Play all audios:
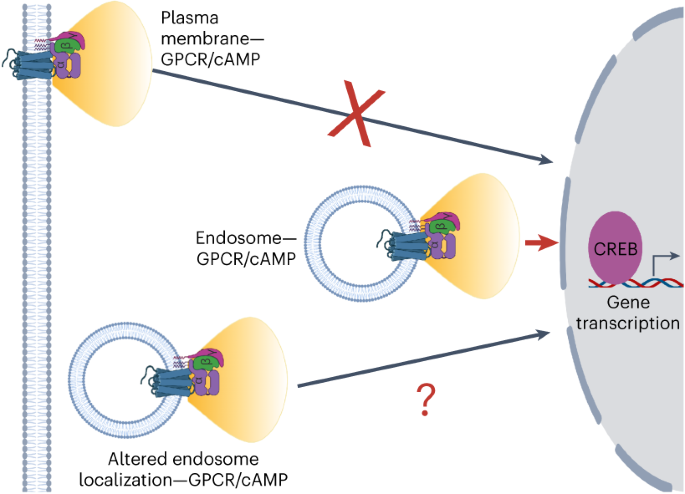
ABSTRACT G-protein-coupled receptors (GPCRs) can initiate unique functional responses depending on the subcellular site of activation. Efforts to uncover the mechanistic basis of
compartmentalized GPCR signaling have concentrated on the biochemical aspect of this regulation. Here we assess the biophysical positioning of receptor-containing endosomes as an alternative
salient mechanism. We devise a strategy to rapidly and selectively redistribute receptor-containing endosomes ‘on command’ in intact cells without perturbing their biochemical composition.
Next, we present two complementary optical readouts that enable robust measurements of bulk- and gene-specific GPCR/cyclic AMP (cAMP)-dependent transcriptional signaling with single-cell
resolution. With these, we establish that disruption of native endosome positioning inhibits the initiation of the endosome-dependent transcriptional responses. Finally, we demonstrate a
prominent mechanistic role of PDE-mediated cAMP hydrolysis and local protein kinase A activity in this process. Our study, therefore, illuminates a new mechanism regulating GPCR function by
identifying endosome positioning as the principal mediator of spatially selective receptor signaling. Access through your institution Buy or subscribe This is a preview of subscription
content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access
subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this
article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in
* Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS MOLECULAR MECHANISM OF GPCR SPATIAL ORGANIZATION AT THE PLASMA
MEMBRANE Article 17 July 2023 RAPID WHOLE CELL IMAGING REVEALS A CALCIUM-APPL1-DYNEIN NEXUS THAT REGULATES COHORT TRAFFICKING OF STIMULATED EGF RECEPTORS Article Open access 17 February 2021
SPATIAL DECODING OF ENDOSOMAL CAMP SIGNALS BY A METASTABLE CYTOPLASMIC PKA NETWORK Article 01 March 2021 DATA AVAILABILITY All relevant data supporting the findings are available within the
paper and the Supplementary Data. Source data are provided with the manuscript. Additional information and reagents are available from the corresponding author upon reasonable request.
Source data are provided with this paper. REFERENCES * Sorkin, A. & von Zastrow, M. Endocytosis and signalling: intertwining molecular networks. _Nat. Rev. Mol. Cell Biol._ 10, 609–622
(2009). Article CAS PubMed PubMed Central Google Scholar * Thomsen, A. R. B., Jensen, D. D., Hicks, G. A. & Bunnett, N. W. Therapeutic targeting of endosomal G-protein-coupled
receptors. _Trends Pharmacol. Sci._ 39, 879–891 (2018). Article CAS PubMed PubMed Central Google Scholar * Tsvetanova, N. G., Irannejad, R. & von Zastrow, M. G protein-coupled
receptor (GPCR) signaling via heterotrimeric G proteins from endosomes. _J. Biol. Chem._ 290, 6689–6696 (2015). Article CAS PubMed PubMed Central Google Scholar * Vilardaga, J. P.,
Jean-Alphonse, F. G. & Gardella, T. J. Endosomal generation of cAMP in GPCR signaling. _Nat. Chem. Biol._ 10, 700–706 (2014). Article CAS PubMed PubMed Central Google Scholar *
Godbole, A., Lyga, S., Lohse, M. J. & Calebiro, D. Internalized TSH receptors en route to the TGN induce local Gs-protein signaling and gene transcription. _Nat. Commun._ 8, 443 (2017).
Article PubMed PubMed Central Google Scholar * Tsvetanova, N. G. & von Zastrow, M. Spatial encoding of cyclic AMP signaling specificity by GPCR endocytosis. _Nat. Chem. Biol._ 10,
1061–1065 (2014). Article CAS PubMed PubMed Central Google Scholar * Tsvetanova, N. G. et al. Endosomal cAMP production broadly impacts the cellular phosphoproteome. _J. Biol. Chem._
297, 100907 (2021). Article CAS PubMed PubMed Central Google Scholar * Calebiro, D. et al. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. _PLoS Biol._ 7,
e1000172 (2009). Article PubMed PubMed Central Google Scholar * Ferrandon, S. et al. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. _Nat. Chem. Biol._ 5,
734–742 (2009). Article CAS PubMed PubMed Central Google Scholar * Irannejad, R. et al. Functional selectivity of GPCR-directed drug action through location bias. _Nat. Chem. Biol._ 13,
799–806 (2017). Article CAS PubMed PubMed Central Google Scholar * Nash, C. A., Wei, W., Irannejad, R. & Smrcka, A. V. Golgi localized β1-adrenergic receptors stimulate golgi PI4P
hydrolysis by PLCε to regulate cardiac hypertrophy. _eLife_ 8, e48167 (2019). Article CAS PubMed PubMed Central Google Scholar * Purgert, C. A. et al. Intracellular mGluR5 can mediate
synaptic plasticity in the hippocampus. _J. Neurosci._ 34, 4589–4598 (2014). Article PubMed PubMed Central Google Scholar * Vincent, K. et al. Intracellular mGluR5 plays a critical role
in neuropathic pain. _Nat. Commun._ 7, 10604 (2016). Article CAS PubMed PubMed Central Google Scholar * White, A. D. et al. Spatial bias in cAMP generation determines biological
responses to PTH type 1 receptor activation. _Sci. Signal_ 14, eabc5944 (2021). Article CAS PubMed PubMed Central Google Scholar * Okazaki, M. et al. Prolonged signaling at the
parathyroid hormone receptor by peptide ligands targeted to a specific receptor conformation. _Proc. Natl Acad. Sci. USA_ 105, 16525–16530 (2008). Article CAS PubMed PubMed Central
Google Scholar * Feinstein, T. N. et al. Noncanonical control of vasopressin receptor type 2 signaling by retromer and arrestin. _J. Biol. Chem._ 288, 27849–27860 (2013). Article CAS
PubMed PubMed Central Google Scholar * Kuna, R. S. et al. Glucagon-like peptide-1 receptor-mediated endosomal cAMP generation promotes glucose-stimulated insulin secretion in pancreatic
β-cells. _Am. J. Physiol. Endocrinol. Metab._ 305, E161–E170 (2013). Article CAS PubMed Google Scholar * Tian, X. et al. The α-arrestin ARRDC3 regulates the endosomal residence time and
intracellular signaling of the β2-adrenergic receptor. _J. Biol. Chem._ 291, 14510–14525 (2016). Article CAS PubMed PubMed Central Google Scholar * Thomsen, A. R. et al. GPCR-G
protein-β-arrestin super-complex mediates sustained G protein signaling. _Cell_ 166, 907–919 (2016). Article CAS PubMed PubMed Central Google Scholar * Varandas, K. C., Irannejad, R.
& von Zastrow, M. Retromer endosome exit domains serve multiple trafficking destinations and regulate local G protein activation by GPCRs. _Curr. Biol._ 26, 3129–3142 (2016). Article
CAS PubMed PubMed Central Google Scholar * Bowman, S. L., Shiwarski, D. J. & Puthenveedu, M. A. Distinct G protein-coupled receptor recycling pathways allow spatial control of
downstream G protein signaling. _J. Cell Biol._ 214, 797–806 (2016). Article CAS PubMed PubMed Central Google Scholar * Madamanchi, A. β-Adrenergic receptor signaling in cardiac
function and heart failure. _McGill J. Med._ 10, 99 (2007). PubMed PubMed Central Google Scholar * Mutlu, G. M. & Factor, P. Alveolar epithelial β2-adrenergic receptors. _Am. J.
Respir. Cell Mol. Biol._ 38, 127–134 (2008). Article CAS PubMed Google Scholar * Irannejad, R. et al. Conformational biosensors reveal GPCR signalling from endosomes. _Nature_ 495,
534–538 (2013). Article CAS PubMed Google Scholar * Mayr, B. & Montminy, M. Transcriptional regulation by the phosphorylation-dependent factor CREB. _Nat. Rev. Mol. Cell Biol._ 2,
599–609 (2001). Article CAS PubMed Google Scholar * Peng, G. E., Pessino, V., Huang, B. & von Zastrow, M. Spatial decoding of endosomal cAMP signals by a metastable cytoplasmic PKA
network. _Nat. Chem. Biol._ 17, 558–566 (2021). Article CAS PubMed PubMed Central Google Scholar * Schuster, M., Lipowsky, R., Assmann, M.-A., Lenz, P. & Steinberg, G. Transient
binding of dynein controls bidirectional long-range motility of early endosomes. _Proc. Natl Acad. Sci. USA_ 108, 3618–3623 (2011). Article CAS PubMed PubMed Central Google Scholar *
Reck-Peterson, S. L., Redwine, W. B., Vale, R. D. & Carter, A. P. The cytoplasmic dynein transport machinery and its many cargoes. _Nat. Rev. Mol. Cell Biol._ 19, 382–398 (2018). Article
CAS PubMed PubMed Central Google Scholar * Korolchuk, V. I. et al. Lysosomal positioning coordinates cellular nutrient responses. _Nat. Cell Biol._ 13, 453–460 (2011). Article CAS
PubMed PubMed Central Google Scholar * Sainath, R. & Gallo, G. The dynein inhibitor Ciliobrevin D inhibits the bidirectional transport of organelles along sensory axons and impairs
NGF-mediated regulation of growth cones and axon branches. _Dev. Neurobiol._ 75, 757–777 (2015). Article CAS PubMed Google Scholar * Violin, J. D. et al. β2-Adrenergic receptor signaling
and desensitization elucidated by quantitative modeling of real time cAMP dynamics. _J. Biol. Chem._ 283, 2949–2961 (2008). Article CAS PubMed Google Scholar * Temkin, P. et al. SNX27
mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. _Nat. Cell Biol._ 13, 715–721 (2011). Article PubMed PubMed Central Google Scholar *
Odaka, H., Arai, S., Inoue, T. & Kitaguchi, T. Genetically-encoded yellow fluorescent cAMP indicator with an expanded dynamic range for dual-color imaging. _PLoS ONE_ 9, e100252 (2014).
Article PubMed PubMed Central Google Scholar * Herskovits, J. S., Burgess, C. C., Obar, R. A. & Vallee, R. B. Effects of mutant rat dynamin on endocytosis. _J. Cell Biol._ 122,
565–578 (1993). Article CAS PubMed Google Scholar * Bayle, J. H. et al. Rapamycin analogs with differential binding specificity permit orthogonal control of protein activity. _Chem.
Biol._ 13, 99–107 (2006). Article CAS PubMed Google Scholar * Nagendran, M., Riordan, D. P., Harbury, P. B. & Desai, T. J. Automated cell-type classification in intact tissues by
single-cell molecular profiling. _eLife_ 7, e30510 (2018). Article PubMed PubMed Central Google Scholar * Semesta, K. M., Tian, R., Kampmann, M., von Zastrow, M. & Tsvetanova, N. G.
A high-throughput CRISPR interference screen for dissecting functional regulators of GPCR/cAMP signaling. _PLoS Genet._ 16, e1009103 (2020). Article CAS PubMed PubMed Central Google
Scholar * Gillooly, D. J. et al. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. _EMBO J._ 19, 4577–4588 (2000). Article CAS PubMed PubMed Central Google
Scholar * Zhang, J. Z. et al. Phase separation of a PKA regulatory subunit controls cAMP compartmentation and oncogenic signaling. _Cell_ 182, 1531–1544 (2020). Article CAS PubMed PubMed
Central Google Scholar * Harootunian, A. T. et al. Movement of the free catalytic subunit of cAMP-dependent protein kinase into and out of the nucleus can be explained by diffusion. _Mol.
Biol. Cell_ 4, 993–1002 (1993). Article CAS PubMed PubMed Central Google Scholar * Smith, F. D. et al. Local protein kinase A action proceeds through intact holoenzymes. _Science_ 356,
1288–1293 (2017). Article CAS PubMed PubMed Central Google Scholar * Zhang, J.-F. et al. An ultrasensitive biosensor for high-resolution kinase activity imaging in awake mice. _Nat.
Chem. Biol._ 17, 39–46 (2021). Article CAS PubMed Google Scholar * Bock, A. et al. Optical mapping of cAMP signaling at the nanometer scale. _Cell_ 182, 1519–1530 e1517 (2020). Article
CAS PubMed PubMed Central Google Scholar * Mo, G. C. et al. Genetically encoded biosensors for visualizing live-cell biochemical activity at super-resolution. _Nat. Methods_ 14, 427–434
(2017). Article CAS PubMed PubMed Central Google Scholar * Bers, D. M., Xiang, Y. K. & Zaccolo, M. Whole-cell cAMP and PKA activity are epiphenomena, nanodomain signaling matters.
_Physiology (Bethesda)_ 34, 240–249 (2019). CAS PubMed Google Scholar * Nigg, E., Hilz, H., Eppenberger, H. & Dutly, F. Rapid and reversible translocation of the catalytic subunit of
cAMP‐dependent protein kinase type II from the Golgi complex to the nucleus. _EMBO J._ 4, 2801–2806 (1985). Article CAS PubMed PubMed Central Google Scholar * Anton, S. E. et al.
Receptor-associated independent cAMP nanodomains mediate spatiotemporal specificity of GPCR signaling. _Cell_ 185, 1130–1142 (2022). Article CAS PubMed Google Scholar * Koschinski, A.
& Zaccolo, M. Activation of PKA in cell requires higher concentration of cAMP than in vitro: implications for compartmentalization of cAMP signalling. _Sci. Rep._ 7, 14090 (2017).
Article PubMed PubMed Central Google Scholar * English, A. R. & Voeltz, G. K. Endoplasmic reticulum structure and interconnections with other organelles. _Cold Spring Harb. Perspect.
Biol._ 5, a013227 (2013). Article PubMed PubMed Central Google Scholar * Jia, R. & Bonifacino, J. S. Lysosome positioning influences mTORC2 and AKT signaling. _Mol. Cell_ 75, 26–38
(2019). Article CAS PubMed PubMed Central Google Scholar * Riccio, A., Pierchala, B. A., Ciarallo, C. L. & Ginty, D. D. An NGF-TrkA-mediated retrograde signal to transcription
factor CREB in sympathetic neurons. _Science_ 277, 1097–1100 (1997). Article CAS PubMed Google Scholar * Scerra, G. et al. Lysosomal positioning diseases: beyond substrate storage. _Open
Biol._ 12, 220155 (2022). Article CAS PubMed PubMed Central Google Scholar * Murphy, J. E., Padilla, B. E., Hasdemir, B., Cottrell, G. S. & Bunnett, N. W. Endosomes: a legitimate
platform for the signaling train. _Proc. Natl Acad. Sci. USA_ 106, 17615–17622 (2009). Article CAS PubMed PubMed Central Google Scholar * Kwon, Y. et al. Non-canonical β-adrenergic
activation of ERK at endosomes. _Nature_ 611, 173–179 (2022). Article CAS PubMed PubMed Central Google Scholar * Inda, C. et al. Different cAMP sources are critically involved in G
protein-coupled receptor CRHR1 signaling. _J. Cell Biol._ 214, 181–195 (2016). Article CAS PubMed PubMed Central Google Scholar * Kotowski, S. J., Hopf, F. W., Seif, T., Bonci, A. &
von Zastrow, M. Endocytosis promotes rapid dopaminergic signaling. _Neuron_ 71, 278–290 (2011). Article CAS PubMed PubMed Central Google Scholar * Legland, D., Arganda-Carreras, I.
& Andrey, P. MorphoLibJ: integrated library and plugins for mathematical morphology with ImageJ. _Bioinformatics_ 32, 3532–3534 (2016). Article CAS PubMed Google Scholar * Rizk, A.
et al. Segmentation and quantification of subcellular structures in fluorescence microscopy images using Squassh. _Nat. Protoc._ 9, 586–596 (2014). Article CAS PubMed Google Scholar *
Kamentsky, L. et al. Improved structure, function and compatibility for CellProfiler: modular high-throughput image analysis software. _Bioinformatics_ 27, 1179–1180 (2011). Article CAS
PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank members of the Tsvetanova Lab and R. Irannejad (UCSF) for valuable discussions of the project and
feedback on the manuscript. Immunofluorescence microscopy imaging was performed using resources from the Duke Light Microscopy Core Facility, with specific training under L. Cameron, Y. Gao
and B. Carlson. PKARegIIB-mCherry and EGFP-SNX27 were gifts from R. Irannejad (UCSF). SSF-β2-AR, RAB11a-GFP, DynK44E-mCherry, pcDNA3.0, eGFP-C1 and Endo-bPAC were gifts from M. von Zastrow
(UCSF). MK1200 and dCas9-BFP-KRAB were gifts from M. Kampmann (UCSF). pBa.Kif1a 1-396.GFP was a gift from G. Banker and M. Bentley (Addgene plasmid, 45058; http://n2t.net/addgene:45058;
RRID:Addgene_45058). pBa-KIF5C 559-tdTomato-FKBP was a gift from G. Banker and M. Bentley (Addgene plasmid, 64211; http://n2t.net/addgene:64211; RRID:Addgene_64211). pEGFP-FRB was a gift
from K. Hahn (Addgene plasmid, 25919; http://n2t.net/addgene:25919; RRID:Addgene_25919). GFP-EEA1 wt was a gift from S. Corvera (Addgene plasmid, 42307; http://n2t.net/addgene:42307;
RRID:Addgene_42307). pmCherry-N1-GalT was a gift from L. Lu (Addgene plasmid, 87327; http://n2t.net/addgene:87327; RRID:Addgene_87327). Flamindo2 and nlsFlamindo2 were gifts from T.
Kitaguchi (Addgene plasmid, 73938; http://n2t.net/addgene:73938; RRID:Addgene_73938 and Addgene plasmid, 73939; http://n2t.net/addgene:73939; RRID:Addgene_73939). Figures 1a and 6f were
created using BioRender. Research reported in this publication was supported by the National Institutes of Health (R01NS127847 and R35GM142640 to N.G.T., R01DK073368 to J.Z. and F31NS120567
to B.K.A.W.) and the American Heart Association (Predoctoral Fellowship 834472 to J.F.Z.). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Pharmacology and Cancer Biology, Duke
University, Durham, NC, USA Blair K. A. Willette & Nikoleta G. Tsvetanova * Department of Pharmacology, University of California San Diego, La Jolla, CA, USA Jin-Fan Zhang & Jin
Zhang * Department of Bioengineering, University of California San Diego, La Jolla, CA, USA Jin-Fan Zhang & Jin Zhang * Department of Chemistry and Biochemistry, University of California
San Diego, La Jolla, CA, USA Jin Zhang Authors * Blair K. A. Willette View author publications You can also search for this author inPubMed Google Scholar * Jin-Fan Zhang View author
publications You can also search for this author inPubMed Google Scholar * Jin Zhang View author publications You can also search for this author inPubMed Google Scholar * Nikoleta G.
Tsvetanova View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS N.G.T. supervised the project. N.G.T. and B.K.A.W. conceived the project and
designed experiments. B.K.A.W. and J.F.Z. performed and analyzed all experiments. J.Z. supervised and coordinated experiments involving the generation and characterization of nuclear
ExRai-AKAR2 sensor. N.G.T., B.K.A.W. and J.F.Z. interpreted results. N.G.T. and B.K.A.W. wrote the manuscript. All authors edited the manuscript. CORRESPONDING AUTHOR Correspondence to
Nikoleta G. Tsvetanova. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Chemical Biology_ thanks Davide
Calebiro and the other, anonymous, reviewers for their contribution to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figs. 1–14 and uncropped western blot for
Supplementary Fig. 8c. REPORTING SUMMARY SUPPLEMENTARY DATA Supporting data for Supplementary Figs. 1–14. SUPPLEMENTARY VIDEO 1 Live-cell imaging of HEK293 cells expressing CID components.
The video shows endosome distribution after 5 min of AP21967 (rapalog) treatment. SUPPLEMENTARY VIDEO 2 Live-cell imaging of HEK293 cells expressing Flamindo2-FRB-EEA1 and
Kif1a-tdTomato-FKBP. Cells were pretreated with AP21967 (rapalog) for 30 min. SOURCE DATA SOURCE DATA FIG. 1 Numerical source data and image source data for Fig. 1. SOURCE DATA FIG. 2
Numerical source data and image source data for Fig. 2. SOURCE DATA FIG. 3 Numerical source data and image source data for Fig. 3. SOURCE DATA FIG. 4 Numerical source data and image source
data for Fig. 4. SOURCE DATA FIG. 5 Numerical source data and image source data for Fig. 5. SOURCE DATA FIG. 6 Numerical source data for Fig. 6. RIGHTS AND PERMISSIONS Springer Nature or its
licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the
accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Willette, B.K.A., Zhang, JF., Zhang, J. _et al._ Endosome positioning coordinates spatially selective GPCR signaling. _Nat Chem Biol_ 20, 151–161 (2024).
https://doi.org/10.1038/s41589-023-01390-7 Download citation * Received: 25 May 2022 * Accepted: 29 June 2023 * Published: 27 July 2023 * Issue Date: February 2024 * DOI:
https://doi.org/10.1038/s41589-023-01390-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative