Play all audios:
ABSTRACT Tumour innervation is associated with worse patient outcomes in multiple cancers1,2, which suggests that it may regulate metastasis. Here we observed that highly metastatic mouse
mammary tumours acquired more innervation than did less-metastatic tumours. This enhanced innervation was driven by expression of the axon-guidance molecule SLIT2 in tumour vasculature.
Breast cancer cells induced spontaneous calcium activity in sensory neurons and elicited release of the neuropeptide substance P (SP). Using three-dimensional co-cultures and in vivo models,
we found that neuronal SP promoted breast tumour growth, invasion and metastasis. Moreover, patient tumours with elevated SP exhibited enhanced lymph node metastatic spread. SP acted on
tumoral tachykinin receptors (TACR1) to drive death of a small population of TACR1high cancer cells. Single-stranded RNAs (ssRNAs) released from dying cells acted on neighbouring tumoural
Toll-like receptor 7 (TLR7) to non-canonically activate a prometastatic gene expression program. This SP- and ssRNA-induced _Tlr7_ gene expression signature was associated with reduced
breast cancer survival outcomes. Therapeutic targeting of this neuro–cancer axis with the TACR1 antagonist aprepitant, an approved anti-nausea drug, suppressed breast cancer growth and
metastasis in multiple models. Our findings reveal that tumour-induced hyperactivation of sensory neurons regulates multiple aspects of metastatic progression in breast cancer through a
therapeutically targetable neuropeptide/extracellular ssRNA sensing axis. Access through your institution Buy or subscribe This is a preview of subscription content, access via your
institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $32.99 / 30 days cancel
any time Learn more Subscribe to this journal Receive 51 print issues and online access $199.00 per year only $3.90 per issue Learn more Buy this article * Purchase on SpringerLink *
Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional
subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS SENSORY NERVES ENHANCE TRIPLE-NEGATIVE BREAST CANCER INVASION AND METASTASIS VIA THE AXON
GUIDANCE MOLECULE PLEXINB3 Article Open access 04 November 2022 NOCICEPTIVE NEURONS PROMOTE GASTRIC TUMOUR PROGRESSION VIA A CGRP–RAMP1 AXIS Article 19 February 2025 TARGETING THE PERIPHERAL
NEURAL-TUMOUR MICROENVIRONMENT FOR CANCER THERAPY Article 06 September 2024 DATA AVAILABILITY Raw sequencing data and count tables for transcriptional profiling of 4T1-derived spheroids
have been deposited at the Gene Expression Omnibus under accession number GSE267958. Reads were mapped to the mouse genome assembly GRCm38. Data for the METABRIC study are publicly available
under EGA accession number EGAS00000000083. Data from the TCGA study are publicly available online (https://portal.gdc.cancer.gov). Source data are provided with this paper. CODE
AVAILABILITY All custom computer code is publicly available at GitHub (https://github.com/benostendorf/padmanaban_etal_2024). REFERENCES * Ayala, G. E. et al. Cancer-related axonogenesis and
neurogenesis in prostate cancer. _Clin. Cancer Res._ 14, 7593–7603 (2008). Article CAS PubMed Google Scholar * Huang, D. et al. Nerve fibers in breast cancer tissues indicate aggressive
tumor progression. _Medicine_ 93, e172 (2014). Article PubMed PubMed Central Google Scholar * Oertel, H. Innervation and tumour growth: a preliminary report. _Can. Med. Assoc. J._ 18,
135–139 (1928). CAS PubMed PubMed Central Google Scholar * Renz, B. W. et al. β2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. _Cancer Cell_ 34, 863–867 (2018).
Article CAS PubMed PubMed Central Google Scholar * Latil, A. et al. Quantification of expression of netrins, slits and their receptors in human prostate tumors. _Int. J. Cancer_ 103,
306–315 (2003). Article CAS PubMed Google Scholar * Osswald, M. et al. Brain tumour cells interconnect to a functional and resistant network. _Nature_ 528, 93–98 (2015). Article ADS
CAS PubMed Google Scholar * Venkatesh, H. S. et al. Neuronal activity promotes glioma growth through neuroligin-3 secretion. _Cell_ 161, 803–816 (2015). Article CAS PubMed PubMed
Central Google Scholar * Venkatesh, H. S. et al. Electrical and synaptic integration of glioma into neural circuits. _Nature_ 573, 539–545 (2019). Article ADS CAS PubMed PubMed Central
Google Scholar * Zhao, C. M. et al. Denervation suppresses gastric tumorigenesis. _Sci. Transl. Med._ 6, 250ra115 (2014). Article ADS PubMed PubMed Central Google Scholar * Magnon,
C. et al. Autonomic nerve development contributes to prostate cancer progression. _Science_ 341, 1236361 (2013). Article PubMed Google Scholar * Balood, M. et al. Nociceptor neurons
affect cancer immunosurveillance. _Nature_ 611, 405–412 (2022). Article ADS CAS PubMed PubMed Central Google Scholar * Globig, A. M. et al. The β1-adrenergic receptor links sympathetic
nerves to T cell exhaustion. _Nature_ 622, 383–392 (2023). Article ADS CAS PubMed Google Scholar * Gerendai, I. et al. Transneuronal labelling of nerve cells in the CNS of female rat
from the mammary gland by viral tracing technique. _Neuroscience_ 108, 103–118 (2001). Article CAS PubMed Google Scholar * Hebb, C. & Linzell, J. L. Innervation of the mammary gland.
A histochemical study in the rabbit. _Histochem. J._ 2, 491–505 (1970). Article CAS PubMed Google Scholar * Tavora, B. et al. Tumoural activation of TLR3–SLIT2 axis in endothelium
drives metastasis. _Nature_ 586, 299–304 (2020). Article ADS CAS PubMed PubMed Central Google Scholar * Brose, K. et al. Slit proteins bind Robo receptors and have an evolutionarily
conserved role in repulsive axon guidance. _Cell_ 96, 795–806 (1999). Article CAS PubMed Google Scholar * Nguyen-Ngoc, K. V. et al. ECM microenvironment regulates collective migration
and local dissemination in normal and malignant mammary epithelium. _Proc. Natl Acad. Sci. USA_ 109, E2595–E2604 (2012). Article CAS PubMed PubMed Central Google Scholar * Aslakson, C.
J. & Miller, F. R. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. _Cancer Res._ 52, 1399–1405
(1992). CAS PubMed Google Scholar * Bujak, J. K., Kosmala, D., Szopa, I. M., Majchrzak, K. & Bednarczyk, P. Inflammation, cancer and immunity-implication of TRPV1 channel. _Front.
Oncol._ 9, 1087 (2019). Article PubMed PubMed Central Google Scholar * Liebig, C., Ayala, G., Wilks, J. A., Berger, D. H. & Albo, D. Perineural invasion in cancer: a review of the
literature. _Cancer_ 115, 3379–3391 (2009). Article CAS PubMed Google Scholar * Kastin, A. _Handbook of Biologically Active Peptides_ (Academic, 2013). * Otsuka, M. & Konishi, S.
Release of substance P-like immunoreactivity from isolated spinal cord of newborn rat. _Nature_ 264, 83–84 (1976). Article ADS CAS PubMed Google Scholar * Eshete, F. & Fields, R. D.
Spike frequency decoding and autonomous activation of Ca2+-calmodulin-dependent protein kinase II in dorsal root ganglion neurons. _J. Neurosci._ 21, 6694–6705 (2001). Article CAS PubMed
PubMed Central Google Scholar * Nishikawa, S. et al. Two histidine residues are essential for ribonuclease T1 activity as is the case for ribonuclease A. _Biochemistry_ 26, 8620–8624
(1987). Article CAS PubMed Google Scholar * Robertson, H. D., Webster, R. E. & Zinder, N. D. Purification and properties of ribonuclease III from _Escherichia coli_. _J. Biol. Chem._
243, 82–91 (1968). Article CAS PubMed Google Scholar * Bremnes, R. M., Sirera, R. & Camps, C. Circulating tumour-derived DNA and RNA markers in blood: a tool for early detection,
diagnostics, and follow-up? _Lung Cancer_ 49, 1–12 (2005). Article PubMed Google Scholar * Huang, W. et al. Site-specific RNase A activity was dramatically reduced in serum from multiple
types of cancer patients. _PLoS ONE_ 9, e96490 (2014). Article ADS PubMed PubMed Central Google Scholar * De Lamirande, G. Action of deoxyribonuclease and ribonuclease on the growth of
Ehrlich ascites carcinoma in mice. _Nature_ 192, 52–54 (1961). Article ADS PubMed Google Scholar * Ledoux, L. Action of ribonuclease on two solid tumours in vivo. _Nature_ 176, 36–37
(1955). Article ADS CAS PubMed Google Scholar * Lund, J. M. et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. _Proc. Natl Acad. Sci. USA_ 101, 5598–5603 (2004).
Article ADS CAS PubMed PubMed Central Google Scholar * Kawasaki, T. & Kawai, T. Toll-like receptor signaling pathways. _Front. Immunol._ 5, 461 (2014). Article PubMed PubMed
Central Google Scholar * Ojaniemi, M. et al. Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. _Eur. J. Immunol._ 33,
597–605 (2003). Article CAS PubMed Google Scholar * Ha, T. et al. TLR2 ligands induce cardioprotection against ischaemia/reperfusion injury through a PI3K/Akt-dependent mechanism.
_Cardiovasc. Res._ 87, 694–703 (2010). Article CAS PubMed PubMed Central Google Scholar * Hesketh, P. J. et al. The oral neurokinin-1 antagonist aprepitant for the prevention of
chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin-the Aprepitant Protocol 052 Study
Group. _J. Clin. Oncol._ 21, 4112–4119 (2003). Article CAS PubMed Google Scholar * Rosso, M., Robles-Frias, M. J., Covenas, R., Salinas-Martin, M. V. & Munoz, M. The NK-1 receptor is
expressed in human primary gastric and colon adenocarcinomas and is involved in the antitumor action of L-733,060 and the mitogenic action of substance P on human gastrointestinal cancer
cell lines. _Tumour Biol._ 29, 245–254 (2008). Article CAS PubMed Google Scholar * Munoz, M. & Rosso, M. The NK-1 receptor antagonist aprepitant as a broad spectrum antitumor drug.
_Invest. N. Drugs_ 28, 187–193 (2010). Article CAS Google Scholar * Nagakawa, O. et al. Effect of prostatic neuropeptides on invasion and migration of PC-3 prostate cancer cells. _Cancer
Lett._ 133, 27–33 (1998). Article CAS PubMed Google Scholar * Nizam, E. & Erin, N. Differential consequences of neurokinin receptor 1 and 2 antagonists in metastatic breast carcinoma
cells; effects independent of substance P. _Biomed. Pharmacother._ 108, 263–270 (2018). Article CAS PubMed Google Scholar * Le, T. T. et al. Sensory nerves enhance triple-negative
breast cancer invasion and metastasis via the axon guidance molecule PlexinB3. _NPJ Breast Cancer_ 8, 116 (2022). Article CAS PubMed PubMed Central Google Scholar * Austin, M., Elliott,
L., Nicolaou, N., Grabowska, A. & Hulse, R. P. Breast cancer induced nociceptor aberrant growth and collateral sensory axonal branching. _Oncotarget_ 8, 76606–76621 (2017). Article
PubMed PubMed Central Google Scholar * Jurcak, N. R. et al. Axon guidance molecules promote perineural invasion and metastasis of orthotopic pancreatic tumors in mice. _Gastroenterology_
157, 838–850 (2019). Article CAS PubMed Google Scholar * Kamiya, A. et al. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer
progression. _Nat. Neurosci._ 22, 1289–1305 (2019). Article CAS PubMed Google Scholar * Zahalka, A. H. et al. Adrenergic nerves activate an angio-metabolic switch in prostate cancer.
_Science_ 358, 321–326 (2017). Article ADS CAS PubMed PubMed Central Google Scholar * Partecke, L. I. et al. Subdiaphragmatic vagotomy promotes tumor growth and reduces survival via
TNFα in a murine pancreatic cancer model. _Oncotarget_ 8, 22501–22512 (2017). Article PubMed PubMed Central Google Scholar * Renz, B. W. et al. Cholinergic signaling via muscarinic
receptors directly and indirectly suppresses pancreatic tumorigenesis and cancer stemness. _Cancer Discov._ 8, 1458–1473 (2018). Article CAS PubMed PubMed Central Google Scholar *
Boilly, B., Faulkner, S., Jobling, P. & Hondermarck, H. Nerve dependence: from regeneration to cancer. _Cancer Cell_ 31, 342–354 (2017). Article CAS PubMed Google Scholar * Amit, M.
et al. Loss of p53 drives neuron reprogramming in head and neck cancer. _Nature_ 578, 449–454 (2020). * Kalinichenko, V. V., Mokyr, M. B., Graf, L. H. Jr, Cohen, R. L. & Chambers, D. A.
Norepinephrine-mediated inhibition of antitumor cytotoxic T lymphocyte generation involves a β-adrenergic receptor mechanism and decreased TNF-α gene expression. _J. Immunol._ 163, 2492–2499
(1999). Article CAS PubMed Google Scholar * Mohammadpour, H. et al. β2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor
cells. _J. Clin. Invest._ 129, 5537–5552 (2019). Article CAS PubMed PubMed Central Google Scholar * Benci, J. L. et al. Opposing functions of interferon coordinate adaptive and innate
immune responses to cancer immune checkpoint blockade. _Cell_ 178, 933–948 (2019). Article CAS PubMed PubMed Central Google Scholar * Cao, Y. Q. et al. Primary afferent tachykinins are
required to experience moderate to intense pain. _Nature_ 392, 390–394 (1998). Article ADS CAS PubMed Google Scholar * Wang, Y. et al. Ephrin-B2 controls VEGF-induced angiogenesis and
lymphangiogenesis. _Nature_ 465, 483–486 (2010). Article ADS CAS PubMed Google Scholar * Guy, C. T., Cardiff, R. D. & Muller, W. J. Induction of mammary tumors by expression of
polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. _Mol. Cell. Biol._ 12, 954–961 (1992). CAS PubMed PubMed Central Google Scholar * Maroulakou, I. G.,
Anver, M., Garrett, L. & Green, J. E. Prostate and mammary adenocarcinoma in transgenic mice carrying a rat C3(1) simian virus 40 large tumor antigen fusion gene. _Proc. Natl Acad. Sci.
USA_ 91, 11236–11240 (1994). Article ADS CAS PubMed PubMed Central Google Scholar * Hale, J. J. et al. Structural optimization affording 2-(_R_)-(1-(_R_)-3,
5-bis(trifluoromethyl)phenylethoxy)-3-(_S_)-(4-fluoro)phenyl-4- (3-oxo-1,2,4-triazol-5-yl)methylmorpholine, a potent, orally active, long-acting morpholine acetal human NK-1 receptor
antagonist. _J. Med. Chem._ 41, 4607–4614 (1998). Article CAS PubMed Google Scholar * Padmanaban, V. et al. Organotypic culture assays for murine and human primary and metastatic-site
tumors. _Nat. Protoc._ 15, 2413–2442 (2020). Article CAS PubMed PubMed Central Google Scholar * Young, L., Sung, J., Stacey, G. & Masters, J. R. Detection of Mycoplasma in cell
cultures. _Nat. Protoc._ 5, 929–934 (2010). Article CAS PubMed Google Scholar * Foty, R. A simple hanging drop cell culture protocol for generation of 3D spheroids. _J. Vis. Exp._
https://doi.org/10.3791/2720 (2011). * Heil, F. et al. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. _Science_ 303, 1526–1529 (2004). Article ADS CAS
PubMed Google Scholar * Alexopoulou, L., Holt, A. C., Medzhitov, R. & Flavell, R. A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. _Nature_ 413,
732–738 (2001). Article ADS CAS PubMed Google Scholar * Hemmi, H. et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. _Nat.
Immunol._ 3, 196–200 (2002). Article CAS PubMed Google Scholar * Jurk, M. et al. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. _Nat. Immunol._
3, 499 (2002). Article CAS PubMed Google Scholar * Maira, S. M. et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. _Mol.
Cancer Ther._ 11, 317–328 (2012). Article CAS PubMed Google Scholar * Davies, B. R. et al. Preclinical pharmacology of AZD5363, an inhibitor of AKT: pharmacodynamics, antitumor activity,
and correlation of monotherapy activity with genetic background. _Mol. Cancer Ther._ 11, 873–887 (2012). Article CAS PubMed Google Scholar * Folkes, A. J. et al. The identification of
2-(1_H_-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-_d_]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3
kinase for the treatment of cancer. _J. Med. Chem._ 51, 5522–5532 (2008). Article CAS PubMed Google Scholar * Padmanaban, V. et al. E-cadherin is required for metastasis in multiple
models of breast cancer. _Nature_ 573, 439–444 (2019). Article ADS CAS PubMed PubMed Central Google Scholar * Krause, S., Brock, A. & Ingber, D. E. Intraductal injection for
localized drug delivery to the mouse mammary gland. _J. Vis. Exp._ https://doi.org/10.3791/50692 (2013). * Jancso, G., Kiraly, E., Such, G., Joo, F. & Nagy, A. Neurotoxic effect of
capsaicin in mammals. _Acta Physiol. Hung._ 69, 295–313 (1987). CAS PubMed Google Scholar * Chi, J. et al. Three-dimensional adipose tissue imaging reveals regional variation in beige fat
biogenesis and PRDM16-dependent sympathetic neurite density. _Cell Metab._ 27, 226–236 (2018). Article CAS PubMed Google Scholar * Luppi, P. H., Fort, P. & Jouvet, M. Iontophoretic
application of unconjugated cholera toxin B subunit (CTb) combined with immunohistochemistry of neurochemical substances: a method for transmitter identification of retrogradely labeled
neurons. _Brain Res._ 534, 209–224 (1990). Article CAS PubMed Google Scholar * Gee, K. R. et al. Chemical and physiological characterization of fluo-4 Ca2+-indicator dyes. _Cell Calcium_
27, 97–106 (2000). Article CAS PubMed Google Scholar * Curtis, C. et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. _Nature_ 486,
346–352 (2012). Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank the members of our laboratory for discussions and feedback on the
manuscript text; P. Rajasethupathy for advice on calcium imaging analysis; M. Klatt for technical help with several animal experiments; the members of the various resource centres at
Rockefeller University, including A. North, C. Pyrgaki, Banerjee P., and other staff of the Bio Imaging Resource Center, including C. Zhao, the staff of the Genomics Resource Center, S.
Mazel and the staff of the Flow Cytometry Resource Center. The results published here are in part based on data generated by the TCGA Research Network. This work was supported by
U54CA261701, R35CA274446, the Black Family Metastasis Center, the Breast Cancer Research Foundation and the Reem Kayden award. V.P. was supported by the Hope Funds for Cancer Research
postdoctoral fellowship. I.K. is member of the German Academic Scholarship Foundation (Studienstiftung des deutschen Volkes) and was awarded a fellowship from Boehringer Ingelheim Fonds
(BIF). B.N.O. was supported by a Max Eder grant of the German Cancer Aid (70114327) and is a fellow of the digital clinician scientist program at BIH-Charité. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Laboratory of Systems Cancer Biology, The Rockefeller University, New York, NY, USA Veena Padmanaban, Isabel Keller, Ethan S. Seltzer, Benjamin N. Ostendorf & Sohail F.
Tavazoie * Department of Hematology, Oncology, and Tumor Immunology and Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Berlin, Germany Benjamin N. Ostendorf * Berlin
Institute for Medical Systems Biology, Max Delbrück Center for Molecular Medicine, Berlin, Germany Benjamin N. Ostendorf * Laboratory of Mucosal Immunology, The Rockefeller University, New
York, NY, USA Zachary Kerner Authors * Veena Padmanaban View author publications You can also search for this author inPubMed Google Scholar * Isabel Keller View author publications You can
also search for this author inPubMed Google Scholar * Ethan S. Seltzer View author publications You can also search for this author inPubMed Google Scholar * Benjamin N. Ostendorf View
author publications You can also search for this author inPubMed Google Scholar * Zachary Kerner View author publications You can also search for this author inPubMed Google Scholar * Sohail
F. Tavazoie View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS V.P. and S.F.T. conceptualized the study, designed experiments, supervised
research and wrote the manuscript with input from all of the authors. V.P. performed most of the experiments with technical assistance from I.K., E.S.S. and Z.K.; B.N.O. analysed
mRNA-sequencing data. S.F.T. obtained funding and supervised scientists. CORRESPONDING AUTHOR Correspondence to Sohail F. Tavazoie. ETHICS DECLARATIONS COMPETING INTERESTS The authors
declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature_ thanks Osamu Takeuchi and the other, anonymous, reviewer(s) for their contribution to the peer review of this
work. Peer reviewer reports are available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
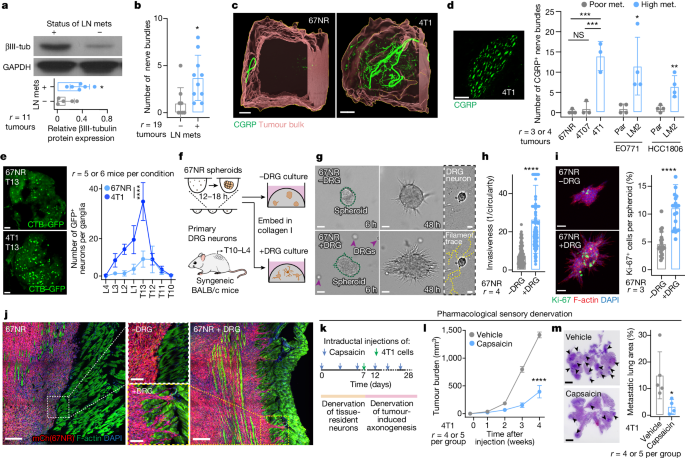
affiliations. EXTENDED DATA FIGURES AND TABLES EXTENDED DATA FIG. 1 BREAST TUMOURS ARE FREQUENTLY INNERVATED BY SENSORY NERVES. (a) We previously uncovered a requirement for
endothelial-derived SLIT2 (ecSLIT2) in metastasis15. (b) Quantification of ecSLIT2 expression in poorly _vs_ highly metastatic breast tumour models. _****p_ < 0.0001, Mann-Whitney test.
Mean ± SD. (c-d) Quantification of total (c) and CGRP+ sensory (d) innervation in 4T1 tumours grown in ecSLIT2 knockout (endothelial-specific depletion of SLIT2) mice. **** _p_ < 0.0001,
*_p_ = 0.0489, t-test. Mean ± SD. (e) Quantification of innervation in SLIT2-knockout mammary tumours in Slit2_fl/fl_; MMTV-PyMT; MMTV-Cre mice. ns_p_ = 0.6414, Mann-Whitney test. Mean ± SD.
(f) Kaplan-Meier plots showing distant metastasis-free survival (DMFS) of breast cancer patients sorted by the median expression level of βIII-tubulin (mRNA, protein) or PGP9.5 (mRNA) of
their tumours. The x-axes were set to have >10 surviving patients in each arm. (g) Nerve bundle abundance in highly metastatic primary tumours relative to corresponding isogenic poorly
metastatic tumours. Between 4 and 8 tumours were analysed per group. **_p_ (67NR vs 4T1) = 0.001, **_p_ (4T07 vs 4T1) = 0.0019, ns_p_ (67NR vs 4T07) = 0.9095, ANOVA; **_p_ (EO771 Par vs LM2)
= 0.0065, *_p_ (HCC1806 Par vs LM2) = 0.0159, Mann-Whitney test. Mean ± SD. (h) Expression of pan-neuronal βIII-tubulin in 67NR _vs_ 4T1 primary tumours, stroma-depleted primary tumour
organoids, or cell lines. **_p_ = 0.0048, t-test. Mean ± SD. MW: βIII-tubulin (50 kDa). (i) Liver metastasis from a 4T1 tumour-bearing mouse immuno-stained for nerve fibres. _r_ = 3/group.
(j) Retrograde tracing of DRG neurons innervating abdominal mammary glands of wild-type mice. Mean ± SEM. (k-l) Sensory, CGRP+ innervation observed within the normal murine mammary gland
(k), murine models of breast cancer (l), and primary human tumours (l). _r_ = 3 tumours/ group. Source Data EXTENDED DATA FIG. 2 SENSORY NEURONS PROMOTE CANCER INVASION AND PROLIFERATION
ACROSS MULTIPLE EX VIVO MURINE AND HUMAN MODELS OF BREAST CANCER. (a) Frequency of apoptosis (cleaved caspase 3, CC3 +) in DRG neurons cultured alone or in the presence of 67NR cancer cells.
(b) Micrographs of mCherry+ 67NR spheroids co-cultured with βIII-tubulin+ DRG neurons. Neuron and spheroid boundaries are manually traced to illustrate little physical contact. _n_ = 10
ROIs/ group_, r_ = 4. (c) 2D invasion assay of 67NR cancer cells that were cultured alone or in the presence of primary DRG neurons. *_p_ = 0.0432, t-test. Mean ± SD. (d) Quantification of
the number of mitotically active cells within regions of 4T1 primary tumours adjacent to or far away from a nerve bundle. **_p_ = 0.0043, Mann-Whitney test. Mean ± SD. (e-f) 3D colony
formation assay. 67NR cancer cells were cultured alone or in the presence of DRG neurons in 3D Matrigel. (e) Schematic. (f) Quantification. **_p_ = 0.0067, Kruskal-Wallis test. Mean ± SD.
(g-i) 3D co-culture assays of MMTV-PyMT mammary tumour organoids and primary DRG neurons. (g) Schematic. (h) Invasion assay. ****_p_ < 0.0001, Mann-Whitney test. (i) Colony formation
assay. **_p_ = 0.0033, t-test. Mean ± SD. (j-m) 3D co-culture assays of 4 independent primary human breast tumours and DRG neurons. (j) Schematic of organoid isolation. (k) Invasion
quantification. ****_p_ < 0.0001, *_p_ = 0.0266, Mann-Whitney test. Mean ± SD. (l) Proliferation quantification. ****_p_ < 0.0001, *_p_ = 0.0401 (#2), 0.0267 (#4), t-test. Mean ± SD.
(m) Basic de-identified clinical information for human tumour samples cultured. Source Data EXTENDED DATA FIG. 3 CO-TRANSPLANTATION OF BREAST CANCER CELLS WITH DRG NEURONS DRIVES METASTASIS.
(a-h) Co-transplantation of mCherry+ (mCh) 67NR cancer cells and DRG neurons. (a) Schematic. (b) Optically cleared 67NR tumours transplanted with or without DRG neurons and immunostained
for CGRP+ sensory nerves. _r_ = 2 tumours/ group. (c) Mean fluorescence intensity (MFI) for SP in 67NR primary tumours transplanted with or without DRG neurons. **_p_ = 0.0046, t-test. Mean
± SD. (d) Tumour growth. **_p_ = 0.0017, t-test. Mean ± SEM. (e) Percentage of the tumour-stroma boundary with a pushing _vs_ invasive morphology. ****_p_ < 0.0001, Chi-square test. (f)
Association of mCh+ 67NR cancer cells with the lung endothelium in mice transplanted with orthotopic 67NR tumours. _r_ = 3 lungs/ group. (g) CTC enumeration in mice transplanted with mCh+
67NR cancer cells with or without DRG neurons. *_p_ = 0.0287, t-test. Mean ± SD. (h) mCh+ micro-metastases. **_p_ = 0.0079, Mann-Whitney test. Mean ± SD. (i) Co-transplantation of 67NR
cancer cells with increasing numbers of DRG neurons. ns_p_ = 0.4124, **_p_ = 0.0026, ****_p_ < 0.0001, ANOVA. Mean ± SEM. Source Data EXTENDED DATA FIG. 4 SENSORY NEURONS DRIVE METASTATIC
COLONIZATION IN MULTIPLE BREAST CANCER MODELS. (a-c) Tail vein injections of mCh+ 67NR cancer cells with or without DRG neurons. (a) Schematic. (b) CGRP + / βIII-tubulin+ neuronal cell
bodies in mice co-injected with DRG neurons. _r_ = 3 lungs/ group. (c) mCh+ metastases. ****_p_ < 0.0001, Mann-Whitney test. Mean ± SD. (d-f) Co-transplantation of EO771 cancer cells and
DRG neurons. (d) Schematic. (e) Tumour growth. *_p_ = 0.0345, t-test. Mean ± SEM. (f) Number of micro-metastases counted by H&E. *_p_ = 0.0249, t-test. Mean ± SD. Source Data EXTENDED
DATA FIG. 5 CAPSAICIN DOES NOT ALTER GROWTH OR INVASION OF CANCER CELLS IN VITRO AND REDUCES TUMOUR GROWTH IN IMMUNE-COMPROMISED MICE IN VIVO. (a) Sensory (CGRP +) and sympathetic (TH +)
innervation in ipsilateral or contralateral mammary glands 7 days after capsaicin administration. (b) Quantification of CGRP+ nerve bundles post-capsaicin administration. *_p_ = 0.0403,
t-test. Mean ± SD. (c-d) 3D culture of 4T1 spheroids in the presence of capsaicin (1–100 μM). (c) Invasion quantification. ns_p_ > 0.9999, Kruskal-Wallis test. (d) Spheroid surface area.
ns_p_ = 0.7108, Kruskal-Wallis test. Mean ± SD. (e-f) Sensory-specific denervation of 4T1 tumours grown in NSG mice. (e) Schematic. (f) Tumour growth. ****_p_ < 0.0001, t-test. Mean ±
SEM. Source Data EXTENDED DATA FIG. 6 NEURONAL SUBSTANCE-P DRIVES BREAST CANCER METASTASIS VIA THE ACTIVATION OF THE TUMORAL TACR1 RECEPTOR. (a) Invasion assay of 67NR cancer cells in
response to SP. *_p_ = 0.0254, t-test. Mean ± SD. (b-c) 67NR cancer cell spheroids cultured with neuropeptides galanin or CGRP, with or without RNase A. (b) Invasion quantification. **_p_ =
0.0011, ****_p_ < 0.0001, ns_p_ (CGRP) = 0.1066, ns_p_ (galanin) > 0.9999, Kruskal-Wallis test. Vehicle-treated 67NR spheroids (−/+RNase A) are also plotted in Fig. 2d and Fig. 3d
respectively since the experiments were conducted together. (c) Proliferation quantification. ***_p_ = 0.0006, Mann-Whitney test. Mean ± SD. (d) ELISA-based quantification for SP levels in
conditioned medium. p values are based on comparisons with base medium (NGM). ns_p_ = 0.7426 (_vs_ DRG only), 0.8724 (_vs_ 67NR only); *p = 0.0213; **_p_ = 0.0024, ANOVA. Mean ± SD. (e-g) In
vivo measurements of SP expression. (e) Mean fluorescence intensity (MFI) of SP in 4T1 _vs_ 67NR primary tumours. ****_p_ < 0.0001, t-test. (f) Plasma SP levels in 67NR _vs_ 4T1
tumour-bearing mice. *_p_ = 0.0359, t-test. (g) MFI of SP in 4T1 vehicle _vs_ capsaicin-treated primary tumours. ****_p_ < 0.0001, t-test. Mean ± SD. (h-k) Effects of an SP-blocking
antibody on 4T1 tumour growth and metastasis. (h) Schematic. (i) MFI of SP in IgG _vs_ anti-SP treated 4T1 tumours. ****_p_ < 0.0001, Mann-Whitney test. Mean ± SD. (j) Tumour growth.
****_p_ < 0.0001, Mann-Whitney test. Mean ± SEM. (k) Metastatic area quantified by H&E. **_p_ = 0.0012, t-test. Mean ± SD. (l-o) Orthotopic transplantation of EO771 LM2 cells into the
abdominal mammary glands of _Tac1-WT_ and _Tac1-null_ host mice. (l) Schematic. (m) MFI of SP in primary tumours. ***_p_ = 0.0003, t-test. Mean ± SD. (n) Tumour growth. ***_p_ = 0.0003,
t-test. Mean ± SEM. (o) Number of macro-metastases quantified by H&E. ***_p_ = 0.0006, t-test. Mean ± SD. (p-r) Depletion of SP’s receptor, TACR1 in 4T1 cancer cell spheroids. (p)
Validation of knockdown. ****_p_ < 0.0001, ANOVA. MW: TACR1 (46 kDa). Quantification of spheroid invasion (q) and proliferation (r). ****_p_ < 0.0001, Mann-Whitney test. Mean ± SD.
(s-u) Orthotopic transplantation of 4T1 cancer cells depleted for TACR1. (s) Schematic. (t) Tumour growth. ****_p_ < 0.0001, ANOVA. Mean ± SEM. (u) Metastatic area quantified by H&E.
***_p_ = 0.0003, ANOVA. Mean ± SD. Source Data EXTENDED DATA FIG. 7 NEURONAL SP DRIVES METASTASIS IN A SSRNA-DEPENDENT MANNER. (a-b) Schematic for isolation of conditioned medium from tumour
only, DRG only, or tumour-DRG cultures (a). Invasion quantification, ns_p_ = 0.1463, ****_p_ < 0.0001, Kruskal-Wallis test (b). Mean ± SD. (c) Calcium fluorescence traces (ΔF/F0) of DRG
neurons cultured alone or in the presence of 67NR cancer cells. F = measured fluorescence, F0 = baseline fluorescence. (d) Invasion quantification of 67NR spheroids co-cultured with DRG
neurons in the presence of a sodium channel blocker, tetrodotoxin (TTX). ns_p_ > 0.9999, ****_p_ < 0.0001, Kruskal-Wallis test. Mean ± SD. (e) Invasion quantification of 67NR spheroids
cultured with DNase-treated or heat inactivated tumour-CM or DRG-CM. ***_p_ = 0.001, ****_p_ < 0.0001, Kruskal-Wallis test. Mean ± SD. (f) Invasion quantification of 67NR spheroids
cultured with solely RNase A, RNase T1 or RNase III. ns_p_ > 0.9999, Kruskal-Wallis test. Mean ± SD. (g) Proliferation quantification of 67NR spheroids cultured with or without ssRNA40.
****_p_ < 0.0001, Mann-Whitney test. Mean ± SD. (h) Invasion quantification of 67NR spheroids cultured with a dsRNA mimetic, Poly (I:C). **_p_ = 0.0072, Kruskal-Wallis test. Mean ± SD.
(i-k) Effect of RNase A treatment on 4T1 tumour growth and metastasis. (i) Schematic. (j) Tumour growth. ****_p_ < 0.0001, ANOVA. Mean ± SEM. (k) Metastatic area quantified by H&E.
*_p_ = 0.0392 (vehicle _vs_ RNase A #1), *_p_ = 0.0347 (vehicle _vs_ RNase A #2), ANOVA. Mean ± SD. (l) MFI of dsRNA (measured using an anti-dsRNA antibody, J2) in vehicle _vs_ RNase A
treated 4T1 primary tumours. ns_p_ = 0.22 (RNase A #1), 0.0625 (RNase A #2), ANOVA. Mean ± SD. (m-o) Intra-cardiac injections of mCherry+ 67NR cancer cells pre-treated with tumour-CM or
DRG-CM. (m) Schematic. (n) Representative mCherry+ metastases. (o) Quantification of mCherry+ metastases. **_p_ = 0.0028, *_p_ = 0.0366 (liver), *_p_ = 0.0313 (brain), t-test. Mean ± SD.
Source Data EXTENDED DATA FIG. 8 SP-DRIVEN ACTIVATION OF TACR1 AND SSRNA-DRIVEN ACTIVATION OF TLR7 PROMOTE BREAST CANCER INVASIVENESS ACROSS MULTIPLE MODELS OF BREAST CANCER. (a) Invasion
quantification of Py8119, 4T1, and MDA-MB-231 breast cancer spheroids in the presence of SP. **_p_ = 0.0013, ****_p_ < 0.0001, Kruskal-Wallis test. Mean ± SD. (b) Invasion quantification
of Py8119, 4T1, and MDA-MB-231 breast cancer spheroids in the presence of ssRNA40. *_p_ = 0.0235, ****_p_ < 0.0001, Kruskal-Wallis test. Mean ± SD. Source Data EXTENDED DATA FIG. 9
NEURONAL SP SIGNALS VIA TUMORAL TLR7 RECEPTORS TO DRIVE METASTASIS. (a) Percent apoptotic area within 67NR spheroids cultured with galanin. ns_p_ = 0.0807, Mann-Whitney test. Mean ± SD. (b)
67NR spheroids cultured with or without DRG neurons and immunostained for TACR1. _n_ = 10 spheroids/ group. (c) Flow cytometry analysis of TACR1 expression in 67NR cancer cells. **_p_ =
0.0089, t-test. Mean ± SD. (d-f) 67NR spheroids depleted for TACR1 and cultured with SP. (e) Percent apoptotic area. _***p_ = 0.0003, Mann-Whitney test, _****p_ < 0.0001, Kruskal-Wallis
test. (f) Invasion quantification. _****p_ < 0.0001, Kruskal-Wallis test. Mean ± SD. (g) Validation of _Tlr7_ knockdown in 67NR cancer cells. ****_p_ < 0.0001, ANOVA. Mean ± SD. MW:
TLR7 (135 kDa). Validation of _Tlr3_ knockdown in 67NR cancer cells. ****_p_ < 0.0001, ANOVA. Mean ± SD. Invasion quantification of 67NR spheroids depleted for TLR3 and cultured with or
without DRG neurons. ****_p_ < 0.0001, Kruskal-Wallis test. Mean ± SD. Invasion quantification of 67NR spheroids cultured in the presence of TLR7 agonists, R837 or R848. ****_p_ <
0.0001, **_p_ = 0.0011, Kruskal-Wallis test. Mean ± SD. Validation of _Tlr7_ knockdown in 4T1 cancer cells. ***_p_ = 0.0006, ANOVA. Mean ± SD. MW: TLR7 (135 kDa). Invasion quantification of
4T1 spheroids depleted of TLR7. ****_p_ < 0.0001, Kruskal-Wallis test. Mean ± SD. Immunostaining for CD45 (all immune), CD3 (T cells), or NK1.1 (NK cells) on 4T1 primary tumours depleted
of TLR7. ns_p_ > 0.9999 (all conditions), Kruskal-Wallis test. Mean ± SD. (n-p) Orthotopic transplantations of 4T1 cancer cells depleted for TLR7 in NSG mice. (n) Schematic. (o) Tumour
growth. ****_p_ < 0.0001, ANOVA. Mean ± SEM. (p) Metastatic area quantified by H&E. ****_p_ < 0.0001, ANOVA. Mean ± SD. (q-s) Orthotopic transplantation of 4T1 cancer cells
depleted for TLR7, followed by periodic injections of RNase A. (q,r) Schematics. (s) Tumour growth. **_p_ = 0.0033, ns_p_ = 0.9923, ANOVA. Mean ± SD. Source Data EXTENDED DATA FIG. 10
SENSORY NEURONS ACTIVATE A NON-CANONICAL TLR7 SIGNALLING AXIS IN CANCER CELLS WHICH CORRELATES WITH POOR PATIENT OUTCOME. (a) mRNA sequencing of control _vs Tlr7_ depleted 4T1 spheroids. Top
five downregulated pathways in 4T1 spheroids depleted for _Tlr7_ as assessed by gene set enrichment analysis (_p_ values according to permutation testing). (b) Validation of MyD88 knockdown
in 67NR cancer cells. MW: MyD88 (33 kDa). _r_ = 3. (c) Invasion quantification of 67NR spheroids depleted for MyD88 and cultured with or without DRG neurons. ****_p_ < 0.0001,
Mann-Whitney test. ns_p_ > 0.9999 (si#1), ns_p_ = 0.0783 (si#2), ns_p_ = 0.4140 (si#3), Kruskal-Wallis test. Mean ± SD. (d) Invasion quantification of 67NR spheroids cultured with or
without SP and a PI3K inhibitor (left to right: buparlisib, capivasertib, or pictilisib). ns_p_ (Veh _vs_ buparlisib) = 0.1273, ns_p_ (Veh _vs_ capivasertib) = 0.6967, ns_p_ (Veh _vs_
pictilisib) > 0.9999, ****_p_ < 0.0001, Kruskal-Wallis test. Mean ± SD. (e-f) Multivariate analysis of the association of age, tumour stage and a _Tlr7_-dependent gene signature with
survival in breast cancer patients from the METABRIC (e) and TCGA (f) datasets (_p_ values according to multivariate Cox proportional hazard models, error bars indicate 95% confidence
intervals). Source Data EXTENDED DATA FIG. 11 APREPITANT IMPAIRS TUMOUR GROWTH AND METASTASIS OF PY8119 AND MMTV-PYMT MODELS OF BREAST CANCER. (a) Invasion quantification of Py8119, 4T1, and
MDA-MB-231 breast cancer spheroids cultured in the presence of aprepitant. ****_p_ < 0.0001, ns_p_ = 0.9621, **_p_ = 0.0013, Kruskal-Wallis test. Mean ± SD. (b-d) Aprepitant was
evaluated for its potential in inhibiting breast cancer progression and metastasis. (b) Schematic. (c) Py8119 tumour growth and metastasis count. **_p_ = 0.0012, *_p_ = 0.0117, Mann-Whitney
test. (d) MMTV-PyMT tumour growth. **_p_ = 0.0047, Mann-Whitney test. Mean ± SEM. Source Data SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figs. 1 and 2. REPORTING
SUMMARY PEER REVIEW FILE SOURCE DATA SOURCE DATA FIG. 1 SOURCE DATA FIG. 2 SOURCE DATA FIG. 3 SOURCE DATA FIG. 4 SOURCE DATA FIG. 5 SOURCE DATA EXTENDED DATA FIG. 1 SOURCE DATA EXTENDED DATA
FIG. 2 SOURCE DATA EXTENDED DATA FIG. 3 SOURCE DATA EXTENDED DATA FIG. 4 SOURCE DATA EXTENDED DATA FIG. 5 SOURCE DATA EXTENDED DATA FIG. 6 SOURCE DATA EXTENDED DATA FIG. 7 SOURCE DATA
EXTENDED DATA FIG. 8 SOURCE DATA EXTENDED DATA FIG. 9 SOURCE DATA EXTENDED DATA FIG. 10 SOURCE DATA EXTENDED DATA FIG. 11 RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a
society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript
version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Padmanaban, V., Keller,
I., Seltzer, E.S. _et al._ Neuronal substance P drives metastasis through an extracellular RNA–TLR7 axis. _Nature_ 633, 207–215 (2024). https://doi.org/10.1038/s41586-024-07767-5 Download
citation * Received: 19 January 2023 * Accepted: 28 June 2024 * Published: 07 August 2024 * Issue Date: 05 September 2024 * DOI: https://doi.org/10.1038/s41586-024-07767-5 SHARE THIS ARTICLE
Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative