteristics of coronavirus disease 2019 in China. _N. Engl. J. Med._ 382, 1708–1720 (2020). CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS The authors thank
E. East for native language editing. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Neurology with Institute of Translational Neurology, University of Muenster, Muenster,
Germany Catharina Korsukewitz & Heinz Wiendl * Department of Neurology, Concord Hospital and The Brain and Mind Centre, University of Sydney, Sydney, Australia Stephen W. Reddel * Center
for Neuroinflammation and Neurotherapeutics and the Department of Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, USA Amit Bar-Or Authors * Catharina
Korsukewitz View author publications You can also search for this author inPubMed Google Scholar * Stephen W. Reddel View author publications You can also search for this author inPubMed
Google Scholar * Amit Bar-Or View author publications You can also search for this author inPubMed Google Scholar * Heinz Wiendl View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS C.K. and H.W. researched data for the article and wrote the article. All authors made substantial contributions to discussion of the content and
reviewed and edited the manuscript before submission. CORRESPONDING AUTHOR Correspondence to Heinz Wiendl. ETHICS DECLARATIONS COMPETING INTERESTS C.K. has received travel support and/or
speaking honoraria from Biogen, Sanofi Genzyme, Roche, Merck and Teva within the past years. S.R. has received funds within the past 5 years for (but not limited to) travel support,
honoraria, trial payments, research and clinical support to the neurology department of which he is a member from several bodies and charities such as Lambert Initiative, Beeren Foundation
and anonymous donors, and from Baxter, Bayer Schering, Biogen Idec, CSL, Sanofi Genzyme, Grifols, Octapharma, Merck, Novartis, Roche, Sanofi Aventis Genzyme, Servier and Teva. S.R. also
declares the following competing interests: co-founder and shareholder of Medical Safety Systems trading as RxMx (including grant and/or contracts with Genzyme, Novartis, Roche and Janssen);
receives payment for contributions to the National IVIg Governance Advisory Council & Specialist Working Group Australia (Neurology) and the Australian Medical Services Advisory
Committee ad hoc sub-committee on IVIg; unpaid member of the Australian Technical Advisory Group on Immunization Varicella Zoster working party; receives a public salary as a staff
specialist neurologist from Concord Hospital Sydney Local Health District; receives private billings from patients and Medicare Australia reimbursement as a private practice neurologist; and
is an unpaid medical adviser to various patient and advocacy groups. A.B.-O. serves on scientific advisory boards for Atara Biotherapeutics, Biogen Idec, Celgene/Receptos, Janssen/Actelion,
Merck/EMD Serono, Novartis, Roche/Genentech and Sanofi Genzyme, and has sponsored research agreements with Biogen Idec, Novartis and Roche/Genentech. H.W. receives honoraria for acting as a
member of scientific advisory boards for Biogen, Evgen, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Roche Pharma AG and Sanofi-Aventis, as well as speaker honoraria and travel
support from Alexion, Biogen, Cognomed, F. Hoffmann-La Roche, Gemeinnützige Hertie-Stiftung, Merck Serono, Novartis, Roche Pharma AG, Genzyme, Teva and WebMD Global. H.W. is also a paid
consultant for Abbvie, Actelion, Biogen, IGES, Johnson & Johnson, Merck, Novartis, Roche and Sanofi. His research is funded by the German Ministry for Education and Research (BMBF),
Deutsche Forschungsgemeinschaft (DFG), Else Kröner Fresenius Foundation, Fresenius Foundation, the European Union, Hertie Foundation, NRW Ministry of Education and Research,
Interdisciplinary Center for Clinical Studies (IZKF) Muenster and RE Children’s Foundation, Biogen, GlaxoSmithKline GmbH, Roche Pharma AG and Sanofi Genzyme. ADDITIONAL INFORMATION PEER
REVIEW INFORMATION _Nature Reviews Neurology_ thanks G. Giovannoni, S. Pittock, V.W. Yong and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RELATED LINKS CORONAVIRUS AND MS REPORTING DATABASE
(COVIMS): https://www.covims.org COVID-19 ASSOCIATED RISKS AND EFFECTS IN MYASTHENIA GRAVIS (CARE-MG): https://myasthenia.org/For-Professionals/Resources-for-Professionals/CARE-MG LEAN
EUROPEAN OPEN SURVEY ON SARS-COV-2 INFECTED PATIENTS (LEOSS): https://leoss.net ONGOING CLINICAL TRIALS OF CORTICOSTEROIDS IN COVID-19:
https://clinicaltrials.gov/ct2/results?cond=covid+&term=corticosteroids&cntry=&state=&city=&dist= SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Korsukewitz, C., Reddel, S.W., Bar-Or, A. _et al._ Neurological immunotherapy in the era of COVID-19 — looking for
consensus in the literature. _Nat Rev Neurol_ 16, 493–505 (2020). https://doi.org/10.1038/s41582-020-0385-8 Download citation * Accepted: 17 June 2020 * Published: 08 July 2020 * Issue Date:
15 September 2020 * DOI: https://doi.org/10.1038/s41582-020-0385-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative