Play all audios:
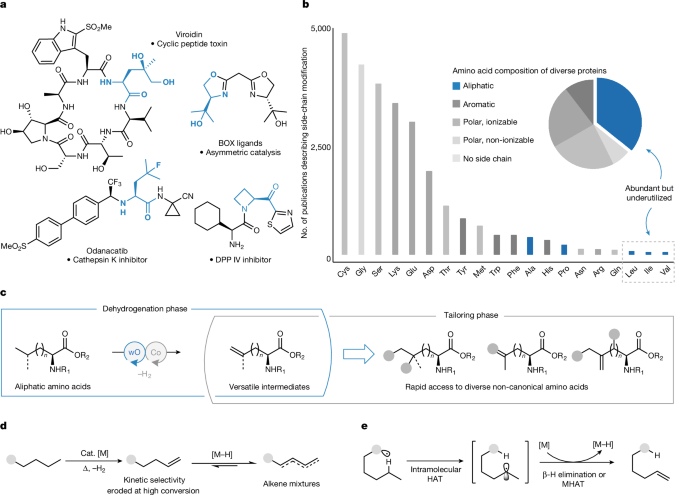
ABSTRACT Amino acids are essential building blocks in biology and chemistry. Whereas nature relies on a small number of amino acid structures, chemists desire access to a vast range of
structurally diverse analogues1,2,3. The selective modification of amino acid side-chain residues represents an efficient strategy to access non-canonical derivatives of value in chemistry
and biology. While semisynthetic methods leveraging the functional groups found in polar and aromatic amino acids have been extensively explored, highly selective and general approaches to
transform unactivated C–H bonds in aliphatic amino acids remain less developed4,5. Here we disclose a stepwise dehydrogenative method to convert aliphatic amino acids into structurally
diverse analogues. The key to the success of this approach lies in the development of a selective catalytic acceptorless dehydrogenation method driven by photochemical irradiation, which
provides access to terminal alkene intermediates for downstream functionalization. Overall, this strategy enables the rapid synthesis of new amino acid building blocks and suggests
possibilities for the late-stage modification of more complex oligopeptides. Access through your institution Buy or subscribe This is a preview of subscription content, access via your
institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel
any time Learn more Subscribe to this journal Receive 51 print issues and online access $199.00 per year only $3.90 per issue Learn more Buy this article * Purchase on SpringerLink *
Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional
subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS BIOMIMETIC 1,2-AMINO MIGRATION VIA PHOTOREDOX CATALYSIS Article Open access 07 March 2025
MODULAR AND DIVERSE SYNTHESIS OF AMINO ACIDS VIA ASYMMETRIC DECARBOXYLATIVE PROTONATION OF AMINOMALONIC ACIDS Article 16 November 2023 HARNESSING TRANSAMINASES TO CONSTRUCT AZACYCLIC
NON-CANONICAL AMINO ACIDS Article 28 March 2024 DATA AVAILABILITY All data supporting the findings of this paper are available in the main text or the Supplementary Information. REFERENCES *
Hruby, V. J. & Qian, X. in _Peptide Synthesis Protocols_ (eds Pennington, M. W. & Dunn, B. M.) 249–286 (Humana, 1995). * Nájera, C. & Sansano, J. M. Catalytic asymmetric
synthesis of α-amino acids. _Chem. Rev._ 107, 4584–4671 (2007). Article PubMed Google Scholar * Almhjell, P. J., Boville, C. E. & Arnold, F. H. Engineering enzymes for noncanonical
amino acid synthesis. _Chem. Soc. Rev._ 47, 8980–8997 (2018). Article PubMed PubMed Central CAS Google Scholar * deGruyter, J. N., Malins, L. R. & Baran, P. S. Residue-specific
peptide modification: a chemist’s guide. _Biochemistry_ 56, 3863–3873 (2017). Article PubMed CAS Google Scholar * Noisier, A. F. M. & Brimble, M. A. C–H functionalization in the
synthesis of amino acids and peptides. _Chem. Rev._ 114, 8775–8806 (2014). Article PubMed CAS Google Scholar * Walsh, C. T., O’Brien, R. V. & Khosla, C. Nonproteinogenic amino acid
building blocks for nonribosomal peptide and hybrid polyketide scaffolds. _Angew. Chem. Int. Ed. Engl._ 52, 7098–7124 (2013). Article PubMed PubMed Central CAS Google Scholar *
Blaskovich, M. A. T. Unusual amino acids in medicinal chemistry. _J. Med. Chem._ 59, 10807–10836 (2016). Article PubMed CAS Google Scholar * Hickey, J. L., Sindhikara, D., Zultanski, S.
L. & Schultz, D. M. Beyond 20 in the 21st century: prospects and challenges of non-canonical amino acids in peptide drug discovery. _ACS Med. Chem. Lett._ 14, 557–565 (2023). Article
PubMed PubMed Central CAS Google Scholar * Reetz, M. T. New approaches to the use of amino acids as chiral building blocks in organic synthesis. _Angew. Chem. Int. Ed. Engl._ 30,
1531–1546 (1991). Article Google Scholar * Rezhdo, A., Islam, M., Huang, M. & Van Deventer, J. A. Future prospects for noncanonical amino acids in biological therapeutics. _Curr. Opin.
Biotechnol._ 60, 168–178 (2019). Article PubMed PubMed Central CAS Google Scholar * Saleh, A. M., Wilding, K. M., Calve, S., Bundy, B. C. & Kinzer-Ursem, T. L. Non-canonical amino
acid labeling in proteomics and biotechnology. _J. Biol. Eng._ 13, 43 (2019). Article PubMed PubMed Central Google Scholar * Lugtenburg, T., Gran-Scheuch, A. & Drienovská, I.
Non-canonical amino acids as a tool for the thermal stabilization of enzymes. _Protein Eng. Des. Sel._ 36, gzad003 (2023). Article PubMed PubMed Central Google Scholar * Aguilar Troyano,
F. J., Merkens, K., Anwar, K. & Gómez‐Suárez, A. Radical‐based synthesis and modification of amino acids. _Angew. Chem. Int. Ed. Engl._ 60, 1098–1115 (2021). Article PubMed CAS
Google Scholar * Boutureira, O. & Bernardes, G. J. L. Advances in chemical protein modification. _Chem. Rev._ 115, 2174–2195 (2015). Article PubMed CAS Google Scholar * Capecchi, A.
& Reymond, J.-L. Peptides in chemical space. _Med. Drug Discov._ 9, 100081 (2021). Article CAS Google Scholar * Voica, A.-F., Mendoza, A., Gutekunst, W. R., Fraga, J. O. & Baran,
P. S. Guided desaturation of unactivated aliphatics. _Nat. Chem._ 4, 629–635 (2012). Article PubMed PubMed Central CAS Google Scholar * Herbort, J. H., Bednar, T. N., Chen, A. D.,
RajanBabu, T. V. & Nagib, D. A. γ C–H functionalization of amines via triple H-atom transfer of a vinyl sulfonyl radical chaperone. _J. Am. Chem. Soc._ 144, 13366–13373 (2022). Article
PubMed PubMed Central CAS Google Scholar * Chuentragool, P., Parasram, M., Shi, Y. & Gevorgyan, V. General, mild, and selective method for desaturation of aliphatic amines. _J. Am.
Chem. Soc._ 140, 2465–2468 (2018). Article PubMed PubMed Central CAS Google Scholar * Wang, K. et al. Selective dehydrogenation of small and large molecules by a chloroiridium catalyst.
_Sci. Adv._ 8, eabo6586 (2022). Article PubMed PubMed Central CAS Google Scholar * Dobereiner, G. E. & Crabtree, R. H. Dehydrogenation as a substrate-activating strategy in
homogeneous transition-metal catalysis. _Chem. Rev._ 110, 681–703 (2010). Article PubMed CAS Google Scholar * Choi, J., MacArthur, A. H. R., Brookhart, M. & Goldman, A. S.
Dehydrogenation and related reactions catalyzed by iridium pincer complexes. _Chem. Rev._ 111, 1761–1779 (2011). Article PubMed CAS Google Scholar * Parasram, M., Chuentragool, P., Wang,
Y., Shi, Y. & Gevorgyan, V. General, auxiliary-enabled photoinduced Pd-catalyzed remote desaturation of aliphatic alcohols. _J. Am. Chem. Soc._ 139, 14857–14860 (2017). Article PubMed
PubMed Central CAS Google Scholar * Stateman, L. M., Dare, R. M., Paneque, A. N. & Nagib, D. A. Aza-heterocycles via copper-catalyzed, remote C–H desaturation of amines. _Chem_ 8,
210–224 (2022). Article PubMed CAS Google Scholar * Zhou, S., Zhang, Z.-J. & Yu, J.-Q. Copper-catalysed dehydrogenation or lactonization of C(_sp_3)–H bonds. _Nature_ 629, 363–369
(2024). Article ADS PubMed CAS Google Scholar * West, J. G., Huang, D. & Sorensen, E. J. Acceptorless dehydrogenation of small molecules through cooperative base metal catalysis.
_Nat. Commun._ 6, 10093 (2015). Article ADS PubMed Google Scholar * Ritu, Kolb, D., Jain, N. & König, B. Synthesis of linear enamides and enecarbamates via photoredox acceptorless
dehydrogenation. _Adv. Synth. Catal._ 365, 605–611 (2023). * Ravelli, D., Fagnoni, M., Fukuyama, T., Nishikawa, T. & Ryu, I. Site-selective C–H functionalization by decatungstate anion
photocatalysis: synergistic control by polar and steric effects expands the reaction scope. _ACS Catal._ 8, 701–713 (2018). Article CAS Google Scholar * Zhao, H. et al. Merging
halogen-atom transfer (XAT) and cobalt catalysis to override E2-selectivity in the elimination of alkyl halides: a mild route toward _contra_-thermodynamic olefins. _J. Am. Chem. Soc._ 143,
14806–14813 (2021). Article PubMed CAS Google Scholar * Occhialini, G., Palani, V. & Wendlandt, A. E. Catalytic, _contra_-thermodynamic positional alkene isomerization. _J. Am. Chem.
Soc._ 144, 145–152 (2022). Article PubMed CAS Google Scholar * Yamase, T., Takabayashi, N. & Kaji, M. Solution photochemistry of tetrakis(tetrabutylammonium) decatungstate(VI) and
catalytic hydrogen evolution from alcohols. _J. Chem. Soc. Dalton Trans._ https://doi.org/10.1039/DT9840000793 (1984). * Wrzyszczyński, A. et al. Unexpected Hofmann elimination in the
benzophenone−(phenylthio)acetic tetrabutylammonium salt photoredox system. _J. Am. Chem. Soc._ 125, 11182–11183 (2003). Article PubMed Google Scholar * Fuse, H., Kojima, M., Mitsunuma, H.
& Kanai, M. Acceptorless dehydrogenation of hydrocarbons by noble-metal-free hybrid catalyst system. _Org. Lett._ 20, 2042–2045 (2018). Article PubMed CAS Google Scholar * Zhou,
M.-J., Zhang, L., Liu, G., Xu, C. & Huang, Z. Site-selective acceptorless dehydrogenation of aliphatics enabled by organophotoredox/cobalt dual catalysis. _J. Am. Chem. Soc._ 143,
16470–16485 (2021). Article PubMed CAS Google Scholar * Zhang, Y.-A. et al. Stereochemical editing logic powered by the epimerization of unactivated tertiary stereocenters. _Science_
378, 383–390 (2022). Article ADS PubMed PubMed Central CAS Google Scholar * Halperin, S. D., Fan, H., Chang, S., Martin, R. E. & Britton, R. A convenient photocatalytic
fluorination of unactivated C–H bonds. _Angew. Chem. Int. Ed. Engl._ 53, 4690–4693 (2014). Article PubMed CAS Google Scholar * Yuan, Z. et al. Site‐selective, late‐stage C–H
18F‐fluorination on unprotected peptides for positron emission tomography imaging. _Angew. Chem. Int. Ed. Engl._ 57, 12733–12736 (2018). Article PubMed CAS Google Scholar * Bogart, J. W.
& Bowers, A. A. Dehydroamino acids: chemical multi-tools for late-stage diversification. _Org. Biomol. Chem._ 17, 3653–3669 (2019). Article PubMed PubMed Central CAS Google Scholar
* Edagwa, B. J. & Taylor, C. M. Peptides containing γ,δ-dihydroxy-l-leucine. _J. Org. Chem._ 74, 4132–4136 (2009). Article PubMed CAS Google Scholar * McLean, J. T., Milbeo, P.,
Lynch, D. M., McSweeney, L. & Scanlan, E. M. Radical‐mediated acyl thiol‐ene reaction for rapid synthesis of biomolecular thioester derivatives. _Eur. J. Org. Chem._ 2021, 4148–4160
(2021). Article CAS Google Scholar * Wakimoto, T. et al. Proof of the existence of an unstable amino acid: pleurocybellaziridine in _Pleurocybella porrigens_. _Angew. Chem. Int. Ed.
Engl._ 50, 1168–1170 (2011). Article PubMed CAS Google Scholar * Zwick, C. R. & Renata, H. Remote C–H hydroxylation by an α-ketoglutarate-dependent dioxygenase enables efficient
chemoenzymatic synthesis of manzacidin C and proline analogs. _J. Am. Chem. Soc._ 140, 1165–1169 (2018). Article PubMed CAS Google Scholar * Tao, H. et al. Stereoselectivity and
substrate specificity of the FeII/α-ketoglutarate-dependent oxygenase TqaL. _J. Am. Chem. Soc._ 144, 21512–21520 (2022). Article PubMed CAS Google Scholar * Gomez, C. A., Mondal, D., Du,
Q., Chan, N. & Lewis, J. C. Directed evolution of an iron(II)‐ and α‐ketoglutarate-dependent dioxygenase for site-selective azidation of unactivated aliphatic C–H bonds. _Angew. Chem.
Int. Ed. Engl._ 62, e202301370 (2023). Article PubMed PubMed Central CAS Google Scholar * Nanjo, T., De Lucca, E. C. & White, M. C. Remote, late-stage oxidation of aliphatic C–H
bonds in amide-containing molecules. _J. Am. Chem. Soc._ 139, 14586–14591 (2017). Article PubMed PubMed Central CAS Google Scholar * Sarver, P. J., Bissonnette, N. B. & MacMillan,
D. W. C. Decatungstate-catalyzed C(_sp_3)–H sulfinylation: rapid access to diverse organosulfur functionality. _J. Am. Chem. Soc._ 143, 9737–9743 (2021). Article PubMed PubMed Central CAS
Google Scholar * Galonić, D. P., Vaillancourt, F. H. & Walsh, C. T. Halogenation of unactivated carbon centers in natural product biosynthesis: trichlorination of leucine during
barbamide biosynthesis. _J. Am. Chem. Soc._ 128, 3900–3901 (2006). Article PubMed Google Scholar * Cudic, M., Marí, F. & Fields, G. B. Synthesis and solid-phase application of
suitably protected γ-hydroxyvaline building blocks. _J. Org. Chem._ 72, 5581–5586 (2007). Article PubMed PubMed Central CAS Google Scholar * Shu, C., Noble, A. & Aggarwal, V. K.
Metal-free photoinduced C(_sp_3)–H borylation of alkanes. _Nature_ 586, 714–719 (2020). Article ADS PubMed CAS Google Scholar * Barbie, P. & Kazmaier, U. Total synthesis of
cyclomarins A, C and D, marine cyclic peptides with interesting anti-tuberculosis and anti-malaria activities. _Org. Biomol. Chem._ 14, 6036–6054 (2016). Article PubMed CAS Google Scholar
* Agami, C. et al. Asymmetric syntheses of enantiopure 4-substituted pipecolic acid derivatives. _Eur. J. Org. Chem._ 2001, 2385–2389 (2001). Article Google Scholar * Ferraboschi, P.,
Mieri, M. D., Grisenti, P., Lotz, M. & Nettekoven, U. Diastereoselective synthesis of an argatroban intermediate, ethyl (2_R_,4_R_)-4-methylpipecolate, by means of a mandyphos/rhodium
complex-catalyzed hydrogenation. _Tetrahedron Asymmetry_ 22, 1626–1631 (2011). Article CAS Google Scholar * Smaligo, A. J. et al. Hydrodealkenylative C(_sp_3)–C(_sp_2) bond fragmentation.
_Science_ 364, 681–685 (2019). Article ADS PubMed PubMed Central CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank J. Yang (MIT) for HPLC separation of product 2P and
S. Garhwal (MIT) for supercritical fluid chromatography data collection. Financial support for this work was provided by the National Institutes of Health (GM146248) and the National Science
Foundation (NSF) through a Graduate Research Fellowship to G.O. (DGE1745303). AUTHOR INFORMATION Author notes * These authors contributed equally: Xin Gu, Yu-An Zhang AUTHORS AND
AFFILIATIONS * Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA, USA Xin Gu, Yu-An Zhang, Shuo Zhang, Leon Wang, Xiyun Ye, Gino Occhialini, Jonah Barbour,
Bradley L. Pentelute & Alison E. Wendlandt Authors * Xin Gu View author publications You can also search for this author inPubMed Google Scholar * Yu-An Zhang View author publications
You can also search for this author inPubMed Google Scholar * Shuo Zhang View author publications You can also search for this author inPubMed Google Scholar * Leon Wang View author
publications You can also search for this author inPubMed Google Scholar * Xiyun Ye View author publications You can also search for this author inPubMed Google Scholar * Gino Occhialini
View author publications You can also search for this author inPubMed Google Scholar * Jonah Barbour View author publications You can also search for this author inPubMed Google Scholar *
Bradley L. Pentelute View author publications You can also search for this author inPubMed Google Scholar * Alison E. Wendlandt View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS A.E.W., X.G. and Y.-A.Z. conceived the work, designed experiments and analysed the data. L.W. and S.Z. contributed equally to substrate synthesis and
characterization. X.Y. and B.L.P. provided expertise on the synthesis and analysis of oligopeptides. G.O. and J.B. performed exploratory experiments establishing the feasibility of
terminal-selective aliphatic dehydrogenation. A.E.W., X.G. and Y.-A.Z. drafted the manuscript with input from all authors. A.E.W. directed the research. All authors have given approval to
the final version of the manuscript. CORRESPONDING AUTHOR Correspondence to Alison E. Wendlandt. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER
REVIEW PEER REVIEW INFORMATION _Nature_ thanks Jonathan Clayden, Christopher Teskey and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. ADDITIONAL
INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY
INFORMATION This file contains Supplementary Information; for details, see Table of Contents. RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds
exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely
governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Gu, X., Zhang, YA., Zhang, S. _et al._ Synthesis of
non-canonical amino acids through dehydrogenative tailoring. _Nature_ 634, 352–358 (2024). https://doi.org/10.1038/s41586-024-07988-8 Download citation * Received: 22 November 2023 *
Accepted: 22 August 2024 * Published: 29 August 2024 * Issue Date: 10 October 2024 * DOI: https://doi.org/10.1038/s41586-024-07988-8 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative