Play all audios:
ABSTRACT Fewer than half of all patients with advanced-stage high-grade serous ovarian cancers (HGSCs) survive more than five years after diagnosis, but those who have an exceptionally long
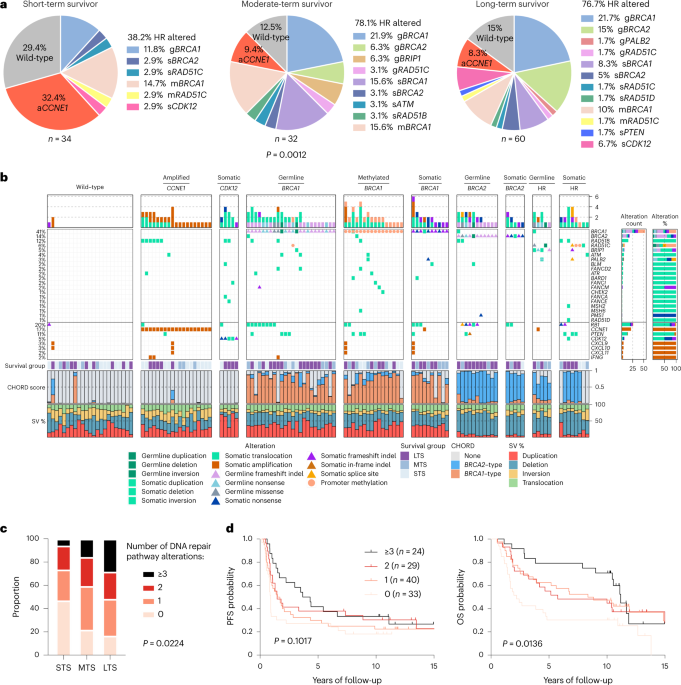
survival could provide insights into tumor biology and therapeutic approaches. We analyzed 60 patients with advanced-stage HGSC who survived more than 10 years after diagnosis using
whole-genome sequencing, transcriptome and methylome profiling of their primary tumor samples, comparing this data to 66 short- or moderate-term survivors. Tumors of long-term survivors were
more likely to have multiple alterations in genes associated with DNA repair and more frequent somatic variants resulting in an increased predicted neoantigen load. Patients clustered into
survival groups based on genomic and immune cell signatures, including three subsets of patients with _BRCA1_ alterations with distinctly different outcomes. Specific combinations of
germline and somatic gene alterations, tumor cell phenotypes and differential immune responses appear to contribute to long-term survival in HGSC. Access through your institution Buy or
subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get
Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and online access $209.00 per year only
$17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout
ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS GENETIC CHARACTERIZATION OF
PRIMARY AND METASTATIC HIGH-GRADE SEROUS OVARIAN CANCER TUMORS REVEALS DISTINCT FEATURES ASSOCIATED WITH SURVIVAL Article Open access 03 July 2023 GENOMIC LANDSCAPE AND IMMUNE-RELATED GENE
EXPRESSION PROFILING OF EPITHELIAL OVARIAN CANCER AFTER NEOADJUVANT CHEMOTHERAPY Article Open access 27 January 2022 MULTIOMIC ANALYSIS OF HOMOLOGOUS RECOMBINATION-DEFICIENT END-STAGE
HIGH-GRADE SEROUS OVARIAN CANCER Article 27 February 2023 DATA AVAILABILITY ICGC datasets: Previously published WGS and RNA-seq data generated as part of the ICGC Ovarian Cancer project14
are available from the European Genome-phenome Archive (EGA) repository (https://ega-archive.org) as a single BAM file for each sample type (tumor/normal) under the accession code
EGAD00001000877. Due to the sensitive nature of these patient data sets, access is subject to approval from the ICGC Data Access Compliance Office
(https://docs.icgc.org/download/data-access/), an independent body who authorizes controlled access to ICGC sequencing data. ICGC SNP array and methylation data sets have been deposited into
the Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/) under accession code GSE65821, without access restrictions. ICGC gene count level transcriptomic data have been
deposited into the GEO under accession code GSE209964. MOCOG datasets: WGS, RNA-seq and SNP array data from long-term survivors generated as part of the MOCOG study have been deposited in
the EGA repository under accession code EGAS00001005984. WGS and RNA-seq data are available as raw FASTQ files for each sample type (tumor/normal) and SNP array data are available as raw
signal intensity files in text format for each sample type (tumor/normal). Access to patient sequence data can be gained for academic use through application to the independent Data Access
Committee ([email protected]). Responses to data requests will be provided within two weeks. Information on how to apply for access is available at the EGA under accession code
EGAS00001005984. The MOCOG cohort raw methylation data sets have been submitted to the GEO under accession code GSE211687, with no access restrictions. Uniformly processed somatic variant
data from the ICGC and MOCOG cohorts have been deposited in Synapse under accession code syn34616347, and processed expression and methylation data from both cohorts have been submitted into
the GEO under accession code GSE211687, without access restrictions. Population frequencies of genetic variants can be accessed via the Genome Aggregation Database (gnomAD) at
https://gnomad.broadinstitute.org/. Supporting evidence for pathogenicity of genomic alterations can be accessed via ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/), BRCA Exchange
(https://brcaexchange.org/) and the _TP53_ Database (https://tp53.isb-cgc.org/). The Ensembl ranked order of severity of variant consequences is available at:
https://m.ensembl.org/info/genome/variation/prediction/predicted_data.html. Precomputed TCGA ovarian serous cystadenocarcinoma survival analysis data can be downloaded from OncoLnc
(http://www.oncolnc.org/). Mutational signature reference databases can be accessed via COSMIC (https://cancer.sanger.ac.uk/signatures/) and Signal
(https://signal.mutationalsignatures.com/). The LM22 signature matrix used for immune cell deconvolution can be downloaded at https://cibersortx.stanford.edu/. The COSMIC Cancer Gene Census
can be accessed at https://cancer.sanger.ac.uk/census. MSigDB hallmark gene sets can be accessed at https://www.gsea-msigdb.org/gsea/msigdb/. Illumina methylation probes that were filtered
out due to poor performance (for example, cross-reactive or nonspecific probes) can be found at https://github.com/sirselim/illumina450k_filtering. Germline polymorphic sites for reference
and variant allele read counts used in FACETS analysis can be found at ftp://ftp.ncbi.nih.gov/snp/organisms/human_9606_b151_GRCh37p13/VCF/common_all_20180423.vcf.gz. The gene transfer format
used for annotation and RNA-seq counts is available at ftp://ftp.ensembl.org/pub/grch37/release-92/. All other data are available within the article and its supplementary information files.
CODE AVAILABILITY No custom code or software was used in the data analyses. All results can be replicated using publicly available tools and software. The tools and versions used are fully
described in the Methods and Supplementary Information. REFERENCES * Millstein, J. et al. Prognostic gene expression signature for high-grade serous ovarian cancer. _Ann. Oncol._ 31,
1240–1250 (2020). Article CAS Google Scholar * Hoppenot, C., Eckert, M. A., Tienda, S. M. & Lengyel, E. Who are the long-term survivors of high grade serous ovarian cancer? _Gynecol.
Oncol._ 148, 204–212 (2018). Article Google Scholar * Fagö-Olsen, C. L. et al. Does neoadjuvant chemotherapy impair long-term survival for ovarian cancer patients? A nationwide Danish
study. _Gynecol. Oncol._ 132, 292–298 (2014). Article Google Scholar * Chi, D. S. et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian
carcinoma (EOC)? _Gynecol. Oncol._ 103, 559–564 (2006). Article CAS Google Scholar * Horowitz, N. S. et al. Does aggressive surgery improve outcomes? Interaction between preoperative
disease burden and complex surgery in patients with advanced-stage ovarian cancer: an analysis of GOG 182. _J. Clin. Oncol._ 33, 937–943 (2015). Article Google Scholar * Alsop, K. et al.
BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: A report from the Australian ovarian cancer study group. _J. Clin. Oncol._ 30,
2654–2663 (2012). Article CAS Google Scholar * The Cancer Genome Atlas Research Network. Integrated genomic analysis of ovarian cancer. _Nature_ 474, 609–615 (2011). Article Google
Scholar * Walsh, T. et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. _Proc. Natl Acad. Sci. USA_
108, 18032–18037 (2011). Article CAS Google Scholar * Ciriello, G. et al. Emerging landscape of oncogenic signatures across human cancers. _Nat. Genet._ 45, 1127–1133 (2013). Article CAS
Google Scholar * Ahmed, A. A. et al. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. _J. Pathol._ 221, 49–56 (2010). Article CAS Google Scholar *
Tothill, R. W. et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. _Clin. Cancer Res._ 14, 5198–5208 (2008). Article CAS Google Scholar *
Etemadmoghadam, D. et al. Integrated genome-wide DNA copy number and expression analysis identifies distinct mechanisms of primary chemoresistance in ovarian carcinomas. _Clin. Cancer Res._
15, 1417–1427 (2009). Article CAS Google Scholar * Hwang, W. T., Adams, S. F., Tahirovic, E., Hagemann, I. S. & Coukos, G. Prognostic significance of tumor-infiltrating T cells in
ovarian cancer: A meta-analysis. _Gynecol. Oncol._ 124, 192–198 (2012). Article Google Scholar * Patch, A. M. et al. Whole-genome characterization of chemoresistant ovarian cancer.
_Nature_ 521, 489–494 (2015). Article CAS Google Scholar * Wang, Y. K. et al. Genomic consequences of aberrant DNA repair mechanisms stratify ovarian cancer histotypes. _Nat. Genet._ 49,
856–864 (2017). Article CAS Google Scholar * Macintyre, G. et al. Copy number signatures and mutational processes in ovarian carcinoma. _Nat. Genet._ 50, 1262–1270 (2018). Article CAS
Google Scholar * Pennington, K. P. et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal
carcinomas. _Clin. Cancer Res._ 20, 764–775 (2014). Article CAS Google Scholar * Farmer, H. et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. _Nature_
434, 917–921 (2005). Article CAS Google Scholar * Fong, P. C. et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with
platinum-free interval. _J. Clin. Oncol._ 28, 2512–2519 (2010). Article CAS Google Scholar * Swisher, E. M. et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma
(ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. _Lancet Oncol._ 18, 75–87 (2017). Article CAS Google Scholar * Bolton, K. L. et al. Association between BRCA1
and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. _JAMA_ 307, 382–390 (2012). Article CAS Google Scholar * Candido-dos-Reis, F. J. et al. Germline
mutation in BRCA1 or BRCA2 and ten-year survival for women diagnosed with epithelial ovarian cancer. _Clin. Cancer Res._ 21, 652–657 (2015). Article CAS Google Scholar * Garsed, D. W. et
al. Homologous recombination DNA repair pathway disruption and retinoblastoma protein loss are associated with exceptional survival in high-grade serous ovarian cancer. _Clin. Cancer Res._
24, 569–580 (2018). Article CAS Google Scholar * Ciriello, G., Cerami, E., Sander, C. & Schultz, N. Mutual exclusivity analysis identifies oncogenic network modules. _Genome Res._ 22,
398–406 (2012). Article CAS Google Scholar * Etemadmoghadam, D. et al. Synthetic lethality between CCNE1 amplification and loss of BRCA1. _Proc. Natl Acad. Sci. USA_ 110, 19489–19494
(2013). Article CAS Google Scholar * Newman, A. M. et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. _Nat. Biotechnol._ 37, 773–782 (2019).
Article CAS Google Scholar * Miller, R. E. et al. ESMO recommendations on predictive biomarker testing for homologous recombination deficiency and PARP inhibitor benefit in ovarian
cancer. _Ann. Oncol._ 31, 1606–1622 (2020). Article CAS Google Scholar * Nguyen, L., W. M. Martens, J., Van Hoeck, A. & Cuppen, E. Pan-cancer landscape of homologous recombination
deficiency. _Nat. Commun._ 11, 1–12 (2020). Article Google Scholar * Joshi, P. M., Sutor, S. L., Huntoon, C. J. & Karnitz, L. M. Ovarian cancer-associated mutations disable catalytic
activity of CDK12, a kinase that promotes homologous recombination repair and resistance to cisplatin and poly(ADP-ribose) polymerase inhibitors. _J. Biol. Chem._ 289, 9247–9253 (2014).
Article CAS Google Scholar * Anaya, J. OncoLnc: Linking TCGA survival data to mRNAs, miRNAs, and lncRNAs. _Peer J. Comp. Sci._ 2, e67 (2016). Article Google Scholar * Norquist, B. et
al. Secondary somatic mutations restoring BRCA1/2 predict chemotherapy resistance in hereditary ovarian carcinomas. _J. Clin. Oncol._ 29, 3008–3015 (2011). Article CAS Google Scholar *
Alexandrov, L. B. et al. The repertoire of mutational signatures in human cancer. _Nature_ 578, 94–101 (2020). Article CAS Google Scholar * Degasperi, A. et al. A practical framework and
online tool for mutational signature analyses show inter-tissue variation and driver dependencies. _Nat. Cancer_ 1, 249–263 (2020). Article CAS Google Scholar * Popova, T. et al. Ovarian
cancers harboring inactivating mutations in CDK12 display a distinct genomic instability pattern characterized by large tandem duplications. _Cancer Res._ 76, 1882–1891 (2016). Article CAS
Google Scholar * Wu, Y. M. et al. Inactivation of CDK12 delineates a distinct immunogenic class of advanced prostate cancer. _Cell_ 173, 1770–1782.e1714 (2018). Article CAS Google
Scholar * Funnell, T. et al. Integrated structural variation and point mutation signatures in cancer genomes using correlated topic models. _PLoS Comput. Biol._ 15, 1–24 (2019). Article
Google Scholar * Zhang, L. et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. _N. Engl. J. Med._ 348, 203–213 (2003). Article CAS Google Scholar *
Ovarian Tumor Tissue Analysis (OTTA) Consortium. Dose-response association of CD8+ tumor-infiltrating lymphocytes and survival time in high-grade serous ovarian cancer. _JAMA Oncol._ 3,
e173290 (2017). * Jiménez-Sánchez, A. et al. Heterogeneous tumor-immune microenvironments among differentially growing metastases in an ovarian cancer patient. _Cell_ 170, 927–938.e920
(2017). Article Google Scholar * Yang, S. Y. C. et al. Landscape of genomic alterations in high-grade serous ovarian cancer from exceptional long- and short-term survivors. _Genome Med_
10, 81 (2018). Article CAS Google Scholar * Korotkevich, G. et al. Fast gene set enrichment analysis. Preprint at _bioRxiv_ https://doi.org/10.1101/060012 (2021). * Liberzon, A. et al.
The molecular signatures database hallmark gene set collection. _Cell Syst._ 1, 417–425 (2015). Article CAS Google Scholar * Saner, F. A. M. et al. Going to extremes: determinants of
extraordinary response and survival in patients with cancer. _Nat. Rev. Cancer_ 19, 339–348 (2019). Article CAS Google Scholar * Wheeler, D. A. et al. Molecular features of cancers
exhibiting exceptional responses to treatment. _Cancer Cell_ 39, 38–53.e37 (2021). Article CAS Google Scholar * Moore, K. et al. Maintenance olaparib in patients with newly diagnosed
advanced ovarian cancer. _N. Engl. J. Med._ 379, 2495–2505 (2018). Article CAS Google Scholar * Ewing, A. et al. Structural Variants at the BRCA1/2 loci are a common source of homologous
repair deficiency in high-grade serous ovarian carcinoma. _Clin. Cancer Res._ 27, 3201–3214 (2021). Article CAS Google Scholar * Swisher, E. M. et al. Characterization of patients with
long-term responses to rucaparib treatment in recurrent ovarian cancer. _Gynecol. Oncol._ 163, 490–497 (2021). Article CAS Google Scholar * Velez-Cruz, R. et al. RB localizes to DNA
double-strand breaks and promotes DNA end resection and homologous recombination through the recruitment of BRG1. _Genes Dev._ 30, 2500–2512 (2016). Article CAS Google Scholar * Fan, W.
et al. MET-independent lung cancer cells evading EGFR kinase inhibitors are therapeutically susceptible to BH3 mimetic agents. _Cancer Res._ 71, 4494–4505 (2011). Article CAS Google
Scholar * Cole, A. NFATC4 promotes quiescence and chemotherapy resistance in ovarian cancer. _JCI Insight_ 5, e131486 (2020). * Sieh, W. et al. Hormone-receptor expression and ovarian
cancer survival: an Ovarian Tumor Tissue Analysis consortium study. _Lancet Oncol._ 14, 853–862 (2013). Article CAS Google Scholar * Gersekowski, K. et al. Germline BRCA variants,
lifestyle and ovarian cancer survival. _Gynecol. Oncol._ 165, 437–445 (2022). Article CAS Google Scholar * Jung, Y. S. et al. Impact of smoking on human natural killer cell activity: A
large cohort study. _J. Cancer Prev._ 25, 13–20 (2020). Article Google Scholar * Cress, R. D., Chen, Y. S., Morris, C. R., Petersen, M. & Leiserowitz, G. S. Characteristics of
long-term survivors of epithelial ovarian cancer. _Obstet. Gynecol._ 126, 491–497 (2015). Article Google Scholar * Schröder, J., Corbin, V. & Papenfuss, A. T. HYSYS: Have you swapped
your samples? _Bioinformatics_ 33, 596–598 (2017). Google Scholar * Song, S. et al. qpure: A tool to estimate tumor cellularity from genome-wide single-nucleotide polymorphism profiles.
_PLoS One_ 7, 5–11 (2012). Google Scholar * Van Loo, P. et al. Allele-specific copy number analysis of tumors. _Proc. Natl Acad. Sci. USA_ 107, 16910–16915 (2010). Article CAS Google
Scholar * Wingett, S. W. & Andrews, S. FastQ Screen: A tool for multi-genome mapping and quality control. _F1000Res._ 7, 1338 (2018). Article Google Scholar * Li, H. & Durbin, R.
Fast and accurate short read alignment with Burrows-Wheeler transform. _Bioinformatics_ 25, 1754–1760 (2009). Article CAS Google Scholar * McKenna, A. et al. The Genome Analysis Toolkit:
a MapReduce framework for analyzing next-generation DNA sequencing data. _Genome Res._ 20, 1297–1303 (2010). Article CAS Google Scholar * Shen, R. & Seshan, V. E. FACETS:
Allele-specific copy number and clonal heterogeneity analysis tool for high-throughput DNA sequencing. _Nucleic Acids Res._ 44, 1–9 (2016). Article Google Scholar * Lai, Z. et al. VarDict:
a novel and versatile variant caller for next-generation sequencing in cancer research. _Nucleic Acids Res._ 44, e108 (2016). Article Google Scholar * Kim, S. et al. Strelka2: fast and
accurate calling of germline and somatic variants. _Nat. Methods_ 15, 591–594 (2018). Article CAS Google Scholar * Koboldt, D. C. et al. VarScan 2: somatic mutation and copy number
alteration discovery in cancer by exome sequencing. _Genome Res._ 22, 568–576 (2012). Article CAS Google Scholar * Danecek, P. et al. Twelve years of SAMtools and BCFtools. _Gigascience_
10, 1–4 (2021). Article CAS Google Scholar * Tan, A., Abecasis, G. R. & Kang, H. M. Unified representation of genetic variants. _Bioinformatics_ 31, 2202–2204 (2015). Article CAS
Google Scholar * Shyr, C. et al. FLAGS, frequently mutated genes in public exomes. _BMC Med. Genomics_ 7, 64 (2014). Article Google Scholar * Chen, X. et al. Manta: Rapid detection of
structural variants and indels for germline and cancer sequencing applications. _Bioinformatics_ 32, 1220–1222 (2016). Article CAS Google Scholar * Cameron, D. L. et al. GRIDSS: Sensitive
and specific genomic rearrangement detection using positional de Bruijn graph assembly. _Genome Res._ 27, 2050–2060 (2017). Article CAS Google Scholar * Wala, J. A. et al. SvABA:
Genome-wide detection of structural variants and indels by local assembly. _Genome Res._ 28, 581–591 (2018). Article CAS Google Scholar * Lawrence, M., Gentleman, R. & Carey, V.
rtracklayer: An R package for interfacing with genome browsers. _Bioinformatics_ 25, 1841–1842 (2009). Article CAS Google Scholar * Bielski, C. M. et al. Genome doubling shapes the
evolution and prognosis of advanced cancers. _Nat. Genet._ 50, 1189–1195 (2018). Article CAS Google Scholar * Robinson, J. T. et al. Integrative genomics viewer. _Nat. Biotechnol._ 29,
24–26 (2011). Article CAS Google Scholar * Sztupinszki, Z. et al. Migrating the SNP array-based homologous recombination deficiency measures to next generation sequencing data of breast
cancer. _NPJ Breast Cancer_ 4, 16 (2018). Article Google Scholar * Nariai, N. et al. HLA-VBSeq: Accurate HLA typing at full resolution from whole-genome sequencing data. _BMC Genomics_ 16,
1–6 (2015). Article Google Scholar * Robinson, J. et al. IPD-IMGT/HLA Database. _Nucleic Acids Res._ 48, D948–D955 (2020). CAS Google Scholar * Hundal, J. et al. PVACtools: A
computational toolkit to identify and visualize cancer neoantigens. _Cancer Immunol. Res._ 8, 409–420 (2020). Article CAS Google Scholar * Dobin, A. et al. STAR: Ultrafast universal
RNA-seq aligner. _Bioinformatics_ 29, 15–21 (2013). Article CAS Google Scholar * Wang, L., Wang, S. & Li, W. RSeQC: Quality control of RNA-seq experiments. _Bioinformatics_ 28,
2184–2185 (2012). Article CAS Google Scholar * Anders, S., Pyl, P. T. & Huber, W. HTSeq-A Python framework to work with high-throughput sequencing data. _Bioinformatics_ 31, 166–169
(2015). Article CAS Google Scholar * Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression
data. _Bioinformatics_ 26, 139–140 (2009). Article Google Scholar * Ritchie, M. E. et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. _Nucleic
Acids Res._ 43, e47 (2015). Article Google Scholar * Aryee, M. J. et al. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays.
_Bioinformatics_ 30, 1363–1369 (2014). Article CAS Google Scholar * Fortin, J.-P. et al. Functional normalization of 450k methylation array data improves replication in large cancer
studies. _Genome Biol._ 15, 503 (2014). Article Google Scholar * Leek, J. T., Johnson, W. E., Parker, H. S., Jaffe, A. E. & Storey, J. D. The sva package for removing batch effects and
other unwanted variation in high-throughput experiments. _Bioinformatics_ 28, 882–883 (2012). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank P. Webb, K. Byth,
R. Lupat, J. Ellul and the Peter MacCallum Cancer Centre Research Computing Facility for their contributions to the study. This work was supported by the U.S. Army Medical Research and
Materiel Command Ovarian Cancer Research Program (Award No. W81XWH-16-2-0010 and W81XWH-21-1-0401), the National Health and Medical Research Council of Australia (1092856, 1117044 and
2008781 to D.D.L.B., and 1186505 to D.W.G.), and the U.S. National Cancer Institute (P30CA046592 for C.L.P. and P30CA008748 for M.C.P.). This research was made possible by generous support
from the Border Ovarian Cancer Awareness Group, the Garvan Research Foundation, the Graf Family Foundation, Mrs Margaret Rose AM, Arthur Coombs and family, and the Piers K Fowler Fund. The
Australian Ovarian Cancer Study (AOCS) gratefully acknowledges the cooperation of participating institutions in Australia and the contribution of study nurses, research assistants and all
clinical and scientific collaborators. The complete AOCS Group can be found at www.aocstudy.org. We would like to thank all of the women who participated in the study. AOCS was supported by
the U.S. Army Medical Research and Materiel Command (DAMD17-01-1-0729), The Cancer Council Victoria, Queensland Cancer Fund, The Cancer Council New South Wales, The Cancer Council South
Australia, The Cancer Council Tasmania, The Cancer Foundation of Western Australia and the National Health and Medical Research Council of Australia (NHMRC; ID199600, ID400413, ID400281).
AOCS gratefully acknowledges additional support from Ovarian Cancer Australia and the Peter MacCallum Cancer Foundation. We thank all the women who participated in the GynBiobank and
gratefully acknowledge the Departments of Gynaecological Oncology, Medical Oncology and Anatomical Pathology at Westmead Hospital, Sydney. The Gynaecological Oncology Biobank at Westmead was
funded by the NHMRC (ID310670, ID628903), the Cancer Institute NSW (12/RIG/1-17, 15/RIG/1-16) and the Department of Gynaecological Oncology, Westmead Hospital, and acknowledges financial
support from the Sydney West Translational Cancer Research Centre, funded by the Cancer Institute NSW (15/TRC/1-01). E.L.C. was supported by NHMRC grant APP1161198. F.A.M.S. was supported by
a Swiss National Foundation EarlyPostdoc Fellowship (P2BEP3-172246), Swiss Cancer Research Foundation grant BIL KFS-3942-08-2016 and a Professor Dr Max Cloëtta and Uniscientia Foundation
grant. A.M.P. and J.D.B. were supported by Cancer Research UK (A22905). B.H.N. was supported by the BC Cancer Foundation, Canada’s Networks of Centres of Excellence (BioCanRx), Genome BC and
the Canada Foundation for Innovation. D.D.L.B. was supported by the U.S. National Cancer Institute U54 program (U54CA209978). AUTHOR INFORMATION Author notes * These authors contributed
equally: Anna DeFazio, David D. L. Bowtell. AUTHORS AND AFFILIATIONS * Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia Dale W. Garsed, Ahwan Pandey, Sian Fereday, Kazuaki
Takahashi, Kathryn Alsop, Joy Hendley, Nadia Traficante, Dinuka Ariyaratne, George Au-Yeung, Leanne Bowes, Elizabeth L. Christie, Orla McNally, Flurina A. M. Saner & David D. L. Bowtell
* Sir Peter MacCallum Department of Oncology, The University of Melbourne, Parkville, Victoria, Australia Dale W. Garsed, Sian Fereday, Kathryn Alsop, Nadia Traficante, George Au-Yeung,
Elizabeth L. Christie & David D. L. Bowtell * The Westmead Institute for Medical Research, Sydney, New South Wales, Australia Catherine J. Kennedy, Yoke-Eng Chiew, Pamela Provan, Jillian
Hung & Anna DeFazio * Department of Gynaecological Oncology, Westmead Hospital, Sydney, New South Wales, Australia Catherine J. Kennedy, Yoke-Eng Chiew, Pamela Provan, Alison Brand,
Jillian Hung & Anna DeFazio * The University of Sydney, Sydney, New South Wales, Australia Catherine J. Kennedy, Yoke-Eng Chiew, Pamela Provan, Alison Brand, Paul Harnett & Anna
DeFazio * Department of Obstetrics and Gynecology, The Jikei University School of Medicine, Tokyo, Japan Kazuaki Takahashi * The Deeley Research Centre, BC Cancer, Victoria, British
Columbia, Canada Phineas T. Hamilton & Brad H. Nelson * Women’s Health Integrated Research Center, Gynecologic Cancer Center of Excellence, Uniformed Services University and Walter Reed
National Military Medical Center, Bethesda, MD, USA Nicholas W. Bateman, Thomas P. Conrads & George L. Maxwell * Henry M. Jackson Foundation for the Advancement of Military Medicine,
Inc., Bethesda, MD, USA Nicholas W. Bateman * The Royal Women’s Hospital, Parkville, Victoria, Australia Leanne Bowes & Orla McNally * Department of Laboratory Medicine and Pathology,
Mayo Clinic, Rochester, MN, USA Julie M. Cunningham * Prince of Wales Clinical School, University of New South Wales, Sydney, New South Wales, Australia Michael Friedlander * Sydney, New
South Wales, Australia Bronwyn Grout * Crown Princess Mary Cancer Centre, Westmead Hospital, Sydney, New South Wales, Australia Paul Harnett * Division of Clinical Trials and Biostatistics,
Department of Quantitative Health Sciences, Mayo Clinic, Rochester, MN, USA Bryan McCauley & Robert A. Vierkant * Department of Obstetrics and Gynaecology, The University of Melbourne,
Parkville, Victoria, Australia Orla McNally * Cancer Research UK Cambridge Institute, University of Cambridge, Cambridge, UK Anna M. Piskorz & James D. Brenton * Department of Obstetrics
and Gynecology, Bern University Hospital and University of Bern, Bern, Switzerland Flurina A. M. Saner * Division of Computational Biology, Department of Quantitative Health Sciences, Mayo
Clinic, Rochester, MN, USA Chen Wang & Stacey J. Winham * Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK Paul D. P. Pharoah * Department of
Oncology, University of Cambridge, Cambridge, UK Paul D. P. Pharoah & James D. Brenton * Women’s Health Integrated Research Center, Women’s Service Line, Inova Health System, Falls
Church, VA, USA Thomas P. Conrads & George L. Maxwell * School of Clinical Medicine, Faculty of Medicine and Health, University of NSW, Sydney, New South Wales, Australia Susan J. Ramus
* Adult Cancer Program, Lowy Cancer Research Centre, University of NSW, Sydney, New South Wales, Australia Susan J. Ramus * Department of Epidemiology and Rogel Cancer Center, University of
Michigan, Ann Arbor, MI, USA Celeste Leigh Pearce * Department of Epidemiology and Biostatistics, Memorial Sloan Kettering Cancer Center, New York, NY, USA Malcolm C. Pike * Department of
Medical Genetics, The University of British Columbia, Vancouver, British Columbia, Canada Brad H. Nelson * Department of Biochemistry and Microbiology, University of Victoria, Victoria,
British Columbia, Canada Brad H. Nelson * Division of Epidemology, Department of Quantitative Health Sciences, Mayo Clinic, Rochester, MN, USA Ellen L. Goode * The Daffodil Centre, The
University of Sydney, a joint venture with Cancer Council NSW, Sydney, New South Wales, Australia Anna DeFazio Authors * Dale W. Garsed View author publications You can also search for this
author inPubMed Google Scholar * Ahwan Pandey View author publications You can also search for this author inPubMed Google Scholar * Sian Fereday View author publications You can also search
for this author inPubMed Google Scholar * Catherine J. Kennedy View author publications You can also search for this author inPubMed Google Scholar * Kazuaki Takahashi View author
publications You can also search for this author inPubMed Google Scholar * Kathryn Alsop View author publications You can also search for this author inPubMed Google Scholar * Phineas T.
Hamilton View author publications You can also search for this author inPubMed Google Scholar * Joy Hendley View author publications You can also search for this author inPubMed Google
Scholar * Yoke-Eng Chiew View author publications You can also search for this author inPubMed Google Scholar * Nadia Traficante View author publications You can also search for this author
inPubMed Google Scholar * Pamela Provan View author publications You can also search for this author inPubMed Google Scholar * Dinuka Ariyaratne View author publications You can also search
for this author inPubMed Google Scholar * George Au-Yeung View author publications You can also search for this author inPubMed Google Scholar * Nicholas W. Bateman View author publications
You can also search for this author inPubMed Google Scholar * Leanne Bowes View author publications You can also search for this author inPubMed Google Scholar * Alison Brand View author
publications You can also search for this author inPubMed Google Scholar * Elizabeth L. Christie View author publications You can also search for this author inPubMed Google Scholar * Julie
M. Cunningham View author publications You can also search for this author inPubMed Google Scholar * Michael Friedlander View author publications You can also search for this author inPubMed
Google Scholar * Bronwyn Grout View author publications You can also search for this author inPubMed Google Scholar * Paul Harnett View author publications You can also search for this
author inPubMed Google Scholar * Jillian Hung View author publications You can also search for this author inPubMed Google Scholar * Bryan McCauley View author publications You can also
search for this author inPubMed Google Scholar * Orla McNally View author publications You can also search for this author inPubMed Google Scholar * Anna M. Piskorz View author publications
You can also search for this author inPubMed Google Scholar * Flurina A. M. Saner View author publications You can also search for this author inPubMed Google Scholar * Robert A. Vierkant
View author publications You can also search for this author inPubMed Google Scholar * Chen Wang View author publications You can also search for this author inPubMed Google Scholar * Stacey
J. Winham View author publications You can also search for this author inPubMed Google Scholar * Paul D. P. Pharoah View author publications You can also search for this author inPubMed
Google Scholar * James D. Brenton View author publications You can also search for this author inPubMed Google Scholar * Thomas P. Conrads View author publications You can also search for
this author inPubMed Google Scholar * George L. Maxwell View author publications You can also search for this author inPubMed Google Scholar * Susan J. Ramus View author publications You can
also search for this author inPubMed Google Scholar * Celeste Leigh Pearce View author publications You can also search for this author inPubMed Google Scholar * Malcolm C. Pike View author
publications You can also search for this author inPubMed Google Scholar * Brad H. Nelson View author publications You can also search for this author inPubMed Google Scholar * Ellen L.
Goode View author publications You can also search for this author inPubMed Google Scholar * Anna DeFazio View author publications You can also search for this author inPubMed Google Scholar
* David D. L. Bowtell View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS D.W.G., S.F., A.D. and D.D.L.B. conceived the project. C.J.K., K.A.,
N.T., G.A.-Y., A.B., M.F., P.R.H., O.M., E.L.G., A.D. and D.D.L.B. provided patient samples and clinical information, and D.W.G., J. Hendley, Y.C. and E.L.C. prepared patient samples.
D.W.G., A.P., S.F., C.J.K., K.A., D.A., L.B., J.M.C., J. Hung, B.M., R.A.V., C.W., S.J.W. and E.L.G. acquired data. A.P. managed and processed genomic data and performed data analysis
together with D.W.G., S.F., K.T., P.P., D.A., E.L.C., B.M., F.A.M.S., R.A.V., C.W. and S.J.W. D.W.G., S.F., K.A., C.L.P., M.C.P. and D.D.L.B. coordinated the study and interpreted the
results along with A.P., K.T., P.T.H., N.W.B., E.L.C., B.G., A.M.P., F.A.M.S., P.D.P.P., J.D.B., T.P.C., G.L.M., S.J.R., B.H.N. and A.D. D.W.G., A.D. and D.D.L.B. supervised the work and
wrote the manuscript together with A.P. and K.T. All authors discussed the results and read the manuscript. CORRESPONDING AUTHORS Correspondence to Dale W. Garsed or David D. L. Bowtell.
ETHICS DECLARATIONS COMPETING INTERESTS S.F., K.A., N.T. and A.D. received grant funding from AstraZeneca for unrelated work. G.A.-Y. received grant funding from AstraZeneca and
Roche-Genentech for unrelated work. M.F. declares honoraria for advisory boards AstraZeneca, GSK, Incyclix, Lilly, MSD, Novartis and Takeda; consultancy for AstraZeneca, Eisai and Novartis;
speaker’s fee and travel from AstraZeneca; speaker’s fee from ACT Genomics; and institutional research funding from AstraZeneca, BeiGene, Novartis; all for unrelated work. J.D.B. received
funding from Aprea and Clovis Oncology for unrelated work. D.D.L.B. received funding from AstraZeneca, Genentech-Roche and BeiGene for unrelated work. The remaining authors declare no
competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Genetics_ thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are
available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. EXTENDED DATA
EXTENDED DATA FIG. 1 PATIENT COHORT. A, Overview of patients (_n_ = 126) and tumor samples analyzed in this study. In addition to paired germline and primary tumor samples in all patients, 5
relapse tumor samples were also analyzed from 4 long-term survivor patients. OS, overall survival. B, Clinical characteristics of patients by survival group. All patients received primary
platinum therapy. aKruskal–Wallis, bChi-square, or clog-rank Mantel–Cox test _P_ values comparing survival groups reported. EXTENDED DATA FIG. 2 FREQUENTLY ALTERED CANCER GENES ACROSS
SURVIVAL GROUPS. A, Overview of somatic alterations in driver genes detected by GRIN, dNdScv, GISTIC, and/or in cancer-associated genes (COSMIC Cancer Gene Census) that are enriched in a
survival group relative to another survival group. From left: two-sided Fisher’s test of the difference in proportions of altered samples between survival groups, triangles and color
indicate direction of the log odds ratio (LOR; blue = down, pink = up), asterisks indicate _P_ value < 0.05 (see Supplementary Table 6 for _P_ values), _P_ values were not adjusted for
multiple comparisons; role of gene in COSMIC Cancer Gene Census (TSG, tumor suppressor gene); genomic alterations split by survival groups, bars at the top indicate the number of alterations
in each listed gene per patient; bar plot of the number of samples with an alteration (alteration type indicated by color); bar plots showing the proportion of alteration types per gene;
_P_ values were calculated using the genomic random interval (GRIN) statistical model (one-sided) for recurrent structural variants (SV) (see Supplementary Data 2 for GRIN _P_ values), the
dNdScv likelihood-ratio test (two-sided) for recurrent base substitutions and small-scale deletions and insertions (see Supplementary Data 1 for dNdScv _P_ values), and GISTIC2
permutation-of-markers test (one-sided) for recurrent copy-number variants (CNV) with red indicating amplification and blue indicating deletion (see Supplementary Data 3 for GISTIC2 _P_
values), _P_ values were adjusted for multiple comparisons using the Benjamini-Hochberg procedure (dNdScv, GISTIC2) or the robust false discovery rate procedure (GRIN) and are shown as
negative log10 _P_ values and capped at 0.001 for display purposes. Each patient (column) is annotated with survival group (LTS, long-term survivor; MTS, moderate-term survivor; STS,
short-term survivor). Below the alterations are bar plots indicating somatic mutation burden in variants per megabase (Mb); SV count including duplications, deletions, inversions and
intrachromosomal rearrangements; and the proportion of the tumor genome that is duplicated (WGD) or lost (WGL). B, Pairwise comparison of the alteration frequencies between survival groups
for genes in the COSMIC Cancer Gene Census. The difference in relative alteration frequency is shown on the x-axis and the _P_ value (Fisher’s test, two-sided) is shown on the y-axis.
Symbols of genes with _P_ values < 0.05 are displayed. Multiple hypothesis correction was not applied in this analysis as adjusted _P_ values were all greater than 0.1. Alterations in
this analysis included non-synonymous mutations, homozygous deletions, amplifications and structural variants in coding genes that are expressed. EXTENDED DATA FIG. 3 KEY FEATURES OF
MUTATIONAL SIGNATURE CLUSTERS AND ASSOCIATED SURVIVAL OUTCOMES. A, Summary of the key clinical and genomic features of each mutational signature cluster. Clusters are ordered top to bottom
by lowest to highest proportion of long-term survivors (LTS) in each cluster. HR, homologous recombination; LOH, loss-of-heterozygosity; SV, structural variant; MTS, moderate-term survivor;
STS, short-term survivor; DUP, duplications; DEL, deletions; INV, inversions. B, Kaplan–Meier analysis of progression-free and C, overall survival in patients stratified by signature
clusters. _P_ values calculated by Mantel–Cox log-rank test and dotted lines indicate median survival. D, Boxplots summarize the proportion (y-axis) of clustered and nonclustered
rearrangements by size (x-axis) and type, for each mutational signature cluster (SIG.1 _n_ = 14, SIG.2 _n_ = 25, SIG.3 _n_ = 13, SIG.4 _n_ = 27, SIG.5 _n_ = 22, SIG.6 _n_ = 9, SIG.7 _n_ =
16); boxes show the interquartile range (25–75th percentiles), central lines indicate the median, whiskers show the smallest/largest values within 1.5 times the interquartile range and
values outside it are shown as individual data points. Del, deletions; tds, tandem duplications; inv, inversions, tra, interchromosomal translocations; Kb, kilobase; Mb, megabase. EXTENDED
DATA FIG. 4 CATEGORICAL FEATURES OF MUTATIONAL SIGNATURE CLUSTERS. A, Proportion of patients affected by gene alterations per mutational signature cluster. Genes are ordered by significance
using Fisher’s exact test (two-sided) and clusters are ordered by the proportion of long-term survivors. B, Proportion of patients with categorical features per cluster. Features are ordered
by significance using Fisher’s exact test (two-sided) and the clusters are arranged by the proportion of long-term survivors. The Fisher’s test _P_ values displayed in (A) and (B) are
Benjamini-Hochberg adjusted _P_ values. Features include homologous recombination (HR) status, homologous recombination deficiency (HRD) type, number of DNA repair pathway alterations,
survival group (LTS, long-term survivor; MTS, moderate-term survivor; STS, short-term survivor), status at last follow-up (D, dead; P, progressed and alive; PF, progression-free and alive),
self-reported smoking status, DeepCC molecular subtype (C1, mesenchymal; C2, immunoreactive; C4, differentiated; C5, proliferative), and neoadjuvant treatment (Y, yes; N, no). EXTENDED DATA
FIG. 5 CLINICAL AND GENOMIC FEATURES OF MUTATIONAL SIGNATURE CLUSTERS. A, Boxplots summarize numerical, clinical and genomic features by mutational signature cluster; points represent each
sample, boxes show the interquartile range (25–75th percentiles), central lines indicate the median, whiskers show the smallest/largest values within 1.5 times the interquartile range, red
triangles indicate the mean, and dotted lines join the means of each cluster to visualize the trend. The Kruskal–Wallis test _P_ values displayed are Benjamini-Hochberg adjusted _P_ values.
Features are ordered by their significance and clusters are ordered by the proportion of long-term survivors. CD8 scores were available for _n_ = 54 primary tumors as previously measured by
immunohistochemistry23 and scored as density of CD8+ T cells (average cells/mm2, y axis) in the tumor epithelium (TE). HRD, homologous recombination deficiency; DEL, deletions; DUP;
duplications; SV, structural variants; Mb, megabase; ITX, intrachromosomal rearrangements; LOH, loss-of-heterozygosity; INV, inversions. B, Bubble plot summary of mutational signature
enrichment across signature clusters. The dendrogram is reused from the signature clustering (Fig. 3) to order the mutational signature types (columns). Mutational signature clusters (rows)
are sorted by the proportion of long-term survivors in each cluster, indicated in brackets. The color and size of bubbles indicate the z-score scaled values of the mean signature exposure
per cluster. Bubbles with a z-score of greater than or equal to 1 have a black border and bubbles with a z-score of greater than 0.5 but less than 1 have a gray border. Bordered bubbles have
asterisks filled in to indicate Kruskal–Wallis test _P_ values adjusted for multiple testing using Benjamini-Hochberg correction. EXTENDED DATA FIG. 6 DNA METHYLATION CLUSTERING OF PRIMARY
TUMOR GENOMES. A, Heatmap of methylation data following consensus clustering of primary tumors (columns) based on the standardized CpG probe intensities (M-values) of the 1% most variable
CpG probes (rows; number of probes = 3,645) across all primary tumor samples (_n_ = 126). The heatmap scale shows the beta values. Five methylation clusters were identified (MET.1—MET.5),
and each patient (column) is annotated with survival group (LTS, long-term survivor; MTS, moderate-term survivor; STS, short-term survivor), age at diagnosis (quartiles), and self-reported
smoking history. Tumor samples are also classified according to _CCNE1_ amplification (amp) status, _BRCA1_ alteration status, CIBERSORTx absolute (abs) immune scores (quartiles), and
molecular subtype11 (C1, mesenchymal; C2, immunoreactive; C4, differentiated; C5, proliferative). Bars in the bottom panel represent the _BRCA1_ (orange) and _BRCA2_ (blue) type homologous
recombination deficiency (CHORD28) scores of each tumor sample. B, Kaplan–Meier analysis of progression-free (PFS) and overall survival (OS) in patients stratified by methylation clusters.
_P_ values calculated by Mantel–Cox log-rank test and dotted lines indicate median survival in years since diagnosis. EXTENDED DATA FIG. 7 TRANSCRIPTIONAL PHENOTYPES IN LONG-TERM SURVIVORS.
A, Clustered heatmap summarizing gene set enrichment analysis (GSEA) using the hallmark Molecular Signatures Database (MSigDB) gene sets. Direction and color of triangles relate to the
normalized enrichment score (NES) as generated by FGSEA. _P_ values (two-sided) were calculated using the FGSEA default Monte Carlo method; the size of the triangles corresponds to the
negative log10 Benjamini-Hochberg adjusted _P_ value (_P_adj). The columns are split by survival groups (STS, short-term survivor; MTS, moderate-term survivor; LTS, long-term survivor), with
the direction of enrichment denoted by the group in the heading (numerator) versus the two other groups labeled below. B, Boxplots summarize expression of _MKI67_ and _PCNA_ proliferation
gene markers across the survival groups (left; STS _n_ = 34, MTS _n_ = 32, LTS _n_ = 60); points represent each sample, boxes show the interquartile range (25–75th percentiles), central
lines indicate the median, and whiskers show the smallest/largest values within 1.5 times the interquartile range. Differential expression analysis was performed using DESeq2 to determine
fold change (right) of gene expression between survival groups (two-tailed Wald test, both unadjusted _P_ values and Benjamini-Hochberg adjusted _P_ values (_P_adj) are shown). C, Forest
plot (left) indicates the hazard ratio (HR, squares) and 95% confidence interval (CI; whiskers) for overall survival calculated using a univariate Cox proportional hazard regression model
based on the LM22 immune cell types detected by CIBERSORTx analysis (_n_ = 126 patients). Cell types are arranged by HR. _P_ values < 0.05 are colored red (*_P_ < 0.05, **_P_ <
0.01) and were not adjusted for multiple comparisons. Absolute enrichment scores per cell type across the cohort are shown in boxplots (right); boxes show the interquartile range (25–75th
percentiles), central lines indicate the median, whiskers show the smallest/largest values within 1.5 times the interquartile range and values outside it are shown as individual data points.
EXTENDED DATA FIG. 8 GENOMIC AND CLINICAL FEATURES OF IMMUNE CLUSTERS. A, A condensed bubble plot of the various LM22 cell types used for the immune clustering (IMM.1 _n_ = 32, IMM.2 _n_ =
23, IMM.3 _n_ = 22, IMM.4 _n_ = 24, IMM.5 _n_ = 25). The dendrogram is reused from the immune clustering (Fig. 5a) to order the cell types. Immune clusters (rows) are sorted by the
proportion of long-term survivors indicated in brackets. The color and size of bubbles indicate z-score scaled values of the mean abundance of cell types per cluster. Bubbles with a z-score
of greater than or equal to 1 have a black border, and those with a z-score of greater than 0.5 but less than 1 have a gray border. Asterisks indicate Kruskal–Wallis test _P_ values adjusted
for multiple testing using Benjamini-Hochberg correction. Boxplots (right) summarize CIBERSORTx absolute scores of each cluster; points represent each sample, boxes show the interquartile
range (25–75th percentiles), central lines indicate the median, and whiskers show the smallest/largest values within 1.5 times the interquartile range. B, Boxplots summarize numerical,
clinical and genomic features by immune cluster (IMM.1 _n_ = 32, IMM.2 _n_ = 23, IMM.3 _n_ = 22, IMM.4 _n_ = 24, IMM.5 _n_ = 25); points represent each sample, boxes show the interquartile
range (25–75th percentiles), central lines indicate the median, whiskers show the smallest/largest values within 1.5 times the interquartile range, red triangles indicate the mean, and
dotted lines join the means of each cluster to visualize the trend. The Kruskal–Wallis test _P_ values displayed are Benjamini-Hochberg adjusted. Features are ordered by their significance
and clusters are ordered by the proportion of long-term survivors. CD8 scores were available for _n_ = 54 primary tumors as previously measured by immunohistochemistry23 and scored as
density of CD8+ T cells (average cells/mm2, y axis) in the tumor epithelium (TE). HRD, homologous recombination deficiency; DEL, deletions; DUP; duplications; SV, structural variants; Mb,
megabase; ITX, intrachromosomal rearrangements; LOH, loss-of-heterozygosity; INV, inversions. EXTENDED DATA FIG. 9 CATEGORICAL FEATURES OF IMMUNE CLUSTERS. A, Proportion of patients with
categorical features per cluster. Features are ordered by significance using Fisher’s exact test (two-sided) and the clusters are arranged by the proportion of long-term survivors. Features
include homologous recombination (HR) status, homologous recombination deficiency (HRD) type, number of DNA repair pathway alterations, survival group (LTS, long-term survivor; MTS,
moderate-term survivor; STS, short-term survivor), status at last follow-up (D, dead; P, progressed and alive; PF, progression-free and alive), self-reported smoking status, DeepCC molecular
subtype (C1, mesenchymal; C2, immunoreactive; C4, differentiated; C5, proliferative), and neoadjuvant treatment (Y, yes; N, no). B, Proportion of patients affected by gene alterations per
immune cluster. Genes are ordered by significance using Fisher’s exact test (two-sided) and clusters are ordered by the proportion of long-term survivors. The Fisher’s test _P_ values
displayed in (A) and (B) are Benjamini-Hochberg adjusted _P_ values. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Note, Figures 1–21 and Tables 18–20. REPORTING SUMMARY
PEER REVIEW FILE SUPPLEMENTARY DATA 1 dNdScv cancer driver gene detection results. The table contains the output from the dNdScv R package that uses maximum-likelihood models (two sided) to
detect genes under selection in the study cohort (_n_ = 126). Only high-confidence base substitutions and small-scale deletions and insertions were used for the analysis. Columns include the
gene symbol (gene_name) and global adjusted _P_ value (qglobal_cv). More details regarding the interpretation of all the columns are available at https://github.com/im3sanger/dndscv.
SUPPLEMENTARY DATA 2 Genomic random interval (GRIN) statistical model (one-sided) for recurrent structural variants. The table contains the output from the gene level GRIN analysis on the
study cohort (_n_ = 126) for high-confidence structural variants filtered for expressed protein coding genes. Columns include the gene symbol (gene.label), the adjusted _P_ value of the
cohort level overlap statistic (q.subjects) for all genes, as well as a re-calculation of the adjusted _P_ value after filtering for the expressed protein coding genes
(q.subjects.after_filter). The table has also been annotated for blacklisted genes for SNVs and indels (snv_indel_blacklist), fragile sites (fragile_site), the fragile site database
(fragile_site.list), presence in the COSMIC database (cosmic), the tier in the COSMIC database (cosmic.tier), and its role in cancer in the COSMIC database (cosmic.tier). More details
regarding the interpretation of the columns output by GRIN are available at https://www.stjude.org/research/departments/biostatistics/software/grin.html. SUPPLEMENTARY DATA 3 Regions of
recurrent copy-number change detected by GISTIC2 (permutation-of-markers test, one-sided). Sheet 1 contains cytoband level results for amplifications and deletions in the overall cohort of
126 primary tumors. Columns include the cytoband (Descriptor) and the adjusted _P_ value of the region (q values). Sheet 2 contains the cytoband level analysis with adjusted _P_ values (q
values) for all 126 primary tumors as well as for tumors grouped by survival category. Sheet 3 contains GISTIC2 results at the gene level, and columns include the gene symbol
(genes.in.wide.peak) and adjusted _P_ values (q values) for all 126 primary tumors well as for tumors grouped by survival category. More details regarding the interpretation of the columns
output by GISTIC2 are available at https://www.genepattern.org/modules/docs/GISTIC_2.0. SUPPLEMENTARY DATA 4 RNA-seq differential expression (DE) and gene set enrichment analysis (GSEA)
across survival groups. Sheet 1 contains GSEA output from the FGSEA tool using a pre-ranked list of differentially expressed genes for each survival group comparison. The analysis was run
using the Molecular Signatures Database (MSigDB) hallmark gene sets. _P_ values (two-sided) were calculated using the FGSEA default Monte Carlo method. Columns include the tumor comparison
set (COMPARISON), hallmark pathways (pathway), adjusted _P_ values (padj) and Normalized Enrichment Scores (NES). Sheets 2–4 contain the DE analyses performed using the R package DESeq2
(two-tailed Wald test); comparisons include long-term survivors versus short-term survivors (Sheet2_LTS_vs_STS), long-term survivors versus moderate-term survivors (Sheet3_LTS_vs_MTS) and
moderate-term survivors versus short-term survivors (Sheet4_MTS_vs_STS). The first survival group in the name of each sheet corresponds to the numerator of the DE analysis. Columns include
the gene symbol (SYMBOL), logged and unlogged fold changes (log2FoldChange, foldChange), and adjusted _P_ values (padj) as output by DESeq2. More details regarding the output of FGSEA can be
found at https://github.com/ctlab/fgsea, and DESeq2 can be found at http://bioconductor.org/packages/devel/bioc/vignettes/DESeq2/inst/doc/DESeq2.html. SUPPLEMENTARY DATA 5 RNA-seq high- and
medium-confidence gene fusion results from the Arriba tool. Columns include the fusion gene pair (gene1, gene2), the breakpoints in the gene pair (breakpoint1, breakpoint2), the confidence
of the fusion assigned by Arriba (confidence) and coordinates of any breakpoint support detected in the whole-genome sequencing list of high-confidence structural variants
(closest_genomic_breakpoint1, closest_genomic_breakpoint2). In-frame fusion gene pairs that were identified in more than one patient are indicated in the column ‘Recurrent in-frame gene
fusion’. More details regarding the interpretation of the Arriba output can be found at https://arriba.readthedocs.io/en/latest/output-files/. SUPPLEMENTARY DATA 6 Differential methylation
(DM) analysis results using the Limma R package in order of long-term survivors versus moderate-term survivors (LTS - MTS), long-term survivors versus short-term survivors (LTS - STS) and
moderate-term survivors versus short-term survivors (MTS - STS). The first survival group in the name of each sheet corresponds to the numerator of the DM analysis. Columns include the
genomic coordinates of the methylation probe (seqname, start, end), the identifier of the probe (Name), the symbol of the gene target (GeneSymbol), the distance to the transcription start
site (TSS) of the gene (distance2TSS), if the probe is in the promoter region of the gene (PROMOTER), if the probe intersects the gene body (GENEBODY), the minimum, maximum, mean, and median
beta value of the probe in the cohort (min_beta, max_beta, mean_beta, median_beta), the minimum, maximum, mean and median RNA expression of the gene in the cohort (min_exp, max_exp,
mean_exp, median_exp), the two-sided Pearson correlation test with gene expression (pearson_cor), the unadjusted Pearson correlation _P_ value (pearson_pval), the Benjamini-Hochberg adjusted
Pearson correlation _P_ value (pearson_qval), the direction of Pearson correlation (pearson_cor_dir), the logged fold-change as output by Limma (logFC), the adjusted _P_ value of the DM
result as output by Limma (adj.P.Val) and a column to indicate a filter for genes that were deemed to be turned off in the numerator comparison group (EXP_TURNED_OFF_IN_LTS or
EXP_TURNED_OFF_IN_MTS). Further details in Supplementary Note. More details regarding the interpretation of the Limma output can be found at
https://bioconductor.org/packages/release/bioc/html/limma.html. SUPPLEMENTARY DATA 7 RNA-seq eifferential expression (DE) and fast gene set enrichment analysis (FGSEA), comparing
transcriptomes of _CCNE1_ amplified tumors in each survival group (short-term survivors _n_ = 11, moderate-term survivors _n_ = 4, long-term survivors _n_ = 6) with a reference group of
tumors with no _CCNE1_ amplification or loss that had no homologous recombination (HR) alterations and were classified as HR proficient (reference _n_ = 21). Sheet 1 contains the output from
the FGSEA tool using a pre-ranked list of differentially expressed genes for each survival group comparison. The analysis was run using the Molecular Signatures Database (MSigDB) hallmark
gene sets. _P_ values (two-sided) were calculated using the FGSEA default Monte Carlo method. Columns include the tumor comparison set (COMPARISON), hallmark pathways (pathway), adjusted _P_
values (padj) and Normalized Enrichment Scores (NES). Sheets 2–4 contain the DE analyses performed using the R package DESeq2 (two-tailed Wald test); comparisons include _CCNE1_ amplified
tumors in long-term survivors versus the reference tumors (Sheet2_LTS_vs_reference), _CCNE1_ amplified tumors in moderate-term survivors versus the reference tumors (Sheet3_MTS_vs_reference)
and _CCNE1_ amplified tumors in short-term survivors versus the reference tumors (Sheet4_STS_vs_reference). The first survival group in the name of each sheet corresponds to the numerator
of the DE analysis. Columns include the gene symbol (SYMBOL), logged and unlogged fold changes (log2FoldChange, foldChange), and adjusted _P_ values (padj) as output by DESeq2. SUPPLEMENTARY
TABLE 1 Supplementary Tables 1–17 RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing
agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement
and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Garsed, D.W., Pandey, A., Fereday, S. _et al._ The genomic and immune landscape of long-term survivors of
high-grade serous ovarian cancer. _Nat Genet_ 54, 1853–1864 (2022). https://doi.org/10.1038/s41588-022-01230-9 Download citation * Received: 06 January 2022 * Accepted: 17 October 2022 *
Published: 01 December 2022 * Issue Date: December 2022 * DOI: https://doi.org/10.1038/s41588-022-01230-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative