Play all audios:
ABSTRACT The ability to learn novel items depends on brain functions that store information about items classified by their associated meanings and outcomes1,2,3,4, but the underlying neural
circuit mechanisms of this process remain poorly understood. Here we show that deep layers of the lateral entorhinal cortex (LEC) contain two groups of ‘item–outcome neurons’: one
developing activity for rewarded items during learning, and another for punished items. As mice learned an olfactory item–outcome association, we found that the neuronal population of LEC
layers 5/6 (LECL5/6) formed an internal map of pre-learned and novel items, classified into dichotomic rewarded versus punished groups. Neurons in the medial prefrontal cortex (mPFC), which
form a bidirectional loop circuit with LECL5/6, developed an equivalent item–outcome rule map during learning. When LECL5/6 neurons were optogenetically inhibited, tangled mPFC
representations of novel items failed to split into rewarded versus punished groups, impairing new learning by mice. Conversely, when mPFC neurons were inhibited, LECL5/6 representations of
individual items were held completely separate, disrupting both learning and retrieval of associations. These results suggest that LECL5/6 neurons and mPFC neurons co-dependently encode item
memory as a map of associated outcome rules. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access
through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to
this journal Receive 51 print issues and online access $199.00 per year only $3.90 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy
now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer
support SIMILAR CONTENT BEING VIEWED BY OTHERS DOPAMINE FACILITATES ASSOCIATIVE MEMORY ENCODING IN THE ENTORHINAL CORTEX Article 22 September 2021 HIPPOCAMPAL AND ORBITOFRONTAL NEURONS
CONTRIBUTE TO COMPLEMENTARY ASPECTS OF ASSOCIATIVE STRUCTURE Article Open access 20 June 2024 FAST UPDATING FEEDBACK FROM PIRIFORM CORTEX TO THE OLFACTORY BULB RELAYS MULTIMODAL IDENTITY AND
REWARD CONTINGENCY SIGNALS DURING RULE-REVERSAL Article Open access 22 January 2025 DATA AVAILABILITY The neurophysiological data generated in this study are available on request. CODE
AVAILABILITY The neurophysiological data and analytical codes are available on request and will be deposited with a subsequent protocol paper. REFERENCES * Suzuki, W. A. Associative learning
signals in the brain. _Prog. Brain Res._ 169, 305–320 (2008). Article PubMed Google Scholar * Osada, T., Adachi, Y., Kimura, H. M. & Miyashita, Y. Towards understanding of the
cortical network underlying associative memory. _Phil. Trans. R. Soc. B_ 363, 2187–2199 (2008). Article PubMed PubMed Central Google Scholar * Ozawa, T. & Johansen, J. P. Learning
rules for aversive associative memory formation. _Curr. Opin. Neurobiol._ 49, 148–157 (2018). Article CAS PubMed Google Scholar * Igarashi, K. M., Lee, J. Y. & Jun, H. Reconciling
neuronal representations of schema, abstract task structure, and categorization under cognitive maps in the entorhinal–hippocampal–frontal circuits. _Curr. Opin. Neurobiol._ 77, 102641
(2022). Article CAS PubMed PubMed Central Google Scholar * Squire, L. R. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. _Psychol. Rev._ 99,
195–231 (1992). Article CAS PubMed Google Scholar * Buzsaki, G. & Moser, E. I. Memory, navigation and theta rhythm in the hippocampal–entorhinal system. _Nat. Neurosci._ 16, 130–138
(2013). Article CAS PubMed PubMed Central Google Scholar * Morris, R. G. in _The Hippocampus Book_ (ed. P. Andersen, P.) 581–714 (Oxford Univ. Press, 2007). * Eichenbaum, H. On the
integration of space, time, and memory. _Neuron_ 95, 1007–1018 (2017). Article CAS PubMed PubMed Central Google Scholar * O’Keefe, J. & Nadel, L. _The Hippocampus as a Cognitive
Map_ (Oxford Univ. Press, 1978). * Moser, E. I., Moser, M. B. & Roudi, Y. Network mechanisms of grid cells. _Phil. Trans. R. Soc. B_ 369, 20120511 (2014). Article PubMed PubMed Central
Google Scholar * Price, J. L. An autoradiographic study of complementary laminar patterns of termination of afferent fibers to the olfactory cortex. _J. Comp. Neurol._ 150, 87–108 (1973).
Article CAS PubMed Google Scholar * Burwell, R. D. The parahippocampal region: corticocortical connectivity. _Ann. N. Y. Acad. Sci._ 911, 25–42 (2000). Article CAS PubMed ADS Google
Scholar * Igarashi, K. M. et al. Parallel mitral and tufted cell pathways route distinct odor information to different targets in the olfactory cortex. _J. Neurosci._ 32, 7970–7985 (2012).
Article CAS PubMed PubMed Central Google Scholar * Young, B. J., Otto, T., Fox, G. D. & Eichenbaum, H. Memory representation within the parahippocampal region. _J. Neurosci._ 17,
5183–5195 (1997). Article CAS PubMed PubMed Central Google Scholar * Deshmukh, S. S. & Knierim, J. J. Representation of non-spatial and spatial information in the lateral entorhinal
cortex. _Front. Behav. Neurosci._ 5, 69 (2011). Article PubMed PubMed Central Google Scholar * Tsao, A., Moser, M. B. & Moser, E. I. Traces of experience in the lateral entorhinal
cortex. _Curr. Biol._ 23, 399–405 (2013). Article CAS PubMed Google Scholar * Igarashi, K. M., Lu, L., Colgin, L. L., Moser, M. B. & Moser, E. I. Coordination of
entorhinal–hippocampal ensemble activity during associative learning. _Nature_ 510, 143–147 (2014). Article CAS PubMed ADS Google Scholar * Lee, J. Y. et al. Dopamine facilitates
associative memory encoding in the entorhinal cortex. _Nature_ 598, 321–326 (2021). Article CAS PubMed PubMed Central ADS Google Scholar * Martin, C., Beshel, J. & Kay, L. M. An
olfacto-hippocampal network is dynamically involved in odor-discrimination learning. _J. Neurophysiol._ 98, 2196–2205 (2007). Article PubMed Google Scholar * Cohen, J. Y., Haesler, S.,
Vong, L., Lowell, B. B. & Uchida, N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. _Nature_ 482, 85–88 (2012). Article CAS PubMed PubMed
Central ADS Google Scholar * Luo, W. et al. Acquiring new memories in neocortex of hippocampal-lesioned mice. _Nat. Commun._ 13, 1601 (2022). Article CAS PubMed PubMed Central ADS
Google Scholar * Insausti, R., Herrero, M. T. & Witter, M. P. Entorhinal cortex of the rat: cytoarchitectonic subdivisions and the origin and distribution of cortical efferents.
_Hippocampus_ 7, 146–183 (1997). Article CAS PubMed Google Scholar * Witter, M. P. & Amaral, D. G. in _The Rat Nervous System_ 3rd edn (ed. Paxinos, G.) 635–704 (Elsevier, 2004). *
Zingg, B. et al. Neural networks of the mouse neocortex. _Cell_ 156, 1096–1111 (2014). Article CAS PubMed PubMed Central Google Scholar * Jun, H. et al. Disrupted place cell remapping
and impaired grid cells in a knockin model of Alzheimer’s disease. _Neuron_ 107, 1095–1112.e6 (2020). Article CAS PubMed PubMed Central Google Scholar * Issa, J. B., Radvansky, B. A.,
Xuan, F. & Dombeck, D. A. Lateral entorhinal cortex subpopulations represent experiential epochs surrounding reward. _Nat. Neurosci._ 27, 536–546 (2024). Article CAS PubMed PubMed
Central Google Scholar * Mulder, A. B., Nordquist, R., Orgut, O. & Pennartz, C. M. Plasticity of neuronal firing in deep layers of the medial prefrontal cortex in rats engaged in
operant conditioning. _Prog. Brain Res._ 126, 287–301 (2000). Article CAS PubMed Google Scholar * Rushworth, M. F., Noonan, M. P., Boorman, E. D., Walton, M. E. & Behrens, T. E.
Frontal cortex and reward-guided learning and decision-making. _Neuron_ 70, 1054–1069 (2011). Article CAS PubMed Google Scholar * Euston, D. R., Gruber, A. J. & McNaughton, B. L. The
role of medial prefrontal cortex in memory and decision making. _Neuron_ 76, 1057–1070 (2012). Article CAS PubMed PubMed Central Google Scholar * Anastasiades, P. G. & Carter, A.
G. Circuit organization of the rodent medial prefrontal cortex. _Trends Neurosci._ 44, 550–563 (2021). Article CAS PubMed PubMed Central Google Scholar * Lillicrap, T. P., Santoro, A.,
Marris, L., Akerman, C. J. & Hinton, G. Backpropagation and the brain. _Nat. Rev. Neurosci._ 21, 335–346 (2020). Article CAS PubMed Google Scholar * Konishi, M. I., Igarashi, K. M.
& Miura, K. Biologically plausible local synaptic learning rules robustly implement deep supervised learning. _Front. Neurosci._ 17, 1160899 (2023). Article PubMed PubMed Central
Google Scholar * Hasegawa, I., Fukushima, T., Ihara, T. & Miyashita, Y. Callosal window between prefrontal cortices: cognitive interaction to retrieve long-term memory. _Science_ 281,
814–818 (1998). Article CAS PubMed ADS Google Scholar * Tomita, H., Ohbayashi, M., Nakahara, K., Hasegawa, I. & Miyashita, Y. Top-down signal from prefrontal cortex in executive
control of memory retrieval. _Nature_ 401, 699–703 (1999). Article CAS PubMed ADS Google Scholar * Frankland, P. W. & Bontempi, B. The organization of recent and remote memories.
_Nat. Rev. Neurosci._ 6, 119–130 (2005). Article CAS PubMed Google Scholar * Tse, D. et al. Schema-dependent gene activation and memory encoding in neocortex. _Science_ 333, 891–895
(2011). Article CAS PubMed ADS Google Scholar * Eichenbaum, H. Prefrontal–hippocampal interactions in episodic memory. _Nat. Rev. Neurosci._ 18, 547–558 (2017). Article CAS PubMed
Google Scholar * Kitamura, T. et al. Engrams and circuits crucial for systems consolidation of a memory. _Science_ 356, 73–78 (2017). Article CAS PubMed PubMed Central ADS Google
Scholar * Tse, D. et al. Schemas and memory consolidation. _Science_ 316, 76–82 (2007). Article CAS PubMed ADS Google Scholar * Baraduc, P., Duhamel, J. R. & Wirth, S. Schema cells
in the macaque hippocampus. _Science_ 363, 635–639 (2019). Article CAS PubMed ADS Google Scholar * Simons, J. S. & Spiers, H. J. Prefrontal and medial temporal lobe interactions in
long-term memory. _Nat. Rev. Neurosci._ 4, 637–648 (2003). Article CAS PubMed Google Scholar * Ito, H. T. Prefrontal–hippocampal interactions for spatial navigation. _Neurosci. Res._
129, 2–7 (2018). Article PubMed Google Scholar * Spellman, T. et al. Hippocampal–prefrontal input supports spatial encoding in working memory. _Nature_ 522, 309–314 (2015). Article CAS
PubMed PubMed Central ADS Google Scholar * Place, R., Farovik, A., Brockmann, M. & Eichenbaum, H. Bidirectional prefrontal–hippocampal interactions support context-guided memory.
_Nat. Neurosci._ 19, 992–994 (2016). Article CAS PubMed PubMed Central Google Scholar * Ito, H. T., Zhang, S. J., Witter, M. P., Moser, E. I. & Moser, M. B. A
prefrontal–thalamo–hippocampal circuit for goal-directed spatial coding. _Nature_ https://doi.org/10.1038/nature14396 (2015). * Feierstein, C. E., Quirk, M. C., Uchida, N., Sosulski, D. L.
& Mainen, Z. F. Representation of spatial goals in rat orbitofrontal cortex. _Neuron_ 51, 495–507 (2006). Article CAS PubMed Google Scholar * Wang, P. Y. et al. Transient and
persistent representations of odor value in prefrontal cortex. _Neuron_ 108, 209–224.e6 (2020). Article CAS PubMed PubMed Central ADS Google Scholar * Basu, R. et al. The orbitofrontal
cortex maps future navigational goals. _Nature_ 599, 449–452 (2021). Article CAS PubMed PubMed Central ADS Google Scholar * Jun, H., Chavez, J., Bramian, A. & Igarashi, K. M.
Protocol for remapping of place cells in disease mouse models. _STAR Protoc._ 2, 100759 (2021). Article PubMed PubMed Central Google Scholar * Kvitsiani, D. et al. Distinct behavioural
and network correlates of two interneuron types in prefrontal cortex. _Nature_ 20, 363–366 (2013). Article ADS Google Scholar Download references ACKNOWLEDGEMENTS We thank M. Witter and
A. Treves, and members in the Igarashi laboratory for providing valuable comments on this work. The work was supported by the US National Institutes of Health (NIH) R01 grants (R01MH121736,
R01AG063864, R01AG066806 and R01AG086441) and a BrightFocus Foundation Research grant (A2019380S) to K.M.I. H.J. was supported by the University of California, Irvine Medical Scientist
Training Program (MSTP; T32GM008620) and the NIH F31 grant (F31AG069500). J.Y.L. was supported by the NIH F31 grant (F31AG074650). AUTHOR INFORMATION Author notes * These authors contributed
equally: Heechul Jun, Jason Y. Lee AUTHORS AND AFFILIATIONS * Department of Anatomy and Neurobiology, School of Medicine, University of California Irvine, Irvine, CA, USA Heechul Jun, Jason
Y. Lee, Nicholas R. Bleza, Ayana Ichii, Jordan D. Donohue & Kei M. Igarashi * Department of Biomedical Engineering, Samueli School of Engineering, University of California Irvine,
Irvine, CA, USA Kei M. Igarashi * Center for Neural Circuit Mapping, School of Medicine, University of California Irvine, Irvine, CA, USA Kei M. Igarashi * Center for the Neurobiology of
Learning and Memory, University of California Irvine, Irvine, CA, USA Kei M. Igarashi * Institute for Memory Impairments and Neurological Disorders, University of California Irvine, Irvine,
CA, USA Kei M. Igarashi Authors * Heechul Jun View author publications You can also search for this author inPubMed Google Scholar * Jason Y. Lee View author publications You can also search
for this author inPubMed Google Scholar * Nicholas R. Bleza View author publications You can also search for this author inPubMed Google Scholar * Ayana Ichii View author publications You
can also search for this author inPubMed Google Scholar * Jordan D. Donohue View author publications You can also search for this author inPubMed Google Scholar * Kei M. Igarashi View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS H.J., J.Y.L. and K.M.I. conceived the project and designed the experiments. H.J., J.Y.L., N.R.B., A.I.
and J.D.D. performed the experiments. H.J., J.Y.L. and K.M.I. performed the analyses. H.J., J.Y.L. and K.M.I. wrote the paper with input from all authors. CORRESPONDING AUTHOR
Correspondence to Kei M. Igarashi. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature_ thanks Thomas McHugh and
the other, anonymous, reviewer(s) for their contribution to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to
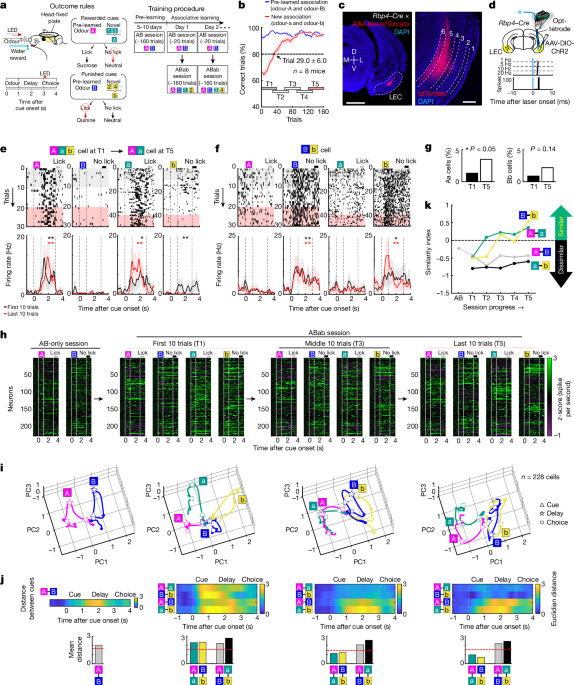
jurisdictional claims in published maps and institutional affiliations. EXTENDED DATA FIGURES AND TABLES EXTENDED DATA FIG. 1 HISTOLOGICAL VALIDATION OF IMPLANTED SITES. (A) Recording
positions in LEC of _Rbp4_-_Cre_ mice for opt-tagging experiment (Figs. 1 and 4). Positions were marked with electrolytic lesioning. D, dorsal, V, ventral, M, medial, L, lateral. (B) Optic
fiber positions in LEC of _Rbp4_-_Cre_ mice injected with AAV-flex-Jaws-GFP into LEC for inhibition experiments (Fig. 3). Arrowhead, estimated tip of optic fibers. (C) Optic fiber positions
in LEC of _Rbp4_-_Cre_ mice injected with retroAAV-flex-Jaws-GFP into mPFC for inhibition of mPFC-projecting LEC cells (Extended Data Fig. 14). (D) Optic fiber positions in LEC of WT mice
injected with AAV-hSyn-Jaws-GFP into mPFC for inhibition of mPFC terminals in the LEC (Fig. 4 and Extended Data Fig. 17). (E) Recording positions in mPFC of WT mice (Fig. 2). Positions were
marked with electrolytic lesioning. (F) Optic fiber positions in mPFC of WT mice injected with AAV-hSyn-Jaws-GFP into mPFC for inhibition experiments (Fig. 4). *Mice used for recording
LECL5/6 cells + mPFC terminal inhibition; **Mice used for recording mPFC cells + LECL5/6 inhibition. EXTENDED DATA FIG. 2 VERIFICATION OF OPTTAGGING PROCEDURE. (A) Spike raster plots of a
typical opttagged neuron which is seen to follow 1, 2, 5, and 10 Hz pulse trains (5 ms pulse, 10 pulses per train). (B) “Opto-PSTH” of 6 example opttagged neurons. Neurons i-v) feature
optically evoked spikes with low latency and jitter (Bottom right) An excluded opttagged cell (vi), with a long latency of 9.1 ms, suggesting a synaptically tagged cell. (C) Latencies of all
opttagged neurons plotted against the jitter of first spike after stimulation. Opttagged neurons had a wide variety of latencies (2-8.5 ms), making it difficult to delineate real opttagged
cells and potential synaptically tagged cells. (D) PCA trajectories, Euclidian distances, and SI plot of LECL5/6 population as in Fig. 1i, excluding opttagged cells with latency greater than
8.5 ms (n = 213 cells). (E) PCA trajectories, Euclidian distances, and SI plot of LECL5/6 population as in Fig. 1i, excluding opttagged cells with latency greater than 5 ms (n = 153 cells).
These results show a similar Odor-A and -a representation, and similar Odor-B and -b representation at T5. Thus, although the total population analysis may include some synaptically
opttagged neurons, it is likely that our conclusion is not affected by their marginal numbers. EXTENDED DATA FIG. 3 CUE-OUTCOME PROFILE OF LECL5/6 NEURONS ALONG THE DORSOVENTRAL AXIS.
Recorded positions along dorsoventral axis of LEC. Distances were calculated from rhinal sulcus border to approximate location of amygdalopiriform transition area and normalized from 0
(dorsal) to 1 (ventral). The result demonstrated that while Aa cells were observed along the D-V axis, Bb cells were found only in the ventral part of the LEC. EXTENDED DATA FIG. 4 SPIKE
PROPERTIES OF LECL5/6 CELLS DURING CORRECT ASSOCIATIVE LEARNING. (A-D) Spike properties of LECL5/6 cells. LECL5/6 cells were recorded in a session with Odor-A and Odor-B (AB-only session).
After ~20 trials in AB session, associative learning (ABab) session was tested (T1-T5). (A) Spike firing of 228 LECL5/6 cells. Mean spike activity was averaged in 50 ms bins and shown in
z-score compared with −1 – 0 s before odor onset. In this panel, cells were sorted using a cluster analysis of firing property in T5. (B) Mean firing rate of 228 LECL5/6 cells shown in
z-score. (C) Percent responsive cells for each cue combination. Neurons with significant firing during 0.5-3.0 s after odor onset (odor + delay period) were counted (Wilcoxon signed-rank
test, p < 0.05). (D) Percent responsive cells in periods of 0.5-1.0 s (odor), 2-3 s (delay) and 3-4 s (choice) after odor onset. Neurons with significant firing (p < 0.05) during each
period were counted (two-sided signed-rank test). (E) Trajectories of neural firing of LECL5/6 cell population as in Fig. 1i, but presented throughout timepoints T1 – T5. (F) Euclidian
distance between odor trial types. (G) Mean Euclidian distance during 0.5-3.0 s after odor onset (odor + delay period). Ninety-fifth percentile distance obtained from shuffled data denotes
significant distance (red line). (H) The change of Similarity Index (SI) during associative learning was compared using the bootstrapping method (see Methods). PCA was performed from a
resampled neuronal population, and this procedure was repeated 1000 times to make 1000 bootstraps. SI was calculated for each bootstrap, then SIs in T2–T5 were subtracted by that in T1, to
test if there was a significant distribution above or below zero. SI for Odors A-a increased at T3-5 (p = 0.005, 0.012, 0.0005 respectively, bootstrapping test), as well as SI for Odors B-b
at T3-5 (p = 0.0023, 0.0021, 0.003 respectively), confirming increasing similarity of representations between Odor-A and -a, and between B-b during leaning. *p < 0.05, **p < 0.01, ***p
< 0.001, two-sided bootstrapping test. Data are presented as mean values +/- SEM. EXTENDED DATA FIG. 5 SELECTIVITY PROFILE OF MPFC AND LECL5/6 SINGLE NEURONS DURING LEARNING. (A)
Examples of neurons based on their selectivity profile between T1 vs T5 period. Type 1: Static cells with same selectivity to an odor cue; Type 2: Static cells without selectivity to any
cue; Type 3: Plastic cells with selectivity in T1 to an odor cue but no selectivity in T5; Type 4: Plastic cells with no selectivity in T1 but gained selectivity to an odor cue in T5; Type
5: Plastic cells with selectivity to an odor cue in T1 but gained distinct selectivity in T5. Significant selectivity (p < 0.05) was assessed using spike response during 0.5 – 3 s after
cue onset, compared to 1 s pre-cue period (two-sided rank sum test). Data are presented as mean values +/- SEM. (B) (Top) Percentage of static (54%) or plastic (46%) mPFC cells. Static cells
were further subdivided into type1 (4%) and type 2 (50%). Plastic cells were further subdivided into type 3 (18%), type 4 (14%), and type 5 (14%). (Bottom) Percentage of static (50%) or
plastic (50%) LEC5/6 cells. Static cells were further subdivided into type1 (4%) and type 2 (46%). Plastic cells were further subdivided into type 3 (19%), type 4 (13%), and type 5 (18%).
EXTENDED DATA FIG. 6 ADDITIONAL PRINCIPAL COMPONENT ANALYSES FOR LECL5/6 CELL RECORDINGS. (a) Error-exclusion analysis. Trajectories of neural firing of LECL5/6 cell population using only
correct (hit) trials for Odors-A and -a and correct rejection (CR) trials for Odors-B and -b. The pattern of A-a vs. B-b dichotomic classification was again observed when the incorrect
trials were removed from the PC analysis, excluding the possibility that the representations in Fig. 1i emerged from the increasing rate of correct trials. (b) Movement analysis.
Trajectories of neural firing of LECL5/6 cell population using only lick (hit) trials for Odors-A and -a, and error lick (false alarm, FA) trials for Odors-B and -b. Although all of them are
trials in which mice made lick responses, the A-a vs. B-b dichotomic classification similar to Fig. 1i was observed, suggesting that LECL5/6 cells do not simply represent lick-related motor
information. EXTENDED DATA FIG. 7 EFFECT OF INDIVIDUAL AA AND BB CELLS ON POPULATION ACTIVITY. PCA trajectories, Euclidian distances, and SI plot of LECL5/6 population, excluding individual
Aa and Bb cells (n = 137 cells). The result showed unstable grouping between Odor-A and Odor-a (shown by unstable SIA-b in the SI plot), and no development of grouping between Odor-B and
Odor-b (shown by low SIB-b throughout the session). This result suggests that Aa cells and Bb cells contribute to the generation of outcome rule representation. EXTENDED DATA FIG. 8
CHARACTERISTICS OF _RBP4_+ LECL5/6 CELLS. (A) Electrophysiological features for classifying putative principal neurons. Peak to valley time of spike waveform was used to distinguish putative
interneurons from principal neurons in _Rbp4_+ LECL5/6 cells. (Left) Dashed line (230µs) represents cut off for wide spike (WS) excitatory neurons (n = 176; 77%) to narrow spike (NS)
interneurons (n = 52; 23%) classification (Bartho et al., 2004). (Right) Independent trajectories of neural firing of LECL5/6 WS neurons and NS neurons both demonstrated the grouping of
Odors-A and -a and grouping of Odors-B and -b. (B) LECL5/6 labeling with excitatory cell marker. (Left) AAV-flex-tdTomato (red) and AAV-flex-CaMKIIa-GFP (green) were injected into LEC5/6 of
_Rbp4_-_Cre_ mice. (Middle) Coronal sections of LEC layer 5/6. CaMKIIa (green) GFP signal reveals excitatory neurons. TdTomato (red) signal reveals _Rbp4_+ LECL5/6 cells. Bottom panel
demonstrates magnified windows from top panel. Yellow arrow points to example LECL5/6 excitatory cell expressing both CaMKIIa GFP and tdTomato. White arrow points to example LECL5/6
non-excitatory cell expressing only tdTomato. (Right) Percentage of double-positive neurons among tdTomato+ neurons (80.08 ± 2.75 % from 8 sections obtained from n = 2 mice). Data are
presented as mean values +/- SEM. EXTENDED DATA FIG. 9 SPIKE PROPERTIES OF LECL5/6 CELLS DURING SPONTANEOUS ERROR SESSIONS. (A) Behavioral performance of LEC _Rbp4_-_Cre_ recording mice in
correct sessions (Fig. 1) and error sessions where mice spontaneously could not reach >80% performance criteria. Data are presented as mean values +/- SEM. (A1) Top, learning curves
during correct and error sessions. Familiar cue performance (A/B) in blue, novel cue performance (a/b) in red. Bottom, percent correct trials in T5 for correct vs. error sessions (p =
8.8e-8, two-way ANOVA; A/B correct vs. a/b error, *p = 3.8e-9 or less, post-hoc Tukey test; n = 8 mice). (A2) (Left) Percentage of correct trials plotted in each odor trial type. (Right)
Performance of mice in the last 10 trials (p = 4.8e-28, two-way ANOVA; ***p = 6e-8 between Odor-a correct vs. odor-a error, post-hoc Tukey test; n = 8 mice). (B-E) Same as in Extended Data
Fig. 4a–d, but for error sessions (n = 72 cells). (F) Proportion of Aa cells (left) and Bb cells (right) in correct vs. error sessions. Both response types were missing in error sessions.
(G) Same as in in Extended Data Fig. 4e–g, but for error sessions. SI plot shows that SIA-a did not increase to positive, indicating Odor-a was not classified together with Odor-A. (H)
Bootstrapping analysis. (Top) Same plot as in Extended Data Fig. 4h, but for error sessions. LECL5/6 cells did not develop similar representations between Odors-A and -a, and between Odors-B
and -b observed in correct sessions. (Bottom) Direct comparison between correct and error sessions confirmed the disappearance of similar representations between Odors-A and -a at T3-T5 of
error sessions (p = 0.27, 0.14, 0.13 respectively). The comparison also indicates more similar representations between Odors-a and -b throughout error sessions T1-T5 (p = 0.043, 0.013,
0.0056, 0.026, 0.0054 respectively). *p < 0.05, **p < 0.01, ***p < 0.001, two-sided bootstrapping test. Data are presented as mean values +/- SEM. EXTENDED DATA FIG. 10 SPIKE
PROPERTIES OF MPFC CELLS DURING CORRECT ASSOCIATIVE LEARNING SESSIONS. (A) Spike firing of 779 mPFC cells. Mean spike activity was averaged in 50 ms bins and shown in z-score compared with
−1 – 0 s before odor onset. In this panel, cells were sorted using a cluster analysis of firing property in T5. (B) Mean firing rate of 779 mPFC cells shown in z-score. (C) Percent
responsive cells in correct T5 (top) and error T5 (bottom). Neurons with significant firing (p < 0.05) during 0.5-3.0 s after odor onset (odor + delay period) were counted (two-sided
signed-rank test). (D) Percent responsive cells in periods of 0.5-1.0 s (odor), 2-3 s (delay) and 3-4 s (choice) after odor onset. Neurons with significant firing (p < 0.05) during each
period were counted (two-sided signed-rank test). (E) Trajectories of neural firing of mPFC cell population as shown in Fig. 2h, but for timepoints T1 – T5. (F) Euclidian distance between
odor trial types. (G) Mean Euclidian distance during 0.5-3.0 s after odor onset (odor + delay period) for timepoints T1 – T5 of correct sessions. Ninety-fifth percentile distance obtained
from shuffled data denotes significant distance (red line). (H) Bootstrapping analysis for Fig. 2j. SI for Odors A-a and B-b showed significant increases in T5 compared to T1 (p = 0.0039 and
p = 1.7e-8, respectively, bootstrapping test), while SI for Odors A-B and a-b showed significant decreases (p = 3.1e-5 and p = 0.029, respectively), confirming increasing similarity between
odors of the same outcome, and decreasing similarity between odors of the different outcome. *p < 0.05, **p < 0.01, ***p < 0.001, two-sided test, n = 1000 bootstraps each. Data are
presented as mean values +/- SEM. EXTENDED DATA FIG. 11 EARLY EMERGENCE OF GROUPING OF ODOR-A AND ODOR-A REWARDED CUES. (A) Trajectories of neural firing of LECL5/6 cell population as in
Fig. 1i, but presented throughout timepoints T1a (first five trials of T1) and T1b (second five trials of T1). (B) Trajectories of neural firing of mPFC cell population as above. EXTENDED
DATA FIG. 12 ADDITIONAL PRINCIPAL COMPONENT ANALYSES FOR MPFC CELL RECORDINGS. (A) Error-exclusion analysis. Trajectories of neural firing of mPFC cell population using only correct (hit)
trials for Odors-A and -a, and correct rejection (CR) trials for Odors-B and -b. The pattern of A-a vs. B-b dichotomic classification was again observed when the incorrect trials were
removed from the PC analysis, excluding the possibility that the representations in Fig. 2h emerged from the increasing rate of correct trials. (B) Movement analysis. Trajectories of neural
firing of mPFC cell population using only lick (hit) trials for Odors-A and -a and error lick (false alarm, FA) trials for Odors-B and -b. Although all of them are trials in which mice made
lick responses, the A-a vs. B-b dichotomic classification similar to Fig. 2h was observed, suggesting that mPFC cells do not simply represent lick-related motor information. EXTENDED DATA
FIG. 13 SPIKE PROPERTIES OF MPFC CELLS DURING SPONTANEOUS ERROR SESSIONS. (A) Behavioral performance of mPFC recording mice in correct sessions (Fig. 2) and error sessions where mice
spontaneously could not reach >80% performance criteria. Data are presented as mean values +/- SEM. (A1) Behavioral performance of mPFC recording mice in correct vs. error sessions. Top,
learning curves during correct and error sessions. Familiar cue performance (A/B) in blue, novel cue performance (a/b) in red. Bottom, percentage of correct trials in T5 for correct vs.
error sessions (p = 2.4e-7, ANOVA; p = 1.9e-8 or less, post-hoc Tukey test; n = 6 mice). (A2) mPFC recording mice data plotted for percent correct trials in each odor trial type as a
function of trial number for each odor type. (Right) Performance of mice in the last 10 trials (p = 1.99e-11, ANOVA; ***p = 6e-8 between Odor-a correct vs. odor-a error, post-hoc Tukey test;
n = 6 mice). (B-E) Same as in Extended Data Fig. 10a–d, but for error sessions (n = 116 cells). (F) Proportion of mPFC Aa cells (left) and Bb cells (right) in correct vs. error sessions.
Aa: p = 1.8e-7, Bb: p = 2.2e-13, two-sided binomial test. (G) Same as in Extended Data Fig. 10e–g, but for error sessions. (H) Bootstrapping analysis. (Top) Same plot as in Extended Data
Fig. 10h, but for error sessions. During the error sessions, mPFC cells did not develop similar representations between Odors-A and -a (T2-T5: p = 0.24, 0.39, 0.36, 0.36, respectively) and
between Odors-B and -b (T2-T5: p = 0.36, 0.034, 0.089, 0.43, respectively) observed in correct sessions. (Bottom) Direct comparison between correct and error sessions confirmed the
disappearance of similar representations between Odors-A and -a (T3-T5: p = 0.006, 0.0005, 6.3e-8, respectively), and similar representations between Odors-B and -b (T5, p = 0.0002). The
comparison also indicates more similar representations between Odors-A and -B (T1-T5: p = 3e-6, 1.8e-6, 6.2e-8. 4e-7, 1.2e-7, respectively), and between Odors-a and -b throughout the error
sessions (T1-T5: p = 7e-6, 1e-16, 3e-15, 1e-16, 1e-16, respectively). *p < 0.05, **p < 0.01, ***p < 0.001, bootstrapping test, n = 1000 bootstraps each. Data are presented as mean
values +/- SEM. EXTENDED DATA FIG. 14 BEHAVIORAL PERFORMANCE IN LECL5/6 INHIBITION EXPERIMENTS. (A) Behavioral performance of LEC _Rbp4_-_Cre_ mice in Fig. 3a,b, but plotted for each odor
trial type. Learning curves during control (left) and LECL5/6 inhibition (middle) sessions. (Right) Performance of mice in the last 10 trials (p = 3.8e-13, two-way ANOVA; p = 6e-8 between
Odor-a in control vs. Odor-a inhibition sessions, post-hoc Tukey test; n = 10 mice). Data are presented as mean values +/- SEM. (B-D) mPFC-projecting LECL5/6 cell inhibition experiments.
Data are presented as mean values +/- SEM. (C) (Left) Learning curves during control and inhibition sessions. (Middle) Percent correct trials in T5 for control vs. inhibition sessions (p =
3.4e-6, two-way ANOVA; A/B control vs. a/b inhibition, p = 3.8e-9; a/b correct vs. a/b error, p = 7.2e-9; A/B error vs. a/b error, p = 3.9e-9, post-hoc Tukey test; n = 10 mice). (Right)
Proportion of correct sessions between control and inhibition conditions (p = 0.0064, two-sided binomial test; n = 30 control and 30 inhibition sessions). (D) Percent correct trials in each
odor trial type as a function of trial number for each odor type. (Right) Performance of mice in the last 10 trials (p = 1.5e-16, two-way ANOVA; p = 6e-8 for Odor-a in correct sessions vs.
odor-a in error sessions, post-hoc Tukey test; n = 10 mice). EXTENDED DATA FIG. 15 MODULATION OF NEURONAL ACTIVITY WITH JAWS INHIBITION. (A) (Top) Recording of mPFC cells during inhibition.
Jaws were expressed in mPFC cells using synapsin promoter driven AAVs. (Middle) Correlation between change in firing rate and pre-stimulation firing rate divided into principal neurons and
interneurons based on their spike waveform peak-to-trough width threshold of 230 μs. Each dot represents a single cell. Cells were classified as inhibited, unaffected, or disinhibited using
two-sided rank sum test (p < 0.05 threshold) between pre-stimulation firing rate and firing rate during inhibition. (Bottom) Percentage of inhibited (21.9%), unaffected (60.3%), or
disinhibited (17.8%) mPFC cells during inhibition. (B) (Top) Opt-tag recording of LECL5/6 cells during inhibition. ChR2 and Jaws were expressed in LECL5/6 of _Rbp4_-_Cre_ mice. (Middle)
Correlation between change in firing rate and pre-stimulation firing rate. Each dot represents a single cell. Cells were classified as inhibited, unaffected, or disinhibited using two-sided
rank sum test (p < 0.05 threshold) between pre-stimulation firing rate and firing rate during inhibition. (Bottom) Percentage of inhibited (12.5%), unaffected (75%), or disinhibited
(12.5%) LEC5/6 cells during inhibition. EXTENDED DATA FIG. 16 DETAILED ANALYSES FROM RECORDING OF MPFC CELLS WITH LECL5/6 INHIBITION. (A-B) Behavioral performance only from n = 4 mice used
in mPFC cell recording with simultaneous inhibition of LECL5/6 cells. (B1) (Top) Learning curves during control and inhibition sessions. (Bottom left) Percentage of correct trials in T5 for
control vs. inhibition sessions (p = 7.5e-9, two-way ANOVA; p = 5.6e-9 or less, post-hoc Tukey test; n = 4 mice). (Bottom right) Proportion of correct sessions between control and inhibition
conditions (p = 1.6e-10, two-sided binomial test; n = 24 control and 24 inhibition sessions). Data are presented as mean values +/- SEM. (B2) Percent correct trials in each odor trial type
as a function of trial number for each odor type. (Right) Performance of mice in the last 10 trials (p = 2.8e-18, two-way ANOVA; p = 6e-6 between Odor-a in correct sessions vs. Odor-a in
error sessions, post-hoc Tukey test; n = 4 mice). Data are presented as mean values +/- SEM. (C) (Top) Trajectories of neural firing of n = 497 mPFC cell population in no-laser control
sessions. (Middle) Euclidian distance between odor trial types. (Bottom) Mean Euclidian distance during 0.5-3.0 s after odor onset (odor + delay period) for timepoints T1 – T5 of correct
sessions. Ninety-fifth percentile distance obtained from shuffled data denotes significant distance (red line). (Right) Similarity index. (D) Same as (c), but for inhibition sessions (laser
on). (E) Bootstrapping analysis. (Top) In control sessions, SI between Odors A-a and between Odors B-b showed significant increase from T1 to T5 (p = 0.014, p = 2.6e-6, respectively). SI for
Odors A-B and a-b showed significant decrease from T1 to T5 (p = 5.4e-6, p = 0.0027, respectively, bootstrapping test). (Middle) In inhibition sessions, SI for all odor pairs did not show
significant differences from T1 to T5 (Aa: p = 0.15, Bb: p = 0.08, AB: p = 0.45, ab: p = 0.34), confirming the impairment of outcome classification. (Bottom) Direct comparison between
control and inhibition sessions confirmed the disappearance of similar representations between Odors-A and -a (T5: p = 7e-6). The comparison also indicates disappearance of separate
representations between Odors-A and -B and between Odors-a and -b in inhibition sessions (p = 2e-4, 8e-13, respectively). n = 1000 bootstraps; *p < 0.05, **p < 0.01, ***p < 0.001,
bootstrapping test. Data are presented as mean values +/- SEM. EXTENDED DATA FIG. 17 BEHAVIORAL PERFORMANCE FOR MPFC INHIBITIONS. (A) Behavioral performance during mPFC inhibition in Fig.
4a,b, but plotted for each odor trial type. Learning curves during control (left) and mPFC inhibition (middle) sessions. (Right) Performance of mice in the last 10 trials (p = 1e-32, two-way
ANOVA; p = 6e-8 between Odor-A in control sessions vs. Odor-A in inhibition sessions; p = 6e-8 between Odor-a in control sessions vs. Odor-a in inhibition sessions, post-hoc Tukey test; n =
13 mice). Data are presented as mean values +/- SEM. (B-D) Behavior performance during mPFC terminal inhibition. Data are presented as mean values +/- SEM. (C) (Left) Learning curves during
control and inhibition sessions. (Middle) Percent correct trials in T5 for control vs. inhibition sessions (p = 0.029, two-way ANOVA; p = 0.0088 or less, post-hoc Tukey test; n = 7 mice).
(Right) Proportion of correct sessions between control and inhibition conditions (p = 0.0001, two-way binomial test; n = 31 control and 21 inhibition sessions). (D) Percent correct trials in
each odor trial type as a function of trial number for each odor type. (Right) Performance of mice in the last 10 trials (p = 0.0037, two-way ANOVA; p = 0.0003 between Odor-a in correct
sessions vs. Odor-a in error sessions, post-hoc Tukey test; n = 7 mice). Although not significant, we observed a trend of decrease in the performance for Odor-A during inhibition (p = 0.63,
Odor-A in correct sessions vs. Odor-A in error sessions, post-hoc Tukey test). EXTENDED DATA FIG. 18 DETAILED ANALYSES FROM RECORDING OF LECL5/6 CELLS WITH MPFC TERMINAL INHIBITION. (A)
Opt-tag recording of LECL5/6 cells with simultaneous inhibition of mPFC inputs in LEC. (B) (Top) Trajectories of neural firing of n = 181 LECL5/6 cell population in no-laser control
sessions. (Middle) Euclidian distance between odor trial types. (Bottom) Mean Euclidian distance during 0.5-3.0 s after odor onset (odor + delay period) for timepoints T1 – T5 of correct
sessions. Ninety-fifth percentile distance obtained from shuffled data denotes significant distance (red line). (Right) Similarity index. (C) Same as (b), but for n = 249 LECL5/6 cell
population during mPFC terminal inhibition sessions (laser on). (D) Bootstrapping analysis. (Top) In control sessions, SI between Odors A-a and between Odors B-b showed significant increase
from T1 to T5 (p = 0.012, p = 0.0095, respectively). (Middle) In inhibition sessions, SI for all odor pairs did not show significant differences from T1 to T5 (Aa: p = 0.41, Bb: p = 0.07,
AB: p = 0.15, ab: p = 0.23), confirming the impairment of outcome classification. (Bottom) Direct comparison between control and inhibition sessions confirmed the disappearance of similar
representations between Odors-A and -a (p = 0.0014 at T5). *p < 0.05, **p < 0.01, ***p < 0.001, two-sided bootstrapping test, n = 1000 bootstraps each. Data are presented as mean
values +/- SEM. EXTENDED DATA FIG. 19 FAST DEVELOPMENT OF ODOR OUTCOME REPRESENTATION IN MPFC POPULATION. (A) Development of task performance, Similarity Index (SI) between Odors-A and a
(SIA-a) for LECL5/6 cell population, and SI between Odors B and b (SI B-b) for LECL5/6 cell population. Variables are normalized onto a scale from 0 (T1a) to 1 (T5). (B) Development of task
performance, SIA-a for mPFC population and SIB-b for mPFC population as above. EXTENDED DATA FIG. 20 DECODING ANALYSES OF LECL5/6 AND MPFC POPULATION ACTIVITY. (A-B) A support vector machine
(SVM) decoder was trained to discriminate novel Odor-a vs. Odor-b, using neural responses to familiar Odor-A vs. Odor-B in the same timepoint (T) as training data. For each 100 ms time bin,
100 SVMs were trained and their performances averaged to achieve the final % correct value. Decoding performance increases quickly in mPFC between T1-T2 (~90% at Trials 6-10 (T1b)), while
performance only gradually reached ~90% at T3 in LECL5/6, indicating a quicker development of outcome representation in mPFC. T1A = trials 1-5; T1B = trials 6-10. A) Decoder performance for
Correct sessions. (Top row) LECL5/6 population. (Bottom row) mPFC population. Right: Average performance from the 0.5-3 s window of each timepoint. B) As above, but for Error sessions. (C-D)
The decoder was trained to discriminate within outcome categories (Odor-A vs. Odor-a OR Odor-B vs. Odor-b), using half the trials of one timepoint to train and the other half to test.
Although the discriminatory power between Odor-A and Odor-a gradually decreased from T1 to T5, it remained at ~75% at T5. This result suggests that LEC neural activities still have
information distinct enough for the decoder to discriminate between Odor-A and -a at T5. This was also the case for Odor-B vs Odor-b (78% at T5), as well as mPFC (82% at T5 for A vs a, 98%
at T5 for B vs b). (C) Decoder performance for LECL5/6 population during Correct sessions. (D) Decoder performance for mPFC population during Correct sessions. EXTENDED DATA FIG. 21
CHARACTERIZING MONOSYNAPTIC INPUT TO _RBP4_+ LECL5/6 CELLS USING RETROGRADE RABIES TRACING. Monosynaptic retrograde tracing of _Rbp4_+ LECL5/6 neurons. (Left) Cre-depndent glycoprotein and
TVA-GFP virus (green) and G-deleted rabies virus expressing mCherry (red) were injected into the LECL5/6 of _Rbp4_-_Cre_ mice. (Right) Top panel shows coronal sections revealing starter
_Rbp4_+ cells (indicated by white arrows) that are double positive (red and green) near the injection site. The remaining panels demonstrate coronal sections with examples of presynaptically
labelled neurons (red only) from local LEC, posterior piriform cortex, amygdala and hippocampal CA1 of the intermediate-ventral tiers (indicated by white arrows). (BLA = Basolateral
amygdala, BMP = Basomedial posterior amygdala, BLV = Basolateral ventral amygdala, LaVL = Lateral amygdala ventrolateral, LaVM = Lateral amygdala ventromedial and LaDL = Lateral amygdala
dorsolateral). SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Results and Discussion which report and discuss additional results that are not logically connected to the
main text. REPORTING SUMMARY SUPPLEMENTARY TABLES Supplementary Tables 1–5 RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to
this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the
terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Jun, H., Lee, J.Y., Bleza, N.R. _et al._ Prefrontal and lateral
entorhinal neurons co-dependently learn item–outcome rules. _Nature_ 633, 864–871 (2024). https://doi.org/10.1038/s41586-024-07868-1 Download citation * Received: 02 November 2023 *
Accepted: 23 July 2024 * Published: 21 August 2024 * Issue Date: 26 September 2024 * DOI: https://doi.org/10.1038/s41586-024-07868-1 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative