Play all audios:
ABSTRACT The progression of chronic liver disease to hepatocellular carcinoma is caused by the acquisition of somatic mutations that affect 20–30 cancer genes1,2,3,4,5,6,7,8. Burdens of
somatic mutations are higher and clonal expansions larger in chronic liver disease9,10,11,12,13 than in normal liver13,14,15,16, which enables positive selection to shape the genomic
landscape9,10,11,12,13. Here we analysed somatic mutations from 1,590 genomes across 34 liver samples, including healthy controls, alcohol-related liver disease and non-alcoholic fatty liver
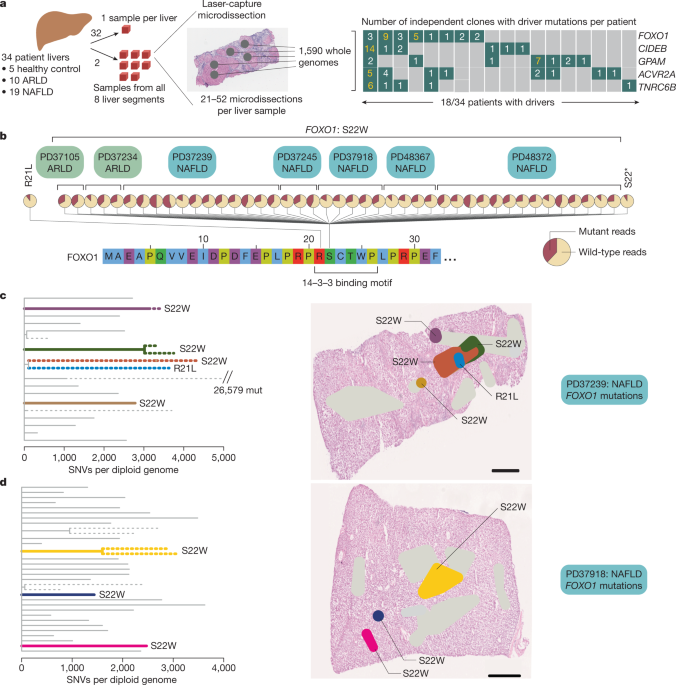
disease. Seven of the 29 patients with liver disease had mutations in _FOXO1_, the major transcription factor in insulin signalling. These mutations affected a single hotspot within the
gene, impairing the insulin-mediated nuclear export of FOXO1. Notably, six of the seven patients with _FOXO1__S22W_ hotspot mutations showed convergent evolution, with variants acquired
independently by up to nine distinct hepatocyte clones per patient. _CIDEB_, which regulates lipid droplet metabolism in hepatocytes17,18,19, and _GPAM_, which produces storage
triacylglycerol from free fatty acids20,21, also had a significant excess of mutations. We again observed frequent convergent evolution: up to fourteen independent clones per patient with
_CIDEB_ mutations and up to seven clones per patient with _GPAM_ mutations. Mutations in metabolism genes were distributed across multiple anatomical segments of the liver, increased clone
size and were seen in both alcohol-related liver disease and non-alcoholic fatty liver disease, but rarely in hepatocellular carcinoma. Master regulators of metabolic pathways are a frequent
target of convergent somatic mutation in alcohol-related and non-alcoholic fatty liver disease. Access through your institution Buy or subscribe This is a preview of subscription content,
access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99
/ 30 days cancel any time Learn more Subscribe to this journal Receive 51 print issues and online access $199.00 per year only $3.90 per issue Learn more Buy this article * Purchase on
SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about
institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS DEEP WHOLE-GENOME ANALYSIS OF 494 HEPATOCELLULAR CARCINOMAS Article 14 February
2024 DIET-INDUCED REWIRING OF THE WNT GENE REGULATORY NETWORK CONNECTS ABERRANT SPLICING TO FATTY LIVER AND LIVER CANCER IN DIAMOND MICE Article Open access 31 October 2023 INTEGRATIVE
COMMON AND RARE VARIANT ANALYSES PROVIDE INSIGHTS INTO THE GENETIC ARCHITECTURE OF LIVER CIRRHOSIS Article Open access 17 April 2024 DATA AVAILABILITY WGS data in the form of BAM files
across samples reported in this study have been deposited in the European Genome-Phenome Archive (accession number EGAD00001006255). RNA-sequencing data have been deposited in the European
Nucleotide Archive (https://www.ebi.ac.uk/ena/browser/home) with accession number ERP123192. CODE AVAILABILITY Detailed methods and custom R scripts for the analysis of clinical features,
telomere lengths and metabolomics data are available in the Supplementary Code. Other packages used in the analysis are listed below: R: v.3.5.1, Perl: v.5.3.0, Python: v.3.8.5, MATLAB:
v.R2019b, BWA-MEM: v.0.7.17 (https://sourceforge.net/projects/bio-bwa/), cgpCaVEMan: v.1.11.2/1.13.14/1.15.1 (https://github.com/cancerit/CaVEMan), cgpPindel: v.2.2.2/2.2.4/2.2.5/3.2.0/3.3.0
(https://github.com/cancerit/cgpPindel), Brass: v.5.4.1/6.0.5/6.1.2/6.2.0/6.3.4 (https://github.com/cancerit/BRASS), ASCAT NGS: v.4.0.1/ 4.1.2/4.2.1 (https://github.com/cancerit/ascatNgs),
JBrowse: v.1.16.1 (https://jbrowse.org/), cgpVAF: v.2.4.0 (https://github.com/cancerit/vafCorrect), alleleCount: v.4.1.0 (https://github.com/cancerit/alleleCount), SigProfiler:
v.1.0.0-GRCh37 (https://github.com/AlexandrovLab), HDP: v.0.1.5 (https://github.com/nicolaroberts/hdp), dNdScv: v.0.0.1 (https://github.com/im3sanger/dndscv), Telomerecat: v.3.4.0
(https://github.com/jhrf/telomerecat), STAR: v.2.7.6a (https://github.com/alexdobin/STAR), Picard-tools: v.2.20.7 (https://broadinstitute.github.io/picard/), Samtools: v.1.12
(http://www.htslib.org/), TrimGalore: v.0.6.4 (https://github.com/FelixKrueger/TrimGalore), GATK: v.4.1.4.1 (https://gatk.broadinstitute.org/hc/en-us), GSEA: v.3.0
(https://www.gsea-msigdb.org/gsea/index.jsp), XGBoost: v.0.82.1 (https://xgboost.readthedocs.io/en/latest/), NDP.view2 (https://www.hamamatsu.com/eu/en/product/type/U12388-01/index.html),
label.switching: v.1.8 (https://cran.r-project.org/web/packages/label.switching/index.html), philentropy: v.0.3.0 (https://cran.r-project.org/web/packages/philentropy/index.html), MCMCglmm:
v.2.29 (https://cran.r-project.org/web/packages/MCMCglmm/index.html), Magick: v.2.0 (https://cran.r-project.org/web/packages/magick/index.html), Pheatmap: v.1.0.12
(https://cran.r-project.org/web/packages/pheatmap/index.html), Thermo Fisher software Tracefinder: v.5.0
(https://www.thermofisher.com/uk/en/home/industrial/mass-spectrometry/liquid-chromatography-mass-spectrometry-lc-ms/lc-ms-software/lc-ms-data-acquisition-software/tracefinder-software.html),
CellProfiler: v.4.0.3 (https://cellprofiler.org/), PerkinElmer Harmony: v.4.9 (https://www.perkinelmer.com/category/cellular-imaging-software). REFERENCES * The Cancer Genome Atlas Research
Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. _Cell_ 169, 1327–1341 (2017). Article PubMed Central CAS Google Scholar * Schulze, K. et al.
Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. _Nat. Genet._ 47, 505–511 (2015). Article CAS PubMed PubMed Central
Google Scholar * Totoki, Y. et al. Trans-ancestry mutational landscape of hepatocellular carcinoma genomes. _Nat. Genet._ 46, 1267–1273 (2014). Article CAS PubMed Google Scholar *
Fujimoto, A. et al. Whole-genome sequencing of liver cancers identifies etiological influences on mutation patterns and recurrent mutations in chromatin regulators. _Nat. Genet._ 44, 760–764
(2012). Article CAS PubMed Google Scholar * Letouzé, E. et al. Mutational signatures reveal the dynamic interplay of risk factors and cellular processes during liver tumorigenesis.
_Nat. Commun._ 8, 1315 (2017). Article ADS PubMed PubMed Central CAS Google Scholar * Guichard, C. et al. Integrated analysis of somatic mutations and focal copy-number changes
identifies key genes and pathways in hepatocellular carcinoma. _Nat. Genet._ 44, 694–698 (2012). Article CAS PubMed PubMed Central Google Scholar * Fujimoto, A. et al. Whole-genome
mutational landscape and characterization of noncoding and structural mutations in liver cancer. _Nat. Genet._ 48, 500–509 (2016). Article CAS PubMed Google Scholar * Pinyol, R. et al.
Molecular characterization of hepatocellular carcinoma in patients with non-alcoholic steatohepatitis. _J. Hepatol._ 75, 865–878 (2021). Article CAS PubMed Google Scholar * Nault, J. C.
et al. Telomerase reverse transcriptase promoter mutation is an early somatic genetic alteration in the transformation of premalignant nodules in hepatocellular carcinoma on cirrhosis.
_Hepatology_ 60, 1983–1992 (2014). Article CAS PubMed Google Scholar * Torrecilla, S. et al. Trunk mutational events present minimal intra- and inter-tumoral heterogeneity in
hepatocellular carcinoma. _J. Hepatol._ 67, 1222–1231 (2017). Article PubMed Google Scholar * Zhu, M. et al. Somatic mutations increase hepatic clonal fitness and regeneration in chronic
liver disease. _Cell_ 177, 608–621 (2019). Article CAS PubMed PubMed Central Google Scholar * Kim, S. K. et al. Comprehensive analysis of genetic aberrations linked to tumorigenesis in
regenerative nodules of liver cirrhosis. _J. Gastroenterol._ 54, 628–640 (2019). Article CAS PubMed Google Scholar * Brunner, S. F. et al. Somatic mutations and clonal dynamics in
healthy and cirrhotic human liver. _Nature_ 574, 538–542 (2019). Article ADS CAS PubMed PubMed Central Google Scholar * Blokzijl, F. et al. Tissue-specific mutation accumulation in
human adult stem cells during life. _Nature_ 538, 260–264 (2016). Article ADS CAS PubMed PubMed Central Google Scholar * Yizhak, K. et al. RNA sequence analysis reveals macroscopic
somatic clonal expansion across normal tissues. _Science_ 364, eaaw0726 (2019). Article CAS PubMed PubMed Central Google Scholar * Brazhnik, K. et al. Single-cell analysis reveals
different age-related somatic mutation profiles between stem and differentiated cells in human liver. _Sci. Adv._ 6, eaax2659 (2020). Article CAS PubMed PubMed Central Google Scholar *
Barneda, D. et al. The brown adipocyte protein CIDEA promotes lipid droplet fusion via a phosphatidic acid-binding amphipathic helix. _Elife_ 4, e07485 (2015). Article PubMed PubMed
Central Google Scholar * Sun, Z. et al. Perilipin1 promotes unilocular lipid droplet formation through the activation of Fsp27 in adipocytes. _Nat. Commun._ 4, 1594 (2013). Article ADS
PubMed CAS Google Scholar * Li, J. Z. et al. Cideb regulates diet-induced obesity, liver steatosis, and insulin sensitivity by controlling lipogenesis and fatty acid oxidation. _Diabetes_
56, 2523–2532 (2007). Article CAS PubMed Google Scholar * Hammond, L. E. et al. Mitochondrial glycerol-3-phosphate acyltransferase-1 is essential in liver for the metabolism of excess
acyl-CoAs. _J. Biol. Chem._ 280, 25629–25636 (2005). Article CAS PubMed Google Scholar * Wendel, A. A., Cooper, D. E., Ilkayeva, O. R., Muoio, D. M. & Coleman, R. A.
Glycerol-3-phosphate acyltransferase (GPAT)−1, but not GPAT4, incorporates newly synthesized fatty acids into triacylglycerol and diminishes fatty acid oxidation. _J. Biol. Chem._ 288,
27299–27306 (2013). Article CAS PubMed PubMed Central Google Scholar * Jeon, S. & Carr, R. Alcohol effects on hepatic lipid metabolism. _J. Lipid Res._ 61, 470–479 (2020). Article
CAS PubMed PubMed Central Google Scholar * Friedman, S. L., Neuschwander-Tetri, B. A., Rinella, M. & Sanyal, A. J. Mechanisms of NAFLD development and therapeutic strategies. _Nat.
Med._ 24, 908–922 (2018). Article CAS PubMed PubMed Central Google Scholar * Clugston, R. D. et al. Altered hepatic lipid metabolism in C57BL/6 mice fed alcohol: a targeted lipidomic
and gene expression study. _J. Lipid Res._ 52, 2021–2031 (2011). Article CAS PubMed PubMed Central Google Scholar * Puri, P. et al. A lipidomic analysis of nonalcoholic fatty liver
disease. _Hepatology_ 46, 1081–1090 (2007). Article CAS PubMed Google Scholar * Meister, G. et al. Identification of novel argonaute-associated proteins. _Curr. Biol._ 15, 2149–2155
(2005). Article CAS PubMed Google Scholar * Rheinbay, E. et al. Analyses of non-coding somatic drivers in 2,658 cancer whole genomes. _Nature_ 578, 102–111 (2020). Article ADS CAS
PubMed PubMed Central Google Scholar * Yaffe, M. B. et al. The structural basis for 14-3-3:phosphopeptide binding specificity. _Cell_ 91, 961–971 (1997). Article CAS PubMed Google
Scholar * Saline, M. et al. AMPK and AKT protein kinases hierarchically phosphorylate the N-terminus of the FOXO1 transcription factor, modulating interactions with 14-3-3 proteins. _J.
Biol. Chem._ 294, 13106–13116 (2019). Article CAS PubMed PubMed Central Google Scholar * Kleiner, D. E. et al. Design and validation of a histological scoring system for nonalcoholic
fatty liver disease. _Hepatology_ 41, 1313–1321 (2005). Article PubMed Google Scholar * Ishak, K. et al. Histological grading and staging of chronic hepatitis. _J. Hepatol._ 22, 696–699
(1995). Article CAS PubMed Google Scholar * Ellis, P. et al. Reliable detection of somatic mutations in solid tissues by laser-capture microdissection and low-input DNA sequencing. _Nat.
Protoc._ 16, 841–871 (2021). Article CAS PubMed Google Scholar * Jones, D. et al. cgpCaVEManWrapper: simple execution of CaVEMan in order to detect somatic single nucleotide variants in
NGS data. _Curr. Protoc. Bioinformatics_ 56, 15.10.1–15.10.18 (2016). Google Scholar * Yoshida, K. et al. Tobacco smoking and somatic mutations in human bronchial epithelium. _Nature_ 578,
266–272 (2020). Article ADS CAS PubMed PubMed Central Google Scholar * Papastamoulis, P. label.switching: an R package for dealing with the label switching problem in MCMC outputs.
_J. Stat. Softw._ 69, Code Snippet 1 (2015). Google Scholar * Nik-Zainal, S. et al. The life history of 21 breast cancers. _Cell_ 149, 994–1007 (2012). Article CAS PubMed PubMed Central
Google Scholar * Martincorena, I. et al. Universal patterns of selection in cancer and somatic tissues. _Cell_ 171, 1029–1041 (2017). Article CAS PubMed PubMed Central Google Scholar
* Raine, K. M. et al. cgpPindel: identifying somatically acquired insertion and deletion events from paired end sequencing. _Curr. Protoc. Bioinformatics_ 52, 15.7.1–15.7.12 (2015). Google
Scholar * Campbell, P. J. et al. Identification of somatically acquired rearrangements in cancer using genome-wide massively parallel paired-end sequencing. _Nat. Genet._ 40, 722–729
(2008). Article CAS PubMed PubMed Central Google Scholar * Stephens, P. J. et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. _Cell_
144, 27–40 (2011). Article MathSciNet CAS PubMed PubMed Central Google Scholar * Sohlenius-Sternbeck, A. K. Determination of the hepatocellularity number for human, dog, rabbit, rat
and mouse livers from protein concentration measurements. _Toxicol. Vitr._ 20, 1582–1586 (2006). Article CAS Google Scholar * Lipscomb, J. C., Fisher, J. W., Confer, P. D. &
Byczkowski, J. Z. In vitro to in vivo extrapolation for trichloroethylene metabolism in humans. _Toxicol. Appl. Pharmacol._ 152, 376–387 (1998). Article CAS PubMed Google Scholar *
Bergstrom, E. N. et al. SigProfilerMatrixGenerator: a tool for visualizing and exploring patterns of small mutational events. _BMC Genomics_ 20, 685 (2019). Article PubMed PubMed Central
Google Scholar * Alexandrov, L. B. et al. The repertoire of mutational signatures in human cancer. _Nature_ 578, 94–101 (2020). Article ADS CAS PubMed PubMed Central Google Scholar *
Drost, H.-G. Philentropy: information theory and distance quantification with R. _J. Open Source Softw._ 3, 765 (2018). Article ADS Google Scholar * Qiao, W. et al. PERT: a method for
expression deconvolution of human blood samples from varied microenvironmental and developmental conditions. _PLoS Comput. Biol_. 8, (2012). * Farmery, J. H. R. et al. Telomerecat: a
ploidy-agnostic method for estimating telomere length from whole genome sequencing data. _Sci. Rep._ 8, 1300 (2018). Article ADS PubMed PubMed Central CAS Google Scholar * Hadfield, J.
D. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. _J. Stat. Softw._ 33, v033i02 (2010). Article Google Scholar * Hoare, M. et al. NOTCH1 mediates
a switch between two distinct secretomes during senescence. _Nat. Cell Biol._ 18, 979–992 (2016). Article CAS PubMed PubMed Central Google Scholar * Liao, Y., Smyth, G. K. & Shi,
W. FeatureCounts: an efficient general purpose program for assigning sequence reads to genomic features. _Bioinformatics_ 30, 923–930 (2014). Article CAS PubMed Google Scholar *
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. _Bioinformatics_ 26, 139–140 (2009).
Article PubMed PubMed Central CAS Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by a Cancer Research UK Grand Challenge Award (C98/A24032) and the Wellcome
Trust. S.W.K.N. holds an EMBO Long Term Fellowship (ALTF 721-2019). S.F.B. was supported by the Swiss National Science Foundation (P2SKP3-171753 and P400PB-180790). M.A.S. is supported by a
Rubicon fellowship from NWO (019.153LW.038). The Cambridge Human Research Tissue Bank is supported by the NIHR Cambridge Biomedical Research Centre. M.H. is supported by a CRUK Clinician
Scientist Fellowship (C52489/A19924) and a CRUK Accelerator Award (C18873/A26813). P.J.C. was supported by a Wellcome Senior Clinical Fellowship until 2020 (WT088340MA). AUTHOR INFORMATION
AUTHORS AND AFFILIATIONS * Cancer Genome Project, Wellcome Sanger Institute, Hinxton, UK Stanley W. K. Ng, Foad J. Rouhani, Simon F. Brunner, Natalia Brzozowska, Federico Abascal, Luiza
Moore, Lia Chappell, Daniel Leongamornlert, Aleksandra Ivovic, Philip Robinson, Timothy Butler, Mathijs A. Sanders, Nicholas Williams, Tim H. H. Coorens, Jon Teague, Keiran Raine, Adam P.
Butler, Yvette Hooks, Beverley Wilson, Natalie Birtchnell, Michael R. Stratton, Iñigo Martincorena, Raheleh Rahbari & Peter J. Campbell * Department of Surgery, Addenbrooke’s Hospital,
Cambridge, UK Foad J. Rouhani & Huw Naylor * CRUK Cambridge Institute, Cambridge, UK Sarah J. Aitken & Matthew Hoare * Department of Pathology, Addenbrooke’s Hospital, Cambridge, UK
Sarah J. Aitken & Susan E. Davies * MRC Toxicology Unit, University of Cambridge, Cambridge, UK Sarah J. Aitken * MRC Cancer Unit, University of Cambridge, Cambridge, UK Ming Yang,
Efterpi Nikitopoulou & Christian Frezza * Department of Hematology, Erasmus University Medical Center, Rotterdam, The Netherlands Mathijs A. Sanders * Department of Medicine, University
of Cambridge, Addenbrooke’s Hospital, Cambridge, UK Matthew Hoare * Stem Cell Institute, University of Cambridge, Cambridge, UK Peter J. Campbell Authors * Stanley W. K. Ng View author
publications You can also search for this author inPubMed Google Scholar * Foad J. Rouhani View author publications You can also search for this author inPubMed Google Scholar * Simon F.
Brunner View author publications You can also search for this author inPubMed Google Scholar * Natalia Brzozowska View author publications You can also search for this author inPubMed Google
Scholar * Sarah J. Aitken View author publications You can also search for this author inPubMed Google Scholar * Ming Yang View author publications You can also search for this author
inPubMed Google Scholar * Federico Abascal View author publications You can also search for this author inPubMed Google Scholar * Luiza Moore View author publications You can also search for
this author inPubMed Google Scholar * Efterpi Nikitopoulou View author publications You can also search for this author inPubMed Google Scholar * Lia Chappell View author publications You
can also search for this author inPubMed Google Scholar * Daniel Leongamornlert View author publications You can also search for this author inPubMed Google Scholar * Aleksandra Ivovic View
author publications You can also search for this author inPubMed Google Scholar * Philip Robinson View author publications You can also search for this author inPubMed Google Scholar *
Timothy Butler View author publications You can also search for this author inPubMed Google Scholar * Mathijs A. Sanders View author publications You can also search for this author inPubMed
Google Scholar * Nicholas Williams View author publications You can also search for this author inPubMed Google Scholar * Tim H. H. Coorens View author publications You can also search for
this author inPubMed Google Scholar * Jon Teague View author publications You can also search for this author inPubMed Google Scholar * Keiran Raine View author publications You can also
search for this author inPubMed Google Scholar * Adam P. Butler View author publications You can also search for this author inPubMed Google Scholar * Yvette Hooks View author publications
You can also search for this author inPubMed Google Scholar * Beverley Wilson View author publications You can also search for this author inPubMed Google Scholar * Natalie Birtchnell View
author publications You can also search for this author inPubMed Google Scholar * Huw Naylor View author publications You can also search for this author inPubMed Google Scholar * Susan E.
Davies View author publications You can also search for this author inPubMed Google Scholar * Michael R. Stratton View author publications You can also search for this author inPubMed Google
Scholar * Iñigo Martincorena View author publications You can also search for this author inPubMed Google Scholar * Raheleh Rahbari View author publications You can also search for this
author inPubMed Google Scholar * Christian Frezza View author publications You can also search for this author inPubMed Google Scholar * Matthew Hoare View author publications You can also
search for this author inPubMed Google Scholar * Peter J. Campbell View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS P.J.C., M.H. and
S.W.K.N. designed the experiments. S.W.K.N. performed mutation calling and computational analyses including visualization of results for mutation calling; identification of SNV clusters and
the inference of phylogenetic relationships between them; assignment of indels and _FOXO1_ hotspot mutations to SNV clusters; clone size estimation and comparisons; mutational signature
extraction; identification of protein-coding and non-coding drivers; telomere length estimation; processing and normalization of RNA-sequencing data; gene set enrichment analysis; and
estimation of the liver-wide mass of driver-mutation-bearing hepatocytes. S.W.K.N. developed software for the refinement of indel calling, phylogenetic inference and visualization of clonal
structure, and clone size estimation, visualization and mapping to histological images. P.J.C. assisted with the filtering of structural variants, performed statistical inference of factors
that affect telomere length using mixed effects models and supervised all statistical analyses. N. Brzozowska performed telomere length estimation. F.A. and I.M. provided support for running
variants of dNdScv. M.R.S. advised on mutational signature extraction. T.H.H.C. provided support for running beta-binomial-based variant filtering. M.A.S. provided support and advice for
performing LCM-specific variant-filtering algorithms for SNV and structural variant calls. D.L. and T.B. provided insights into indel filtering associated with homopolymers and problematic
genomic loci. F.J.R., S.F.B., Y.H., B.W. and N. Birtchnell performed tissue sectioning, fixing, staining and histology image generation. S.F.B. also performed LCM and submission for WGS, and
was responsible for the initial development of source code for producing diagnostic plots to facilitate the manual determination of clonal relationships, and the visualization of
phylogenetic tree structures. P.R., A.I. and T.B. provided wet laboratory support. N.W., J.T., K.R. and A.P.B. provided technical support for computational analyses. M.H. and F.J.R. provided
biological samples used in this study, and the associated clinical annotations were curated with assistance from S.J.A. and S.E.D. S.J.A. and S.E.D. analysed histology sections of
background liver and HCC from all patients in the study, and L.M. supervised microdissection of tissue samples for sequencing. M.H. coordinated all validation experiments relating to _FOXO1_
hotspot mutations using HCC cell lines, with additional support from H.N. M.Y., E.N. and C.F. performed analysis of metabolites from HCC cell lines. L.C. and R.R. performed processing and
quality control of RNA-sequencing samples and data. P.J.C., S.W.K.N. and M.H. drafted the manuscript with input and guidance from M.R.S. and I.M., and updated the paper after contributions
from all authors. CORRESPONDING AUTHORS Correspondence to Matthew Hoare or Peter J. Campbell. ETHICS DECLARATIONS COMPETING INTERESTS A patent has been filed by CRUK’s technology transfer
office, with support from that of Wellcome Sanger Institute (named inventors: S.W.K.N., M.H. and P.J.C.), covering the use of somatic mutations in liver tissue for stratifying diagnosis and
treatment of patients with metabolic diseases. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature_ thanks the anonymous reviewers for their contribution to the peer review of this work.
Peer reviewer reports are available. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. EXTENDED DATA
FIGURES AND TABLES EXTENDED DATA FIG. 1 MUTATIONS IN _ACVR2A_. A, Distribution of somatic mutations in _ACVR2A_ according to genomic location. Pie charts show fraction of sequencing reads
reporting the mutant allele in each microdissection. B, Two microdissections in different patients showing structural variants generating copy loss of _ACVR2A_. Black points represent
corrected read depth along the chromosome. Lines and arcs represent structural variants, coloured by the orientation of the joined ends (purple, deletion-type orientation; brown,
tandem-duplication-type orientation; turquoise, head-to-head inverted; green, tail-to-tail inverted). EXTENDED DATA FIG. 2 MUTATIONS IN _TNRC6B_ AND _NEAT1_. A, Distribution of somatic
mutations in _CLCN5_ according to genomic location. Pie charts show fraction of sequencing reads reporting the mutant allele in each microdissection. B, Distribution of somatic mutations in
the long non-coding RNA, _NEAT1_, according to genomic location. Pie charts show fraction of sequencing reads reporting the mutant allele in each microdissection. EXTENDED DATA FIG. 3
STRUCTURAL VARIANTS AFFECTING _FOXO1_ AND _GPAM_. A, A chromothripsis event affecting chromosome 13 in one of the microdissections from PD37907, a patient with NAFLD. Black points represent
corrected read depth along the chromosome. Lines and arcs represent structural variants, coloured by the orientation of the joined ends (purple, deletion-type orientation; brown,
tandem-duplication-type orientation; turquoise, head-to-head inverted; green, tail-to-tail inverted). The structural variant that breaks _FOXO1_ is highlighted, and would be predicted to
break the gene within the first intron, preserving the first coding exon but deleting the remaining coding exons. B, A tandem duplication upstream of _GPAM_ in a microdissection from
PD37110, a patient with ARLD. _GPAM_ is left intact, but the tandem duplication starts 20kb upstream of the gene. EXTENDED DATA FIG. 4 MULTIPLE INDEPENDENT ACQUISITIONS OF _FOXO1_ MUTATIONS
IN PD37239. The clone map from Fig. 1b is shown, laid onto an H&E-stained section. On the left of the figure, raw sequencing data from representative samples with and without _FOXO1_
mutations are shown, with their physical locations on the H&E section shown by the arrows. In the sequencing data, reads mapping to the forward strand of the reference genome are in
pink; the reverse strand in blue. Base calls that do not match the reference genome are shown as coloured squares. The locations of the S22W and R21L mutations are marked with arrows. The
scatterplots arranged around the H&E section represent VAF plots of mutations in pairs of samples. The colours of the x and y axis titles match the clone map colours of the H&E
section. Individual mutations called in either sample are shown in orange, according to their VAF, with the _FOXO1_ S22W mutation shown in dark green. In clonally related pairs of samples,
most of the mutations are shared by both samples, evident as a cloud of mutations with non-zero VAF. In clonally unrelated samples, the mutations line the _x_ and _y_ axes, with the one
exception being the _FOXO1_ mutation, indicating that it is independently acquired in the two clones. EXTENDED DATA FIG. 5 FURTHER EXAMPLES OF _FOXO1_ MUTATIONS IN PATIENTS WITH CHRONIC
LIVER DISEASE. A–C, Phylogenetic trees and clone maps are shown for PD37234 (A), PD37105 (B) and PD37245 (C). The left panel shows the phylogenetic tree, with coloured branches showing
independently acquired mutations. Solid lines indicate that nesting is in accordance with the pigeonhole principle; dashed lines indicate that nesting is in accordance with the pigeonhole
principle, assuming that hepatocytes represent < 100% of cells. The right panel shows the clones from the phylogenetic tree mapped onto an H&E-stained photomicrograph of the liver,
with _FOXO1-_mutant clones coloured to match the tree. EXTENDED DATA FIG. 6 SOMATIC MUTATIONS OF FOXO1 IMPAIR ITS PHOSPHORYLATION AND NUCLEAR EXPORT. A, HepG2 cells were transfected with the
indicated wild-type or mutant constructs of FOXO1 fused with a C-terminal GFP. Cells were counterstained with DAPI to highlight the nucleus, and imaged after overnight serum starvation
conditions (left) and after 15 min of exposure to 100 nM insulin (right). Studies were performed in triplicate. B, HepG2 cells, expressing ectopic eGFP-tagged wild-type or mutant FOXO1
constructs as indicated and treated for 15 min with vehicle or insulin (100nM), were analysed for the indicated proteins by immunoblotting. Molecular weight markers (kDa) indicated. Studies
were performed in triplicate. Uncropped versions of the blots are shown in Supplementary Fig. 4. EXTENDED DATA FIG. 7 NUCLEAR–CYTOPLASMIC RATIOS FOR WILD-TYPE AND MUTANT FOXO1-GFP CONSTRUCTS
IN HCC CELL LINES. A, B, Wide-field view of Hep3B (A) and PLC/PRF5 (B) cells pseudocoloured on a blue-to-red scale by the nuclear-cytoplasmic ratio of FOXO1-GFP. Cells were imaged under
conditions of serum starvation (left), after exposure to insulin 100nM for 15 min (middle) or foetal calf serum (FCS) for 15 min (right). EXTENDED DATA FIG. 8 RNA SEQUENCING FROM CELL LINES
TRANSDUCED WITH EITHER WILD-TYPE OR MUTANT FOXO1-GFP CONSTRUCTS. A, Heat map showing gene expression levels for genes in the ‘Canonical Glycolysis’ gene set from GO (GO:0061621). The order
of genes on the x axis is determined by the level of significance (and direction of change) and the order of samples on the y axis is by condition (_FOXO1_ status and insulin status). B,
Heat map showing gene expression levels for genes in the ‘Cell cycle, mitotic’ gene set from Reactome (R-HSA-69278). The order of genes on the x axis is determined by the level of
significance (and direction of change) and the order of samples on the y axis is by condition (_FOXO1_ status and insulin status). C–E, Enrichment plots for the ‘FOXO-mediated transcription
of oxidative stress, metabolic and neuronal genes’ gene set of Reactome (9615017) (C); ‘Lipid catabolic process’ gene set of GO (0016042) (D); and ‘Apoptotic process’ gene set of GO
(0006915) (E). In each, the top panel reflects the cumulative enrichment score as the gene set is traversed from most up-regulated to most down-regulated in the presence of _FOXO1_-mutant
constructs. The bottom panel in each shows the ranking of each gene in the gene set across all genes measured. EXTENDED DATA FIG. 9 _CIDEB_ MUTATIONS IN PATIENTS WITH CHRONIC LIVER DISEASE.
A, Distribution of somatic mutations in _CIDEB_. Amino acid residues are coloured by type, with observed mutations in chronic liver disease shown above the wild-type protein sequence. B,
Phylogenetic trees and clone maps are shown for one of the Couinaud segments of PD48367 with _CIDEB_ mutations. The left panel shows the phylogenetic tree, with coloured branches showing
independently acquired driver mutations. Solid lines indicate that nesting is in accordance with the pigeonhole principle; dashed lines indicate that nesting is in accordance with the
pigeonhole principle, assuming that hepatocytes represent < 100% of cells. The right panel shows the clones from the phylogenetic tree mapped onto an H&E-stained photomicrograph of
the liver, with mutant clones coloured to match the tree. EXTENDED DATA FIG. 10 _GPAM_ MUTATIONS IN PATIENTS WITH CHRONIC LIVER DISEASE. A, Distribution of somatic mutations in _GPAM_
according to genomic location. Pie charts show fraction of sequencing reads reporting the mutant allele in each microdissection. B, Phylogenetic trees and clone maps are shown for a biopsy
from PD37111 with _GPAM_ mutations. The left panel shows the phylogenetic tree, with coloured branches showing independently acquired driver mutations. Solid lines indicate that nesting is
in accordance with the pigeonhole principle; dashed lines indicate that nesting is in accordance with the pigeonhole principle, assuming that hepatocytes represent < 100% of cells. The
right panel shows the clones from the phylogenetic tree mapped onto an H&E-stained photomicrograph of the liver, with mutant clones coloured to match the tree. EXTENDED DATA FIG. 11
PROPERTIES OF CLONES AND PATIENTS WITH DRIVER MUTATIONS. A, Stacked bar chart showing the estimated cumulative liver mass carrying driver mutations, extrapolated from samples analysed in
each patient. The calculations assume a total liver mass of 1500g for each patient. Bars are coloured for each of the 6 recurrently mutated genes identified in the study, and patient codes
on the x axis are coloured for disease status. B, Estimated clone size for the 4 most frequently mutated genes compared to wild-type clones. The points are overlaid on box-and-whisker plots
where the median is marked with a heavy black line and the interquartile range in a thin black box. The whiskers denote mark the full range of the data or 25th/75th centile plus 1.5x the
interquartile range (whichever is smaller). The p values are two-sided, derived from Wilcoxon rank-sum tests and have not been corrected for multiple hypothesis testing. Sample sizes are n =
25 mutant clones for _FOXO1_; n = 17 mutant clones for _CIDEB_; n = 15 mutant clones for _GPAM_; and n = 32 mutant clones for _ACVR2A_. C, Scatter plot showing the distribution of ages of
patients in the cohort by whether they carried clones with mutations in the specified genes or not. The p values are two-sided, derived from Wilcoxon rank-sum tests and have not been
corrected for multiple hypothesis testing. Sample sizes were n = 7 _FOXO1_ mutant versus n = 22 _FOXO1_ wild-type; n = 6 _CIDEB_ mutant versus n = 23 _CIDEB_ wild-type; and n = 7 _GPAM_
mutant versus n = 22 _GPAM_ wild-type. D, Stacked bar charts showing the proportion of patients with or without type 2 diabetes by whether they carried driver mutations in each gene. The p
values are two-sided, derived from Fisher’s exact tests and have not been corrected for multiple hypothesis testing. Sample sizes were as for C. E, Stacked bar charts showing the
distribution of the NAFLD Activity Score (NAS) by whether they carried driver mutations in each gene, with low scores denoting a low degree of histological abnormality. The p values are
two-sided, derived from chi-squared tests for trend and have not been corrected for multiple hypothesis testing. Sample sizes were as for C. EXTENDED DATA FIG. 12 ANALYSIS OF TELOMERE
LENGTHS. A, Scatter plot showing the distribution of telomere lengths for samples grouped by disease status, and ranked from lowest to highest age within each disease category. B, Posterior
distributions of the effect size of clone size (per log10(μm2)), age (per decade of life) and disease state (NAFLD and ARLD versus normal) on telomere lengths. Density plots are shown from
the MCMC sampler, coloured by decile. Posterior ‘p values’ are calculated from the posterior samples of the MCMC chain and are two-sided and not corrected for multiple hypothesis testing. C,
Telomere lengths layered onto two representative phylogenetic trees from patients with ARLD. Branches are coloured on a yellow-to-blue scale according to telomere lengths of the sample with
the highest VAF assigned to that branch. The internal nodes are estimated using maximum likelihood and colours are interpolated along each branch. EXTENDED DATA FIG. 13 DISTRIBUTION OF
MUTATIONAL SIGNATURES ACROSS THE PHYLOGENETIC TREES WITHIN THE COHORT. Estimated proportional contributions of each mutational signature to each phylogenetically defined cluster of somatic
substitutions. Stacked bar plots show proportional contributions of signatures in normal controls (top), patients with ARLD (middle), and patients with NAFLD (bottom). EXTENDED DATA FIG. 14
DISTRIBUTION OF THE NEW T>A SIGNATURE ACROSS THREE SAMPLES. A, Signatures for a sample with high rates of the novel signature (PD37240). The left panel shows phylogenetic trees with each
branch coloured by the proportion of mutations in that branch assigned to the different mutational signatures. The contribution from the new signature is coloured purple. The middle panel
shows the overlay of clones onto an H&E-stained liver section. Clones are coloured on a grey-to-purple scale according to the proportion of mutations attributed to the novel signature.
The right panel shows observed mutation spectra for representative clones with low (top) or high (bottom) burden of the novel signature, laid out as for Fig. 4b. Purple arrows indicate parts
of the mutation spectrum that are characteristic of the new mutational signature. B, C, In one patient with NAFLD, we had three samples from 2008 (not shown as the signature was absent),
2011 (B) and 2013 (C), with the relative contribution of the signature increasing over time. The photomicrograph of the H&E section in C was captured after the microdissections were
excised, hence the white gaps in the tissue. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION This file contains Supplementary Notes 1 and 2, Supplementary Methods including further
details on indel calling and mutational signature extraction not included in the main Methods section, Supplementary References and Supplementary Figures 1–6. REPORTING SUMMARY SUPPLEMENTARY
TABLES This file contains Supplementary Tables 1–9 and a Supplementary Table Guide. SUPPLEMENTARY DATA This file contains the Supplementary Code: HTMLs of Jupyter notebooks outlining key
statistical analyses presented in the manuscript, including analysis of clinical variables, telomere lengths and metabolomics data. PEER REVIEW FILE RIGHTS AND PERMISSIONS Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ng, S.W.K., Rouhani, F.J., Brunner, S.F. _et al._ Convergent somatic mutations in metabolism genes in chronic liver disease. _Nature_ 598,
473–478 (2021). https://doi.org/10.1038/s41586-021-03974-6 Download citation * Received: 17 June 2020 * Accepted: 31 August 2021 * Published: 13 October 2021 * Issue Date: 21 October 2021 *
DOI: https://doi.org/10.1038/s41586-021-03974-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative