Play all audios:
ABSTRACT Rheumatic diseases have complex aetiologies that are not fully understood, which makes the study of pathogenic mechanisms in these diseases a challenge for researchers.
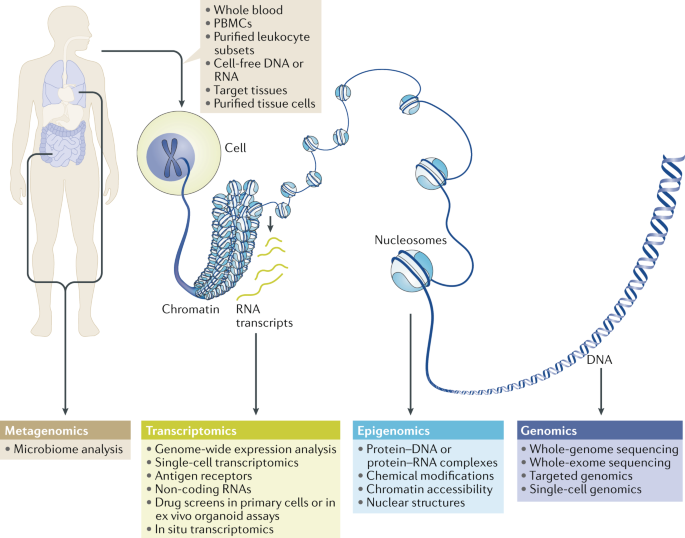
Next-generation sequencing (NGS) and related omics technologies, such as transcriptomics, epigenomics and genomics, provide an unprecedented genome-wide view of gene expression,
environmentally responsive epigenetic changes and genetic variation. The integrated application of NGS technologies to samples from carefully phenotyped clinical cohorts of patients has the
potential to solve remaining mysteries in the pathogenesis of several rheumatic diseases, to identify new therapeutic targets and to underpin a precision medicine approach to the diagnosis
and treatment of rheumatic diseases. This Review provides an overview of the NGS technologies available, showcases important advances in rheumatic disease research already powered by these
technologies and highlights NGS approaches that hold particular promise for generating new insights and advancing the field. KEY POINTS * Next-generation sequencing (NGS) technologies have
the potential to provide insight into the interaction between environmental factors and genetics in the pathogenesis of rheumatic diseases. * Transcriptomic studies have revealed
disease-related pathways and novel pathogenic cell types in rheumatic diseases. * Epigenomic studies have revealed memory-related phenomena that might help to explain the chronicity of
disease and have linked enhancers harbouring disease-associated allelic variants with target genes. * Whole-genome sequencing and exome sequencing have revealed causal mutations in rare
Mendelian autoinflammatory diseases. * NGS approaches will substantially contribute to the application of precision medicine in rheumatology. Access through your institution Buy or subscribe
This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our
best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue
Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL
ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS IDENTIFYING DISEASE-CRITICAL CELL TYPES
AND CELLULAR PROCESSES BY INTEGRATING SINGLE-CELL RNA-SEQUENCING AND HUMAN GENETICS Article 29 September 2022 THE EXPANDING DIAGNOSTIC TOOLBOX FOR RARE GENETIC DISEASES Article 18 January
2024 GENETIC ASSOCIATION ANALYSIS OF 77,539 GENOMES REVEALS RARE DISEASE ETIOLOGIES Article Open access 16 March 2023 REFERENCES * Banchereau, R., Cepika, A. M., Banchereau, J. &
Pascual, V. Understanding human autoimmunity and autoinflammation through transcriptomics. _Annu. Rev. Immunol._ 35, 337–370 (2017). CAS PubMed PubMed Central Google Scholar * Ermann,
J., Rao, D. A., Teslovich, N. C., Brenner, M. B. & Raychaudhuri, S. Immune cell profiling to guide therapeutic decisions in rheumatic diseases. _Nat. Rev. Rheumatol._ 11, 541–551 (2015).
CAS PubMed PubMed Central Google Scholar * Byron, S. A., Van Keuren-Jensen, K. R., Engelthaler, D. M., Carpten, J. D. & Craig, D. W. Translating RNA sequencing into clinical
diagnostics: opportunities and challenges. _Nat. Rev. Genet._ 17, 257–271 (2016). CAS PubMed PubMed Central Google Scholar * Davis, M. M. & Brodin, P. Rebooting human immunology.
_Annu. Rev. Immunol._ 36, 843–864 (2018). CAS PubMed PubMed Central Google Scholar * Donlin, L. T. et al. Methods for high-dimensional analysis of cells dissociated from cryopreserved
synovial tissue. _Arthritis Res. Ther._ 20, 139 (2018). PubMed PubMed Central Google Scholar * Der, E. et al. Tubular cell and keratinocyte single-cell transcriptomics applied to lupus
nephritis reveal type I IFN and fibrosis relevant pathways. _Nat. Immunol_. (in the press). * Rao, D. A. et al. A protocol for single-cell transcriptomics from cryopreserved renal tissue and
urine for the Accelerating Medicine Partnership (AMP) RA/SLE network. Preprint at _bioRxiv_ https://www.biorxiv.org/content/10.1101/275859v1 (2018). * Eikrem, O. et al. Transcriptome
sequencing (RNAseq) enables utilization of formalin-fixed, paraffin-embedded biopsies with clear cell renal cell carcinoma for exploration of disease biology and biomarker development. _PLOS
ONE_ 11, e0149743 (2016). PubMed PubMed Central Google Scholar * Banchereau, R. et al. Personalized immunomonitoring uncovers molecular networks that stratify lupus patients. _Cell_ 165,
1548–1550 (2016). CAS PubMed Google Scholar * Dennis, G. Jr. et al. Synovial phenotypes in rheumatoid arthritis correlate with response to biologic therapeutics. _Arthritis Res. Ther._
16, R90 (2014). PubMed PubMed Central Google Scholar * Costa-Silva, J., Domingues, D. & Lopes, F. M. RNA-Seq differential expression analysis: An extended review and a software tool.
_PLOS ONE_ 12, e0190152 (2017). PubMed PubMed Central Google Scholar * Wang, X. et al. Three-dimensional intact-tissue sequencing of single-cell transcriptional states. _Science_ 361,
eaat5691 (2018). PubMed PubMed Central Google Scholar * Carlucci, P. M. et al. Neutrophil subsets and their gene signature associate with vascular inflammation and coronary
atherosclerosis in lupus. _JCI Insight_ 3, 99276 (2018). PubMed Google Scholar * Cole, S. et al. Integrative analysis reveals CD38 as a therapeutic target for plasma cell-rich pre-disease
and established rheumatoid arthritis and systemic lupus erythematosus. _Arthritis Res. Ther._ 20, 85 (2018). PubMed PubMed Central Google Scholar * Orange, D. E. et al. Identification of
three rheumatoid arthritis disease subtypes by machine learning integration of synovial histologic features and RNA sequencing data. _Arthritis Rheumatol._ 70, 690–701 (2018). CAS PubMed
PubMed Central Google Scholar * Walsh, A. M. et al. Triple DMARD treatment in early rheumatoid arthritis modulates synovial T cell activation and plasmablast/plasma cell differentiation
pathways. _PLOS ONE_ 12, e0183928 (2017). PubMed PubMed Central Google Scholar * Cuppen, B. V. J. et al. RNA sequencing to predict response to TNF-alpha inhibitors reveals possible
mechanism for nonresponse in smokers. _Expert Rev. Clin. Immunol._ 14, 623–633 (2018). CAS PubMed Google Scholar * Teitsma, X. M. et al. Identification of differential co-expressed gene
networks in early rheumatoid arthritis achieving sustained drug-free remission after treatment with a tocilizumab-based or methotrexate-based strategy. _Arthritis Res. Ther._ 19, 170 (2017).
PubMed PubMed Central Google Scholar * Ter Haar, N. M. et al. Reversal of sepsis-like features of neutrophils by interleukin-1 blockade in patients with systemic-onset juvenile
idiopathic arthritis. _Arthritis Rheumatol._ 70, 943–956 (2018). PubMed Google Scholar * Mandelin, A. M. 2nd et al. Transcriptional profiling of synovial macrophages using minimally
invasive ultrasound-guided synovial biopsies in rheumatoid arthritis. _Arthritis Rheumatol._ 70, 841–854 (2018). CAS PubMed PubMed Central Google Scholar * Giladi, A. & Amit, I.
Single-cell genomics: a stepping stone for future immunology discoveries. _Cell_ 172, 14–21 (2018). CAS PubMed Google Scholar * Landhuis, E. Single-cell approaches to immune profiling.
_Nature_ 557, 595–597 (2018). CAS PubMed Google Scholar * Cheung, P., Khatri, P., Utz, P. J. & Kuo, A. J. Single-cell technologies — studying rheumatic diseases one cell at a time.
_Nat. Rev. Rheumatol_. https://doi.org/10.1038/s41584-019-0220-z (2019). * Mizoguchi, F. et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. _Nat.
Commun._ 9, 789 (2018). PubMed PubMed Central Google Scholar * Stephenson, W. et al. Single-cell RNA-seq of rheumatoid arthritis synovial tissue using low-cost microfluidic
instrumentation. _Nat. Commun._ 9, 791 (2018). PubMed PubMed Central Google Scholar * Rao, D. A. et al. Pathologically expanded peripheral T helper cell subset drives B cells in
rheumatoid arthritis. _Nature_ 542, 110–114 (2017). CAS PubMed PubMed Central Google Scholar * Kim, T. H., Choi, S. J., Lee, Y. H., Song, G. G. & Ji, J. D. Gene expression profile
predicting the response to anti-TNF treatment in patients with rheumatoid arthritis; analysis of GEO datasets. _Joint Bone Spine_ 81, 325–330 (2014). CAS PubMed Google Scholar * Gaujoux,
R. et al. Cell-centred meta-analysis reveals baseline predictors of anti-TNFα non-response in biopsy and blood of patients with IBD. _Gut_ 68, 604–614 (2018). PubMed Google Scholar *
Sweeney, T. E. et al. Unsupervised analysis of transcriptomics in bacterial sepsis across multiple datasets reveals three robust clusters. _Crit. Care Med._ 46, 915–925 (2018). PubMed
PubMed Central Google Scholar * Zhang, F. et al. Defining inflammatory cell states in rheumatoid arthritis joint tissues by integrating single-cell transcriptomics and mass cytometry.
_Nat. Immunol_. (in the press). * Arazi, A., R. D. et al. The immune cell landscape in kidneys of lupus nephritis patients. _Nat. Immunol_. (in the press). * Musters, A. et al. In rheumatoid
arthritis, synovitis at different inflammatory sites is dominated by shared but patient-specific T cell clones. _J. Immunol._ 201, 417–422 (2018). CAS PubMed Google Scholar * Sakurai, K.
et al. HLA-DRB1 shared epitope alleles and disease activity are correlated with reduced T cell receptor repertoire diversity in CD4+T cells in rheumatoid arthritis. _J. Rheumatol._ 45,
905–914 (2018). CAS PubMed Google Scholar * Kinslow, J. D. et al. Elevated IgA plasmablast levels in subjects at risk of developing rheumatoid arthritis. _Arthritis Rheumatol._ 68,
2372–2383 (2016). CAS PubMed PubMed Central Google Scholar * Sakakibara, S. et al. Clonal evolution and antigen recognition of anti-nuclear antibodies in acute systemic lupus
erythematosus. _Sci. Rep._ 7, 16428 (2017). PubMed PubMed Central Google Scholar * Lu, D. R. et al. T cell-dependent affinity maturation and innate immune pathways differentially drive
autoreactive B cell responses in rheumatoid arthritis. _Arthritis Rheumatol._ 70, 1732–1744 (2018). CAS PubMed PubMed Central Google Scholar * Elliott, S. E. et al. Affinity maturation
drives epitope spreading and generation of proinflammatory anti-citrullinated protein antibodies in rheumatoid arthritis. _Arthritis Rheumatol._ 70, 1946–1958 (2018). CAS PubMed PubMed
Central Google Scholar * Titcombe, P. J. et al. Pathogenic citrulline-multispecific B cell receptor clades in rheumatoid arthritis. _Arthritis Rheumatol._ 70, 1933–1945 (2018). CAS PubMed
PubMed Central Google Scholar * Wang, J. J. et al. Molecular profiling and clonal tracking of secreted rheumatoid factors in primary Sjogren’s syndrome. _Arthritis Rheumatol._ 70,
1617–1625 (2018). CAS PubMed Google Scholar * Gee, M. H. et al. Antigen identification for orphan T cell receptors expressed on tumor-infiltrating lymphocytes. _Cell_ 172, 549–563 (2018).
CAS PubMed Google Scholar * Allis, C. D. & Jenuwein, T. The molecular hallmarks of epigenetic control. _Nat. Rev. Genet._ 17, 487–500 (2016). CAS PubMed Google Scholar * Rivera,
C. M. & Ren, B. Mapping human epigenomes. _Cell_ 155, 39–55 (2013). CAS PubMed Google Scholar * Alvarez-Errico, D., Vento-Tormo, R., Sieweke, M. & Ballestar, E. Epigenetic control
of myeloid cell differentiation, identity and function. _Nat. Rev. Immunol._ 15, 7–17 (2015). CAS PubMed Google Scholar * Smale, S. T., Tarakhovsky, A. & Natoli, G. Chromatin
contributions to the regulation of innate immunity. _Annu. Rev. Immunol._ 32, 489–511 (2014). CAS PubMed Google Scholar * Ivashkiv, L. B. & Park, S. H. Epigenetic regulation of
myeloid cells. _Microbiol. Spectr._ https://doi.org/10.1128/microbiolspec.MCHD-0010-2015 (2016). Article PubMed Google Scholar * Wang, K. C. & Chang, H. Y. Epigenomics: technologies
and applications. _Circ. Res._ 122, 1191–1199 (2018). CAS PubMed PubMed Central Google Scholar * Ballestar, E. & Li, T. New insights into the epigenetics of inflammatory rheumatic
diseases. _Nat. Rev. Rheumatol._ 13, 593–605 (2017). CAS PubMed Google Scholar * Shi, L. et al. Monocyte enhancers are highly altered in systemic lupus erythematosus. _Epigenomics_ 7,
921–935 (2015). CAS PubMed PubMed Central Google Scholar * Zhang, Z. et al. H3K4 tri-methylation breadth at transcription start sites impacts the transcriptome of systemic lupus
erythematosus. _Clin. Epigenet._ 8, 14 (2016). Google Scholar * Scharer, C. D. et al. ATAC-seq on biobanked specimens defines a unique chromatin accessibility structure in naive SLE B
cells. _Sci. Rep._ 6, 27030 (2016). CAS PubMed PubMed Central Google Scholar * Ai, R. et al. Comprehensive epigenetic landscape of rheumatoid arthritis fibroblast-like synoviocytes.
_Nat. Commun._ 9, 1921 (2018). PubMed PubMed Central Google Scholar * Netea, M. G., Latz, E., Mills, K. H. & O’Neill, L. A. Innate immune memory: a paradigm shift in understanding
host defense. _Nat. Immunol._ 16, 675–679 (2015). CAS PubMed Google Scholar * Biswas, S. K. & Lopez-Collazo, E. Endotoxin tolerance: new mechanisms, molecules and clinical
significance. _Trends Immunol._ 30, 475–487 (2009). CAS PubMed Google Scholar * Saeed, S. et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate
immunity. _Science_ 345, 1251086 (2014). PubMed PubMed Central Google Scholar * Park, S. H. et al. Type I interferons and the cytokine TNF cooperatively reprogram the macrophage epigenome
to promote inflammatory activation. _Nat. Immunol._ 18, 1104–1116 (2017). CAS PubMed PubMed Central Google Scholar * Novakovic, B. et al. Beta-glucan reverses the epigenetic state of
LPS-induced immunological tolerance. _Cell_ 167, 1354–1368 (2016). CAS PubMed PubMed Central Google Scholar * Vatanen, T. et al. Variation in microbiome LPS immunogenicity contributes to
autoimmunity in humans. _Cell_ 165, 842–853 (2016). CAS PubMed PubMed Central Google Scholar * Shi, L. et al. Endotoxin tolerance in monocytes can be mitigated by alpha2-interferon. _J.
Leukoc. Biol._ 98, 651–659 (2015). CAS PubMed PubMed Central Google Scholar * Wendeln, A. C. et al. Innate immune memory in the brain shapes neurological disease hallmarks. _Nature_
556, 332–338 (2018). CAS PubMed PubMed Central Google Scholar * ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. _Nature_ 489, 57–74 (2012).
Google Scholar * Sanyal, A., Lajoie, B. R., Jain, G. & Dekker, J. The long-range interaction landscape of gene promoters. _Nature_ 489, 109–113 (2012). CAS PubMed PubMed Central
Google Scholar * Ye, C. J. et al. Intersection of population variation and autoimmunity genetics in human T cell activation. _Science_ 345, 1254665 (2014). PubMed PubMed Central Google
Scholar * Raj, T. et al. Polarization of the effects of autoimmune and neurodegenerative risk alleles in leukocytes. _Science_ 344, 519–523 (2014). CAS PubMed PubMed Central Google
Scholar * Lee, M. N. et al. Common genetic variants modulate pathogen-sensing responses in human dendritic cells. _Science_ 343, 1246980 (2014). PubMed PubMed Central Google Scholar *
Farh, K. K. et al. Genetic and epigenetic fine mapping of causal autoimmune disease variants. _Nature_ 518, 337–343 (2015). CAS PubMed Google Scholar * Raj, P. et al. Regulatory
polymorphisms modulate the expression of HLA class II molecules and promote autoimmunity. _eLife_ 5, e12089 (2016). PubMed PubMed Central Google Scholar * Martinez-Bueno, M. et al.
Trans-ethnic mapping of BANK1 identifies two independent SLE-risk linkage groups enriched for co-transcriptional splicing marks. _Int. J. Mol. Sci._ 19, E2331 (2018). PubMed Google Scholar
* Pulecio, J., Verma, N., Mejia-Ramirez, E., Huangfu, D. & Raya, A. CRISPR/Cas9-based engineering of the epigenome. _Cell Stem Cell_ 21, 431–447 (2017). CAS PubMed PubMed Central
Google Scholar * Mumbach, M. R. et al. Enhancer connectome in primary human cells identifies target genes of disease-associated DNA elements. _Nat. Genet._ 49, 1602–1612 (2017). CAS PubMed
PubMed Central Google Scholar * Sokhi, U. K. et al. Dissection and function of autoimmunity-associated TNFAIP3 (A20) gene enhancers in humanized mouse models. _Nat. Commun._ 9, 658
(2018). PubMed PubMed Central Google Scholar * Liao, H. K. et al. In vivo target gene activation via CRISPR/Cas9-mediated _trans_-epigenetic modulation. _Cell_ 171, 1495–1507 (2017). CAS
PubMed PubMed Central Google Scholar * Robertson, K. D. DNA methylation and human disease. _Nat. Rev. Genet._ 6, 597–610 (2005). CAS PubMed Google Scholar * Doody, K. M., Bottini, N.
& Firestein, G. S. Epigenetic alterations in rheumatoid arthritis fibroblast-like synoviocytes. _Epigenomics_ 9, 479–492 (2017). CAS PubMed PubMed Central Google Scholar * Hammaker,
D. & Firestein, G. S. Epigenetics of inflammatory arthritis. _Curr. Opin. Rheumatol._ 30, 188–196 (2018). CAS PubMed PubMed Central Google Scholar * Jin, B., Li, Y. & Robertson,
K. D. DNA methylation: superior or subordinate in the epigenetic hierarchy? _Genes Cancer_ 2, 607–617 (2011). CAS PubMed PubMed Central Google Scholar * Mardis, E. R. Next-generation
DNA sequencing methods. _Annu. Rev. Genomics Hum. Genet._ 9, 387–402 (2008). CAS PubMed Google Scholar * Shendure, J. & Ji, H. Next-generation DNA sequencing. _Nat. Biotechnol._ 26,
1135–1145 (2008). CAS PubMed Google Scholar * Feil, R., Charlton, J., Bird, A. P., Walter, J. & Reik, W. Methylation analysis on individual chromosomes: improved protocol for
bisulphite genomic sequencing. _Nucleic Acids Res._ 22, 695–696 (1994). CAS PubMed PubMed Central Google Scholar * Lister, R. et al. Human DNA methylomes at base resolution show
widespread epigenomic differences. _Nature_ 462, 315–322 (2009). CAS PubMed PubMed Central Google Scholar * Reinders, J. & Paszkowski, J. Bisulfite methylation profiling of large
genomes. _Epigenomics_ 2, 209–220 (2010). CAS PubMed Google Scholar * Meissner, A. et al. Reduced representation bisulfite sequencing for comparative high-resolution DNA methylation
analysis. _Nucleic Acids Res._ 33, 5868–5877 (2005). CAS PubMed PubMed Central Google Scholar * Kurdyukov, S. & Bullock, M. DNA methylation analysis: choosing the right method.
_Biology_ 5, E3 (2016). PubMed Google Scholar * Nakano, K., Whitaker, J. W., Boyle, D. L., Wang, W. & Firestein, G. S. DNA methylome signature in rheumatoid arthritis. _Ann. Rheum.
Dis._ 72, 110–117 (2013). CAS PubMed Google Scholar * Whitaker, J. W. et al. An imprinted rheumatoid arthritis methylome signature reflects pathogenic phenotype. _Genome Med._ 5, 40
(2013). CAS PubMed PubMed Central Google Scholar * Ai, R. et al. DNA methylome signature in synoviocytes from patients with early rheumatoid arthritis compared to synoviocytes from
patients with longstanding rheumatoid arthritis. _Arthritis Rheumatol._ 67, 1978–1980 (2015). CAS PubMed PubMed Central Google Scholar * Frank-Bertoncelj, M. et al. Epigenetically-driven
anatomical diversity of synovial fibroblasts guides joint-specific fibroblast functions. _Nat. Commun._ 8, 14852 (2017). CAS PubMed PubMed Central Google Scholar * Rhead, B. et al.
Rheumatoid arthritis naive T cells share hypermethylation sites with synoviocytes. _Arthritis Rheumatol._ 69, 550–559 (2017). CAS PubMed PubMed Central Google Scholar * Mok, A. et al.
Hypomethylation of CYP2E1 and DUSP22 promoters associated with disease activity and erosive disease among rheumatoid arthritis patients. _Arthritis Rheumatol._ 70, 528–536 (2018). CAS
PubMed PubMed Central Google Scholar * Chung, S. A. et al. Genome-wide assessment of differential DNA methylation associated with autoantibody production in systemic lupus erythematosus.
_PLOS ONE_ 10, e0129813 (2015). PubMed PubMed Central Google Scholar * Mok, A. et al. Genome-wide profiling identifies associations between lupus nephritis and differential methylation of
genes regulating tissue hypoxia and type 1 interferon responses. _Lupus Sci. Med._ 3, e000183 (2016). PubMed PubMed Central Google Scholar * Cole, M. B. et al. Epigenetic signatures of
salivary gland inflammation in Sjogren’s syndrome. _Arthritis Rheumatol._ 68, 2936–2944 (2016). CAS PubMed PubMed Central Google Scholar * Puliti, A., Caridi, G., Ravazzolo, R. &
Ghiggeri, G. M. Teaching molecular genetics: chapter 4—positional cloning of genetic disorders. _Pediatr. Nephrol._ 22, 2023–2029 (2007). PubMed PubMed Central Google Scholar * The
International FMF Consortium. Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. _Cell_ 90, 797–807 (1997). Google Scholar
* Zhu, X. et al. Whole-exome sequencing in undiagnosed genetic diseases: interpreting 119 trios. _Genet. Med._ 17, 774 (2015). CAS PubMed PubMed Central Google Scholar * Zhou, Q. et
al. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early onset autoinflammatory syndrome. _Nat. Genet._ 48, 67–73 (2016). CAS PubMed Google Scholar *
Zappala, Z. & Montgomery, S. B. Non-coding loss-of-function variation in human genomes. _Hum. Hered._ 81, 78–87 (2016). CAS PubMed Google Scholar * Ma, M. et al. Disease-associated
variants in different categories of disease located in distinct regulatory elements. _BMC Genomics_ 16, S3 (2015). PubMed PubMed Central Google Scholar * Tesi, B. et al. A _RAB27A_ 5ʹ
untranslated region structural variant associated with late-onset hemophagocytic lymphohistiocytosis and normal pigmentation. _J. Allergy Clin. Immunol._ 142, 317–321 (2018). CAS PubMed
PubMed Central Google Scholar * Namjou, B. et al. Evaluation of the TREX1 gene in a large multi-ancestral lupus cohort. _Genes Immun._ 12, 270–279 (2011). CAS PubMed PubMed Central
Google Scholar * Beaudoin, M. et al. Deep resequencing of GWAS loci identifies rare variants in CARD9, IL23R and RNF186 that are associated with ulcerative colitis. _PLOS Genet._ 9,
e1003723 (2013). CAS PubMed PubMed Central Google Scholar * Cardinale, C. J. et al. Targeted resequencing identifies defective variants of decoy receptor 3 in pediatric-onset
inflammatory bowel disease. _Genes Immun._ 14, 447 (2013). CAS PubMed Google Scholar * Nakagawa, K. et al. Somatic _NLRP3_ mosaicism in Muckle-Wells syndrome. A genetic mechanism shared
by different phenotypes of cryopyrin-associated periodic syndromes. _Ann. Rheumat. Dis._ 74, 603–610 (2015). CAS PubMed Google Scholar * Tanaka, N. et al. High incidence of NLRP3 somatic
mosaicism in patients with chronic infantile neurologic, cutaneous, articular syndrome: results of an international multicenter collaborative study. _Arthritis Rheum._ 63, 3625–3632 (2011).
CAS PubMed PubMed Central Google Scholar * Zhou, Q. et al. Cryopyrin-associated periodic syndrome caused by a myeloid-restricted somatic NLRP3 mutation. _Arthritis Rheumatol._ 67,
2482–2486 (2015). CAS PubMed PubMed Central Google Scholar * Holzelova, E. et al. Autoimmune lymphoproliferative syndrome with somatic Fas mutations. _N. Engl. J. Med._ 351, 1409–1418
(2004). CAS PubMed Google Scholar * Savola, P. et al. Somatic mutations in clonally expanded cytotoxic T lymphocytes in patients with newly diagnosed rheumatoid arthritis. _Nat. Commun._
8, 15869 (2017). CAS PubMed PubMed Central Google Scholar * Rowczenio, D. M. et al. Late-onset cryopyrin-associated periodic syndromes caused by somatic NLRP3 mosaicism—UK single center
experience. _Front. Immunol._ 8, 1410 (2017). PubMed PubMed Central Google Scholar * Yuri, K. et al. Identification of a high-frequency somatic NLRC4 mutation as a cause of
autoinflammation by pluripotent cell–based phenotype dissection. _Arthritis Rheumatol._ 69, 447–459 (2017). Google Scholar * Chung, J. et al. The minimal amount of starting DNA for
Agilent’s hybrid capture-based targeted massively parallel sequencing. _Sci. Rep._ 6, 26732 (2016). CAS PubMed PubMed Central Google Scholar * Grossman, R. L. et al. Toward a shared
vision for cancer genomic data. _N. Engl. J. Med._ 375, 1109–1112 (2016). PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS The work of L.T.D., L.B.I. and
K.-H.P.-M. was supported by grants from the US National Institutes of Health (NIH). REVIEWER INFORMATION _Nature Reviews Rheumatology_ thanks P. Gaffney and the other anonymous reviewers for
their contribution to the peer review of this work. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Arthritis and Tissue Degeneration Program, Hospital for Special Surgery, New York, NY, USA
Laura T. Donlin, Sung-Ho Park, Eugenia Giannopoulou, Kyung-Hyun Park-Min & Lionel B. Ivashkiv * David Z. Rosensweig Genomics Research Center, Hospital for Special Surgery, New York, NY,
USA Laura T. Donlin, Sung-Ho Park, Eugenia Giannopoulou, Kyung-Hyun Park-Min & Lionel B. Ivashkiv * Department of Medicine, Weill Cornell Medicine, New York, NY, USA Laura T. Donlin,
Kyung-Hyun Park-Min & Lionel B. Ivashkiv * Biological Sciences Department, New York City College of Technology, City University of New York, New York, NY, USA Eugenia Giannopoulou *
Immunoregulation Section, Autoimmunity Branch, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, Bethesda, MD, USA Aleksandra Ivovic &
Richard M. Siegel * Immunology and Microbial Pathogenesis Program, Weill Cornell Graduate School of Medical Sciences, New York, NY, USA Lionel B. Ivashkiv Authors * Laura T. Donlin View
author publications You can also search for this author inPubMed Google Scholar * Sung-Ho Park View author publications You can also search for this author inPubMed Google Scholar * Eugenia
Giannopoulou View author publications You can also search for this author inPubMed Google Scholar * Aleksandra Ivovic View author publications You can also search for this author inPubMed
Google Scholar * Kyung-Hyun Park-Min View author publications You can also search for this author inPubMed Google Scholar * Richard M. Siegel View author publications You can also search for
this author inPubMed Google Scholar * Lionel B. Ivashkiv View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS All authors researched data for
the article, provided substantial contributions to discussions of content and wrote the article. L.T.D., A.I. and L.B.I. reviewed and/or edited the manuscript before submission.
CORRESPONDING AUTHOR Correspondence to Lionel B. Ivashkiv. ETHICS DECLARATIONS COMPETING INTERESTS R.M.S. declares that he is an employee of Novartis. The other authors declare no competing
interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RELATED LINKS AMP RA
AND SLE NETWORK: https://amp-ralupus.stanford.edu/about/ra-lupus-amp-project/ GENE EXPRESSION OMNIBUS: https://www.ncbi.nlm.nih.gov/geo/ HUMAN CELL ATLAS: https://www.humancellatlas.org/
IMMPORT: https://www.immport.org/home/ INTERNATIONAL HUMAN EPIGENOME CONSORTIUM: http://ihec-epigenomes.org/welcome/ PRECISESADS: http://www.precisesads.eu/ THE LIFETIME INITIATIVE:
https://lifetime-fetflagship.eu/ RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Donlin, L.T., Park, SH., Giannopoulou, E. _et al._ Insights into
rheumatic diseases from next-generation sequencing. _Nat Rev Rheumatol_ 15, 327–339 (2019). https://doi.org/10.1038/s41584-019-0217-7 Download citation * Published: 18 April 2019 * Issue
Date: June 2019 * DOI: https://doi.org/10.1038/s41584-019-0217-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative