Play all audios:
ABSTRACT Covalent modulators and covalent degrader molecules have emerged as drug modalities with tremendous therapeutic potential. Toward realizing this potential, mass spectrometry-based
chemoproteomic screens have generated proteome-wide maps of potential druggable cysteine residues. However, beyond these direct cysteine-target maps, the full scope of direct and indirect
activities of these molecules on cellular processes and how such activities contribute to reported modes of action, such as degrader activity, remains to be fully understood. Using
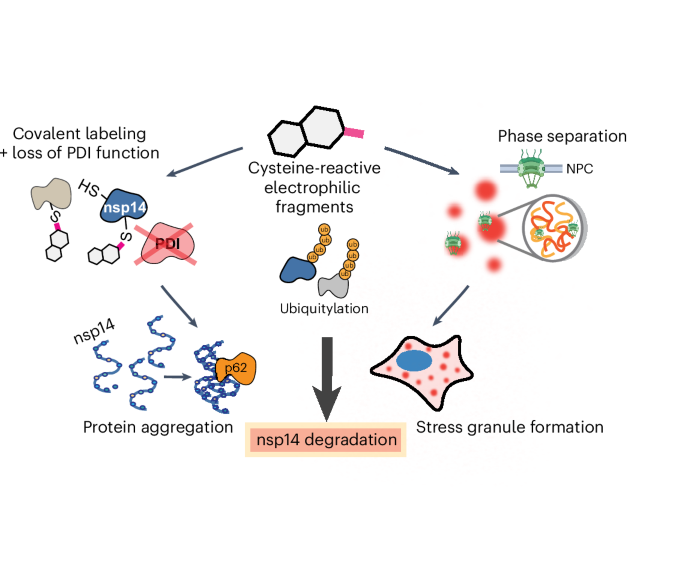
chemoproteomics, we identified a cysteine-reactive small molecule degrader of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nonstructural protein 14 (nsp14), which effects
degradation through direct modification of cysteines in both nsp14 and in host protein disulfide isomerases. This degrader activity was further potentiated by generalized
electrophile-induced global protein ubiquitylation, proteasome activation and widespread aggregation and depletion of host proteins, including the formation of stress granules. Collectively,
we delineate the wide-ranging impacts of cysteine-reactive electrophilic compounds on cellular proteostasis processes. Access through your institution Buy or subscribe This is a preview of
subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value
online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more
Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS:
* Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS A COVALENT CHEMICAL PROBE FOR _CHIKUNGUNYA_ NSP2 CYSTEINE
PROTEASE WITH ANTIALPHAVIRAL ACTIVITY AND PROTEOME-WIDE SELECTIVITY Article Open access 01 March 2025 TARGETED DEGRADATION OF EXTRACELLULAR MITOCHONDRIAL ASPARTYL-TRNA SYNTHETASE MODULATES
IMMUNE RESPONSES Article Open access 22 July 2024 DEUBIQUITINASE-TARGETING CHIMERAS FOR TARGETED PROTEIN STABILIZATION Article 24 February 2022 DATA AVAILABILITY All supporting data for this
study can be found within the article and Supplementary Information. The MS data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the
Proteomics Identification Database (PRIDE)99 partner repository with the dataset identifiers PXD046278 (isoTOP–ABPP data in Figs. 1 and 5), PXD046393 (all other proteomic data corresponding
to Figs. 3–6) and PXD053865 (PDIA6-His AP–MS and Extended Data Fig. 4 proteomics). Publicly available databases used are the UniProtKB Consortium (https://www.uniprot.org/), the 2021 release
of the KEGG pathway database (https://www.genome.jp/kegg/pathway.html) and the 2021 release of the GO Resource (https://geneontology.org/) Molecular Function, Cellular Component and
Biological Process classes. Source data are provided as source data files for Figs. 1–6 and Extended Data Figs. 2, 3, 4, 7 and 9 and within the Supplementary Information for Supplementary
Figs. 1–30. Source data are provided with this paper. CODE AVAILABILITY All code used for this work is available at https://github.com/BackusLab. REFERENCES * Boatner, L. M., Palafox, M. F.,
Schweppe, D. K. & Backus, K. M. CysDB: a human cysteine database based on experimental quantitative chemoproteomics. _Cell Chem. Biol._ 30, 683–698 (2023). Article CAS PubMed PubMed
Central Google Scholar * Backus, K. M. et al. Proteome-wide covalent ligand discovery in native biological systems. _Nature_ 534, 570–574 (2016). Article CAS PubMed PubMed Central
Google Scholar * Weerapana, E. et al. Quantitative reactivity profiling predicts functional cysteines in proteomes. _Nature_ 468, 790–795 (2010). Article CAS PubMed PubMed Central
Google Scholar * Cao, J. et al. Multiplexed CuAAC Suzuki–Miyaura labeling for tandem activity-based chemoproteomic profiling. _Anal. Chem._ 93, 2610–2618 (2021). Article CAS PubMed
PubMed Central Google Scholar * Vinogradova, E. V. et al. An activity-guided map of electrophile–cysteine interactions in primary human T cells. _Cell_ 182, 1009–1026 (2020). Article CAS
PubMed PubMed Central Google Scholar * Yan, T. et al. SP3-FAIMS chemoproteomics for high-coverage profiling of the human cysteinome. _ChemBioChem_ 22, 1841–1851 (2021). Article CAS
PubMed PubMed Central Google Scholar * Kuljanin, M. et al. Reimagining high-throughput profiling of reactive cysteines for cell-based screening of large electrophile libraries. _Nat.
Biotechnol._ 39, 630–641 (2021). Article CAS PubMed PubMed Central Google Scholar * Burton, N. R. et al. Solid-phase compatible silane-based cleavable linker enables custom isobaric
quantitative chemoproteomics. _J. Am. Chem. Soc._ 145, 21303–21318 (2023). Article CAS PubMed PubMed Central Google Scholar * Mader, M. M. et al. Which small molecule? Selecting
chemical probes for use in cancer research and target validation. _Cancer Discov._ 13, 2150–2165 (2023). Article PubMed Google Scholar * Antolin, A. A. et al. The Chemical Probes Portal:
an expert review-based public resource to empower chemical probe assessment, selection and use. _Nucleic Acids Res._ 51, D1492–D1502 (2023). Article PubMed Google Scholar * Hartung, I.
V., Rudolph, J., Mader, M. M., Mulder, M. P. C. & Workman, P. Expanding chemical probe space: quality criteria for covalent and degrader probes. _J. Med. Chem._ 66, 9297–9312 (2023).
Article CAS PubMed PubMed Central Google Scholar * Kavanagh, M. E. et al. Selective inhibitors of JAK1 targeting an isoform-restricted allosteric cysteine. _Nat. Chem. Biol._ 18,
1388–1398 (2022). Article CAS PubMed PubMed Central Google Scholar * Ostrem, J. M., Peters, U., Sos, M. L., Wells, J. A. & Shokat, K. M. K-Ras(G12C) inhibitors allosterically
control GTP affinity and effector interactions. _Nature_ 503, 548–551 (2013). Article CAS PubMed PubMed Central Google Scholar * Janes, M. R. et al. Targeting KRAS mutant cancers with a
covalent G12C-specific inhibitor. _Cell_ 172, 578–589.e17 (2018). Article CAS PubMed Google Scholar * Dickson, P. et al. Physical and functional analysis of the putative rpn13 inhibitor
RA190. _Cell Chem. Biol._ 27, 1371–1382 (2020). Article CAS PubMed PubMed Central Google Scholar * Anchoori, R. K. et al. A bis-benzylidine piperidone targeting proteasome ubiquitin
receptor RPN13/ADRM1 as a therapy for cancer. _Cancer Cell_ 24, 791–805 (2013). Article CAS PubMed Google Scholar * Gamayun, I. et al. Eeyarestatin compounds selectively enhance
Sec61-mediated Ca2+ leakage from the endoplasmic reticulum. _Cell Chem. Biol._ 26, 571–583 (2019). Article CAS PubMed PubMed Central Google Scholar * Zhang, K. et al. Stress granule
assembly disrupts nucleocytoplasmic transport. _Cell_ 173, 958–971.e17 (2018). Article CAS PubMed PubMed Central Google Scholar * Othumpangat, S., Kashon, M. & Joseph, P. Sodium
arsenite-induced inhibition of eukaryotic translation initiation factor 4E (eIF4E) results in cytotoxicity and cell death. _Mol. Cell. Biochem._ 279, 123–131 (2005). Article CAS PubMed
Google Scholar * Kopito, R. R. Aggresomes, inclusion bodies and protein aggregation. _Trends Cell Biol._ 10, 524–530 (2000). Article CAS PubMed Google Scholar * García-Mata, R., Bebök,
Z., Sorscher, E. J. & Sztul, E. S. Characterization and dynamics of aggresome formation by a cytosolic GFP-chimera. _J. Cell Biol._ 146, 1239–1254 (1999). Article PubMed PubMed Central
Google Scholar * Forte, N. et al. Targeted Protein Degradation through E2 Recruitment. _ACS Chem. Biol._ 18, 897–904 (2023). Article CAS PubMed PubMed Central Google Scholar * Zhang,
X., Crowley, V. M., Wucherpfennig, T. G., Dix, M. M. & Cravatt, B. F. Electrophilic PROTACs that degrade nuclear proteins by engaging DCAF16. _Nat. Chem. Biol._ 15, 737–746 (2019).
Article CAS PubMed PubMed Central Google Scholar * Tao, Y. et al. Targeted protein degradation by electrophilic PROTACs that stereoselectively and site-specifically engage DCAF1. _J.
Am. Chem. Soc._ 144, 18688–18699 (2022). Article CAS PubMed PubMed Central Google Scholar * Zhang, X. et al. DCAF11 supports targeted protein degradation by electrophilic proteolysis
targeting chimeras. _J. Am. Chem. Soc._ 143, 5141–5149 (2021). Article CAS PubMed PubMed Central Google Scholar * Spradlin, J. N. et al. Harnessing the anti-cancer natural product
nimbolide for targeted protein degradation. _Nat. Chem. Biol._ 15, 747–755 (2019). Article CAS PubMed PubMed Central Google Scholar * Ward, C. C. et al. Covalent ligand screening
uncovers a RNF4 E3 ligase recruiter for targeted protein degradation applications. _ACS Chem. Biol._ 14, 2430–2440 (2019). Article CAS PubMed PubMed Central Google Scholar * Henning, N.
J. et al. Discovery of a covalent FEM1B recruiter for targeted protein degradation applications. _J. Am. Chem. Soc._ 144, 701–708 (2022). Article CAS PubMed PubMed Central Google
Scholar * Isobe, Y. et al. Manumycin polyketides act as molecular glues between UBR7 and P53. _Nat. Chem. Biol._ 16, 1189–1198 (2020). Article CAS PubMed PubMed Central Google Scholar
* Toriki, E. S. et al. Rational chemical design of molecular glue degraders. _ACS Cent. Sci._ 9, 915–926 (2023). Article CAS PubMed PubMed Central Google Scholar * Sarott, R. C. et al.
Chemical specification of E3 ubiquitin ligase engagement by cysteine-reactive chemistry. _J. Am. Chem. Soc._ 145, 21937–21944 (2023). Article CAS PubMed Google Scholar * Li, Y.-D. et al.
Template-assisted covalent modification underlies activity of covalent molecular glues. _Nat. Chem. Biol._ https://doi.org/10.1038/s41589-024-01668-4 (2024). * Hassan, M. M. et al.
Exploration of the tunability of BRD4 degradation by DCAF16 _trans_-labelling covalent glues. _Eur. J. Med. Chem._ 279, 116904 (2024). Article CAS PubMed PubMed Central Google Scholar *
King, E. A. et al. Chemoproteomics-enabled discovery of a covalent molecular glue degrader targeting NF-κB. _Cell Chem. Biol._ 30, 394–402 (2023). Article CAS PubMed Google Scholar *
Hong, S. H. et al. Exploiting the cullin E3 ligase adaptor protein SKP1 for targeted protein degradation. _ACS Chem. Biol._ 19, 442–450 (2024). Article CAS PubMed PubMed Central Google
Scholar * Littler, D. R., Gully, B. S., Colson, R. N. & Rossjohn, J. Crystal structure of the SARS-CoV-2 non-structural protein 9, Nsp9. _iScience_ 23, 101258 (2020). Article CAS
PubMed PubMed Central Google Scholar * Ma, Y. et al. Structural basis and functional analysis of the SARS coronavirus nsp14–nsp10 complex. _Proc. Natl Acad. Sci. USA_ 112, 9436–9441
(2015). Article CAS PubMed PubMed Central Google Scholar * Decroly, E. et al. Crystal structure and functional analysis of the SARS-coronavirus RNA cap 2′-O-methyltransferase
nsp10/nsp16 complex. _PLoS Pathog._ 7, e1002059 (2011). Article CAS PubMed PubMed Central Google Scholar * Imprachim, N., Yosaatmadja, Y. & Newman, J. A. Crystal structures and
fragment screening of SARS-CoV-2 NSP14 reveal details of exoribonuclease activation and mRNA capping and provide starting points for antiviral drug development. _Nucleic Acids Res._ 51,
475–487 (2023). Article CAS PubMed Google Scholar * Liby, K. et al. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE
signaling. _Cancer Res._ 65, 4789–4798 (2005). Article CAS PubMed Google Scholar * Yang, D. et al. Systematic targeting of protein complexes with molecular COUPLrs. Preprint a _bioRxiv_
https://doi.org/10.1101/2024.07.16.603666 (2024). * Mauvezin, C. & Neufeld, T. P. Bafilomycin A1 disrupts autophagic flux by inhibiting both V-ATPase-dependent acidification and
Ca-P60A/SERCA-dependent autophagosome-lysosome fusion. _Autophagy_ 11, 1437–1438 (2015). Article CAS PubMed PubMed Central Google Scholar * Ohtake, F., Tsuchiya, H., Saeki, Y. &
Tanaka, K. K63 ubiquitylation triggers proteasomal degradation by seeding branched ubiquitin chains. _Proc. Natl Acad. Sci. USA_ 115, E1401–E1408 (2018). Article CAS PubMed PubMed Central
Google Scholar * Gordon, D. E. et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. _Nature_ 583, 459–468 (2020). Article CAS PubMed PubMed Central Google
Scholar * Grantham, J. The molecular chaperone CCT/TRiC: an essential component of proteostasis and a potential modulator of protein aggregation. _Front. Genet._ 11, 172 (2020). Article
CAS PubMed PubMed Central Google Scholar * Bashore, C. et al. Targeted degradation via direct 26S proteasome recruitment. _Nat. Chem. Biol._ 19, 55–63 (2023). Article CAS PubMed
Google Scholar * Tofaris, G. K., Layfield, R. & Spillantini, M. G. α-Synuclein metabolism and aggregation is linked to ubiquitin-independent degradation by the proteasome. _FEBS Lett._
509, 22–26 (2001). Article CAS PubMed Google Scholar * Zhou, M. et al. HUWE1 amplifies ubiquitin modifications to broadly stimulate clearance of proteins and aggregates. Preprint at
_bioRxiv_ https://doi.org/10.1101/2023.05.30.542866 (2023). * Sarkar, A. A. & Zohn, I. E. Hectd1 regulates intracellular localization and secretion of Hsp90 to control cellular behavior
of the cranial mesenchyme. _J. Cell Biol._ 196, 789–800 (2012). Article CAS PubMed PubMed Central Google Scholar * Wang, Q., Li, L. & Ye, Y. Inhibition of p97-dependent protein
degradation by eeyarestatin I. _J. Biol. Chem._ 283, 7445–7454 (2008). Article CAS PubMed PubMed Central Google Scholar * Berkers, C. R. et al. Profiling proteasome activity in tissue
with fluorescent probes. _Mol. Pharm._ 4, 739–748 (2007). Article CAS PubMed Google Scholar * Leestemaker, Y. et al. Proteasome activation by small molecules. _Cell Chem. Biol._ 24,
725–736 (2017). Article CAS PubMed Google Scholar * Vetma, V. et al. Confounding factors in targeted degradation of short-lived proteins. _ACS Chem. Biol._ 19, 1484–1494 (2024). Article
CAS PubMed Google Scholar * Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. _Cell_ 149, 1060–1072 (2012). Article CAS PubMed PubMed Central
Google Scholar * Higgins, L. G. et al. Transcription factor Nrf2 mediates an adaptive response to sulforaphane that protects fibroblasts in vitro against the cytotoxic effects of
electrophiles, peroxides and redox-cycling agents. _Toxicol. Appl. Pharmacol._ 237, 267–280 (2009). Article CAS PubMed Google Scholar * Hetz, C. The unfolded protein response:
controlling cell fate decisions under ER stress and beyond. _Nat. Rev. Mol. Cell Biol._ 13, 89–102 (2012). Article CAS PubMed Google Scholar * Edman, J. C., Ellis, L., Blacher, R. W.,
Roth, R. A. & Rutter, W. J. Sequence of protein disulphide isomerase and implications of its relationship to thioredoxin. _Nature_ 317, 267–270 (1985). Article CAS PubMed Google
Scholar * Plate, L. et al. Small molecule proteostasis regulators that reprogram the ER to reduce extracellular protein aggregation. _eLife_ 5, e15550 (2016). Article PubMed PubMed
Central Google Scholar * Hoffstrom, B. G. et al. Inhibitors of protein disulfide isomerase suppress apoptosis induced by misfolded proteins. _Nat. Chem. Biol._ 6, 900–906 (2010). Article
CAS PubMed PubMed Central Google Scholar * Jean, S., Cox, S., Nassari, S. & Kiger, A. A. Starvation-induced MTMR13 and RAB21 activity regulates VAMP8 to promote
autophagosome-lysosome fusion. _EMBO Rep._ 16, 297–311 (2015). Article CAS PubMed PubMed Central Google Scholar * Liu, Y. et al. A novel mechanism for NF-κB activation via IκB
aggregation: Implications for hepatic Mallory-Denk-body induced inflammation. _Mol. Cell. Proteomics_ 19, 1968–1986 (2020). Article CAS PubMed PubMed Central Google Scholar * Yang, P.
et al. G3BP1 is a tunable switch that triggers phase separation to assemble stress granules. _Cell_ 181, 325–345 (2020). Article CAS PubMed PubMed Central Google Scholar *
Dinkova-Kostova, A. T. et al. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. _Proc. Natl Acad. Sci. USA_
102, 4584–4589 (2005). Article CAS PubMed PubMed Central Google Scholar * Liu, Y. et al. Sulforaphane enhances proteasomal and autophagic activities in mice and is a potential
therapeutic reagent for Huntington’s disease. _J. Neurochem._ 129, 539–547 (2014). Article CAS PubMed PubMed Central Google Scholar * Gromer, S., Arscott, L. D., Williams, C. H.,
Schirmer, R. H. & Becker, K. Human placenta thioredoxin reductase. Isolation of the selenoenzyme, steady state kinetics, and inhibition by therapeutic gold compounds. _J. Biol. Chem._
273, 20096–20101 (1998). Article CAS PubMed Google Scholar * Zhang, J. et al. Systematic identification of anticancer drug targets reveals a nucleus-to-mitochondria ROS-sensing pathway.
_Cell_ 186, 2361–2379 (2023). Article CAS PubMed PubMed Central Google Scholar * Mao, X. et al. Novel multi-targeted ErbB family inhibitor afatinib blocks EGF-induced signaling and
induces apoptosis in neuroblastoma. _Oncotarget_ 8, 1555–1568 (2017). Article PubMed Google Scholar * Huang, F. et al. Repurposing of ibrutinib and quizartinib as potent inhibitors of
necroptosis. _Commun. Biol._ 6, 972 (2023). Article CAS PubMed PubMed Central Google Scholar * Kapoor, I. et al. Resistance to BTK inhibition by ibrutinib can be overcome by preventing
FOXO3a nuclear export and PI3K/AKT activation in B-cell lymphoid malignancies. _Cell Death Dis._ 10, 924 (2019). Article CAS PubMed PubMed Central Google Scholar * Lanning, B. R. et al.
A road map to evaluate the proteome-wide selectivity of covalent kinase inhibitors. _Nat. Chem. Biol._ 10, 760–767 (2014). Article CAS PubMed PubMed Central Google Scholar * Trendel,
J. et al. The human RNA-binding proteome and its dynamics during translational arrest. _Cell_ 176, 391–403 (2019). Article CAS PubMed Google Scholar * Wang, X. et al. Selective depletion
of mutant p53 by cancer chemopreventive isothiocyanates and their structure-activity relationships. _J. Med. Chem._ 54, 809–816 (2011). Article CAS PubMed PubMed Central Google Scholar
* Yasuda, S. et al. Stress- and ubiquitylation-dependent phase separation of the proteasome. _Nature_ 578, 296–300 (2020). Article CAS PubMed Google Scholar * Gwon, Y. et al.
Ubiquitination of G3BP1 mediates stress granule disassembly in a context-specific manner. _Science_ 372, eabf6548 (2021). Article CAS PubMed PubMed Central Google Scholar * Johnston, J.
A., Ward, C. L. & Kopito, R. R. Aggresomes: a cellular response to misfolded proteins. _J. Cell Biol._ 143, 1883–1898 (1998). Article CAS PubMed PubMed Central Google Scholar *
Zhang, C. et al. Autophagic sequestration of SQSTM1 disrupts the aggresome formation of ubiquitinated proteins during proteasome inhibition. _Cell Death Dis._ 13, 615 (2022). Article CAS
PubMed PubMed Central Google Scholar * Yu, C. et al. Afatinib combined with anti-PD1 enhances immunotherapy of hepatocellular carcinoma via ERBB2/STAT3/PD-L1 signaling. _Front. Oncol._
13, 1198118 (2023). Article CAS PubMed PubMed Central Google Scholar * Li, D. et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models.
_Oncogene_ 27, 4702–4711 (2008). Article CAS PubMed PubMed Central Google Scholar * Bar-Peled, L. et al. Chemical proteomics identifies druggable vulnerabilities in a genetically
defined cancer. _Cell_ 171, 696–709 (2017). Article CAS PubMed PubMed Central Google Scholar * Abegg, D. et al. Proteome-wide profiling of targets of cysteine reactive small molecules
by using ethynyl benziodoxolone reagents. _Angew. Chem._ 127, 11002–11007 (2015). Article Google Scholar * Grossman, E. A. et al. Covalent ligand discovery against druggable hotspots
targeted by anti-cancer natural products. _Cell Chem. Biol._ 24, 1368–1376 (2017). Article CAS PubMed PubMed Central Google Scholar * Wang, C., Weerapana, E., Blewett, M. M. &
Cravatt, B. F. A chemoproteomic platform to quantitatively map targets of lipid-derived electrophiles. _Nat. Methods_ 11, 79–85 (2014). Article PubMed Google Scholar * Henning, N. J. et
al. Deubiquitinase-targeting chimeras for targeted protein stabilization. _Nat. Chem. Biol._ 18, 412–421 (2022). Article CAS PubMed PubMed Central Google Scholar * Lazear, M. R. et al.
Proteomic discovery of chemical probes that perturb protein complexes in human cells. _Mol. Cell_ 83, 1725–1742 (2023). Article CAS PubMed Google Scholar * Kathman, S. G. et al.
Remodeling oncogenic transcriptomes by small molecules targeting NONO. _Nat. Chem. Biol._ 19, 825–836 (2023). Article CAS PubMed PubMed Central Google Scholar * Sterling, J., Baker, J.
R., McCluskey, A. & Munoz, L. Systematic literature review reveals suboptimal use of chemical probes in cell-based biomedical research. _Nat. Commun._ 14, 3228 (2023). Article CAS
PubMed PubMed Central Google Scholar * Li, H. et al. Assigning functionality to cysteines by base editing of cancer dependency genes. _Nat. Chem. Biol._ 19, 1320–1330 (2023). Article CAS
PubMed PubMed Central Google Scholar * Chen, Y. et al. Direct mapping of ligandable tyrosines and lysines in cells with chiral sulfonyl fluoride probes. _Nat. Chem._ 15, 1616–1625
(2023). Article CAS PubMed Google Scholar * Resnick, E. et al. Rapid covalent-probe discovery by electrophile-fragment screening. _J. Am. Chem. Soc._ 141, 8951–8968 (2019). Article CAS
PubMed PubMed Central Google Scholar * Dubiella, C. et al. Sulfopin is a covalent inhibitor of Pin1 that blocks Myc-driven tumors in vivo. _Nat. Chem. Biol._ 17, 954–963 (2021). Article
CAS PubMed PubMed Central Google Scholar * Branon, T. C. et al. Efficient proximity labeling in living cells and organisms with TurboID. _Nat. Biotechnol._ 36, 880–887 (2018). Article
CAS PubMed PubMed Central Google Scholar * Liu, H. & Naismith, J. H. An efficient one-step site-directed deletion, insertion, single and multiple-site plasmid mutagenesis protocol.
_BMC Biotechnol._ 8, 91 (2008). Article PubMed PubMed Central Google Scholar * Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis.
_Nat. Methods_ 9, 671–675 (2012). Article CAS PubMed PubMed Central Google Scholar * Chen, E. Y. et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool.
_BMC Bioinformatics_ 14, 128 (2013). Article PubMed PubMed Central Google Scholar * Xie, Z. et al. Gene set knowledge discovery with Enrichr. _Curr. Protoc._ 1, e90 (2021). Article CAS
PubMed PubMed Central Google Scholar * Teo, G. C., Polasky, D. A., Yu, F. & Nesvizhskii, A. I. Fast deisotoping algorithm and its implementation in the msfragger search engine. _J.
Proteome Res._ 20, 498–505 (2021). Article CAS PubMed Google Scholar * Kong, A. T., Leprevost, F. V., Avtonomov, D. M., Mellacheruvu, D. & Nesvizhskii, A. I. MSFragger: ultrafast and
comprehensive peptide identification in mass spectrometry-based proteomics. _Nat. Methods_ 14, 513–520 (2017). Article CAS PubMed PubMed Central Google Scholar * Tyanova, S. et al. The
Perseus computational platform for comprehensive analysis of (prote)omics data. _Nat. Methods_ 13, 731–740 (2016). Article CAS PubMed Google Scholar * Perez-Riverol, Y. et al. The PRIDE
database resources in 2022: a hub for mass spectrometry-based proteomics evidences. _Nucleic Acids Res._ 50, D543–D552 (2022). Article CAS PubMed Google Scholar Download references
ACKNOWLEDGEMENTS This study was supported by a Beckman Young Investigator Award (to K.M.B.), DOD-Advanced Research Projects Agency (D19AP00041 to K.M.B.), National Institutes of Health (DP2
GM146246-02 to K.M.B.), Packard Fellowship (2020-71388 to K.M.B.) and NIGMS UCLA Chemistry Biology Interface (T32GM136614 to A.R.J.). We thank I. Zohn (Center for Genetic Medicine Research,
Children's National Research and Innovation) for providing the HECTD1 plasmid. We additionally thank all members of the Backus Lab for helpful suggestions. We additionally thank Dr. S.
Neumann (Martin Lab, UCLA) and the UCLA Broad Stem Cell Resource Center Microscopy Core for assistance with microscopy. Figure 1a was made using Biorender.com. AUTHOR INFORMATION Author
notes * These authors contributed equally: Ashley R. Julio, Flowreen Shikwana. AUTHORS AND AFFILIATIONS * Department of Biological Chemistry, David Geffen School of Medicine, UCLA, Los
Angeles, CA, USA Ashley R. Julio, Flowreen Shikwana, Cindy Truong, Nikolas R. Burton, Emil R. Dominguez III, Alexandra C. Turmon, Jian Cao & Keriann M. Backus * Department of Chemistry
and Biochemistry, UCLA, Los Angeles, CA, USA Ashley R. Julio, Flowreen Shikwana, Nikolas R. Burton, Alexandra C. Turmon & Keriann M. Backus * DOE Institute for Genomics and Proteomics,
UCLA, Los Angeles, CA, USA Keriann M. Backus * Jonsson Comprehensive Cancer Center, David Geffen School of Medicine, UCLA, Los Angeles, CA, USA Keriann M. Backus * Eli and Edythe Broad
Center of Regenerative Medicine and Stem Cell Research, David Geffen School of Medicine, UCLA, Los Angeles, CA, USA Keriann M. Backus Authors * Ashley R. Julio View author publications You
can also search for this author inPubMed Google Scholar * Flowreen Shikwana View author publications You can also search for this author inPubMed Google Scholar * Cindy Truong View author
publications You can also search for this author inPubMed Google Scholar * Nikolas R. Burton View author publications You can also search for this author inPubMed Google Scholar * Emil R.
Dominguez III View author publications You can also search for this author inPubMed Google Scholar * Alexandra C. Turmon View author publications You can also search for this author inPubMed
Google Scholar * Jian Cao View author publications You can also search for this author inPubMed Google Scholar * Keriann M. Backus View author publications You can also search for this
author inPubMed Google Scholar CONTRIBUTIONS A.R.J., F.S. and K.M.B. conceived of the project. A.R.J., F.S. and K.M.B. designed experiments. A.R.J. and F.S. performed, collected and analyzed
data for all biochemical experiments, including bulk proteomics and protein-directed ABPP, western blot analyses, AP–MS proteomics and cloning of plasmids. F.S. performed, collected and
analyzed data for all isoTOP–ABPP experiments with the help of C.T. and performed all knockdown experiments. A.R.J. performed, collected and analyzed all data for all imaging experiments.
A.C.T. performed, collected and analyzed data for IncuCyte Live-Cell Imaging. N.R.B., E.D. and J.C. performed syntheses of JC19 and all analogs. C.T. developed and implemented software.
A.R.J. and F.S. contributed to the to the preparation and design of figures. A.R.J., F.S. and K.M.B. wrote the manuscript with assistance from all authors. CORRESPONDING AUTHOR
Correspondence to Keriann M. Backus. ETHICS DECLARATIONS COMPETING INTERESTS K.M.B. is a member of the advisory board at Matchpoint Therapeutics. The remaining authors declare no competing
interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Chemical Biology_ thanks Xiaoyu Zhang and the other, anonymous, reviewers for their contribution to the peer review of this work.
ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. EXTENDED DATA EXTENDED DATA
FIG. 1 COMPOUND STRUCTURES. Shown are the structures of all compounds used for the study, ordered in the order of appearance in the text. EXTENDED DATA FIG. 2 JC19 ANALOGS HAVE VARYING
DEGREES OF NSP14 DEPLETION EFFICIENCY. (A) Chemical structures of JC19 analogs. Cysteine-reactive chloroacetamide warhead is colored pink, cysteine-reactive acrylamide warhead is colored
blue and sulfonyl fluoride warhead is colored orange. (B–F) HEK293T cells transiently expressing nsp14-FLAG were treated with the indicated concentrations of JC18 (B), NB92 (C), NB177 (D),
NB179 (E) or JC17 (F) for 0.5 h, and the soluble lysate fraction assayed by immunoblot. (G) HEK293T cells transiently expressing nsp14-FLAG were treated with 100 µm of the indicated
compounds for the indicated times, and the soluble lysate fraction was assayed by immunoblot. All western blot data are representative of 2 independent experiments. Source data EXTENDED DATA
FIG. 3 JC19 INDUCES LINKAGE OF PREDOMINANTLY K48-LINKED POLYUBIQUITIN CHAINS ON NSP14. (A) HEK293T cells transiently expressing nsp14-FLAG were treated with the indicated compounds, lysed
and immunoprecipitated on FLAG resin. (B) HEK293T cells transiently expressing nsp14-FLAG or the indicated ubiquitin construct were treated with DMSO or JC19 (50 µm, 1 h), lysed and
immunoprecipitated on FLAG resin. Immunoblot analysis was used to probe FLAG and ubiquitin expression for the immunoprecipitated fraction. Ubiquitin constructs used include WT (wild-type),
K48R, K63R, K48 (all ubiquitin lysines mutated to arginine except K48) and K63 (all ubiquitin lysines mutated to arginine except K63). (C) Same immunoblot as shown in B, including inputs,
loading control and full-length ubiquitin blot to depict polyubiquitin smears. Polyubiquitylation is primarily due to K48-linked ubiquitin, as indicated by the attenuation (K48R) or
abrogation (K63) of the high-molecular-weight polyubiquitin smear when using ubiquitin constructs containing a mutant K48. (D) ‘Heavy’ SILAC HEK293T cells transiently expressing nsp14-FLAG
were treated with 100 µm JC19 for 1 h (_n_ = 3), while ‘light’ SILAC HEK293T cells transiently expressing nsp14-FLAG were treated with an equal volume of DMSO for 1 h (_n_ = 3). Lysates were
combined, immunoprecipitated on FLAG resin, proteolyzed and subjected to LC–MS/MS analysis. Mean log2(H/L) ratios of detected peptides are depicted, highlighting all nsp14 peptides and
ubiquitinated nsp14 peptides (GlyGly modified). (E) Workflow for AP–MS experiments. All MS data can be found in Supplementary Table 2. Western blot data are representative of 2 independent
experiments. Source data EXTENDED DATA FIG. 4 COMMON CELL STRESS-SENSING PATHWAYS ARE NOT RESPONSIBLE FOR NSP14 DEPLETION. (A) HEK293T cells transiently expressing nsp14-FLAG were treated
with the indicated concentrations of JC19 (1 h). Immunoblot analysis was used to visualize abundance of nsp14 and induction of NRF2 expression in the soluble lysate fraction (0.3% CHAPS in
PBS) for each condition. (B) HEK293T cells transiently expressing nsp14-FLAG were pretreated with either DMSO, tunicamycin (12 µg/mL, 8 h), thapsigargin (2 µm, 8 h) or rapamycin (1 µm, 8 h),
and then either treated with DMSO or JC19 (50 µm, 1 h). Immunoblot analysis was used to visualize abundance of nsp14 and induction of UPR markers in the soluble lysate fraction (0.3% CHAPS
in PBS) for each condition. (C) HEK293T cells were treated with the indicated compounds, and immunoblot analysis was used to visualize induction of UPR markers (lysed in RIPA). (D–F) HEK293T
cells were treated with vehicle DMSO, thapsigargin (2 µm, 15 h) or AA147 (10 µm, 15 h; _n_ = 3 per group; (D) or DMSO, 10 µm JC19 for 1 h or 10 µm JC19 for 15 h (_n_ = 3 per group; (E) or
DMSO, 50 µm JC19 for 1 h or 50 µm JC19 for 15 h (_n_ = 3 per group; (F) and lysed in RIPA lysis buffer. Bulk proteomics sample preparation and label-free quantification proteomics were used
to measure intensities of proteins and p-values generated for each treatment compared to vehicle using a Student’s unpaired t-test. −log10(p-values) were plotted and significance of BiP
(HSPA5) and PDIA4 highlighted. All MS data can be found in Supplementary Table 2. Panel A is representative of one independent measurement, and panels B and C are representative of three
independent measurements. Source data EXTENDED DATA FIG. 5 PDIA6-HIS PULLDOWN. (A) HEK293T cells transiently expressing PDIA6-His protein were treated with DMSO, NB92 or JC19 for 1 h at 50
µm. Cells were lysed, and each condition was split into 2 tubes, one treated with TCEP and one without TCEP. Samples were then incubated with Ni-NTA resin, washed, eluted and prepared for
LC–MS/MS analysis. Proteins significantly enriched by JC19 in both the +TCEP and −TCEP conditions were identified to be true cross-linked proteins to PDIA6. Proteins enriched by JC19 only in
the −TCEP condition but not +TCEP condition were identified to be linked to PDIA6 by disulfide bond. (B) HEK293T cells transiently expressing PDIA6-His8x were affinity purified under
denaturing conditions either in the absence (black border labels) or presence of TCEP reductant (green border labels). Volcano plot displays comparison between enriched proteins for groups
treated with DMSO versus NB92 (50 µm, 1 h) with black colored dots for proteins showing sensitivity to TCEP (_n_ = 3 for each group). An unpaired Student’s t-test was performed to calculate
p-values. All MS data can be found in Supplementary Table 3. EXTENDED DATA FIG. 6 HIGH-RATIO CYSTEINES AS IDENTIFIED BY ISOTOP–ABPP BELONG TO PROTEINS SPANNING VARIOUS SUBCELLULAR
COMPARTMENTS. Heatmap depicting unlogged MS1 isoTOP–ABPP cysteine ratios for high coverage cysteines belonging to proteins with multiple cysteines identified. Cell compartment annotations
from UniProt have been provided for each cysteine identifier. For generation of data, each compound was used in triplicate (n = 3) at 100 µm for 1 h (exception: 4 h treatment for EN450) in
nsp14-expressing HEK293T cells. MS data can be found in Supplementary Table 4. EXTENDED DATA FIG. 7 DELINEATION OF AGGREGATION AND DEGRADATION PROPERTIES OF JC19, EN450 AND NB001. (A)
HEK293T cells transiently expressing nsp14-FLAG were treated with DMSO, a low dose of JC19 (25 µm for 0.5 h) or a high dose of JC19 (100 µm for 1 h) and subjected to immunoblot analysis.
Cells were lysed in 0.3% CHAPS in PBS to generate the soluble lysate, and after clearance by centrifugation, the insoluble debris was solubilized in 8 M urea in PBS to generate ‘insoluble’
lysate. (B) HEK293T cells transiently expressing nsp14-FLAG were treated with the indicated concentrations of JC19 or NB001 for 0.5 h or EN450 for 3 h, and the insoluble lysate was analyzed
by immunoblot. (C) Quantification of nsp14 intensity from replicate western blots as in B. Data are presented as mean ± s.d. (_n_ = 3 per data point). D,E, Nsp14-expressing HEK293T cells
were treated with the indicated concentrations of JC19 for 0.5 h (D) or EN450 for 3 h (E), and were then separated into 3 fractions: ‘soluble’ fraction (cells lysed in 0.3% CHAPS and cleared
by centrifugation), ‘insoluble’ fraction (insoluble debris from CHAPS lysis re-solubilized using 8 M urea) and ‘total’ fraction (cells lysed in 8 M urea with 3× freeze/thaw). (F) HEK293T
cells transiently expressing nsp14-FLAG were treated with the indicated concentrations of JC19 or NB001 for 0.5 h or EN450 for 3 h, and the soluble and insoluble lysates were analyzed by
immunoblot. (G) Quantification of nsp14 intensity from replicate western blots as in F. Data are presented as mean ± s.d. (_n_ = 3 per data point). All western blot data is representative of
three independent measurements. Source data EXTENDED DATA FIG. 8 VARIOUS CYSTEINE-REACTIVE ELECTROPHILES INDUCE STRESS GRANULE FORMATION. (A) U2OS cells stably expressing V5-tagged G3BP1
were treated with DMSO, RA190 (10 µm, 1 h), sulforaphane (50 µm, 1 h), bardoxolone (10 µm, 1 h), ibrutinib (10 µm, 14 h), low afatinib (100 nM for 14 h), high afatinib (10 µm, 1 h) or
auranofin (10 µm, 1 h). Cells were then fixed, permeabilized and subjected to immunofluorescence microscopy. (B) U2OS cells stably expressing V5-tagged G3BP1 were treated with the indicated
concentration of afatinib for 4 h. Cells were then fixed, permeabilized and subjected to immunofluorescence microscopy. (C) Immunofluorescence microscopy of G3BP1 and RANBP2 in response to
DMSO, JC19 (100 µm, 1 h) and EN450 (100 µm, 1 h). (D) Immunofluorescence microscopy of G3BP1 and PSMB2 in response to DMSO and JC19 (100 µm, 1 h). Images were acquired on an LSM880 confocal
microscope at ×63 objective and 2× manual zoom. All scale bars = 10 µm. All images are representative of two independent measurements. EXTENDED DATA FIG. 9 GENETIC KNOCKDOWN OF PDIA3 AND
PDIA6 LEAD TO INCREASES IN AGGREGATED PROTEINS (AGGRESOMES). (A) Immunofluorescence microscopy of HEK293T cells transfected with either a non-targeting control (NTC) siRNA for 48 h, NTC
siRNA for 48 h and treated with MG132 (10 µm, 8 h), PDIA3 siRNA for 48 h or PDIA6 siRNA for 48 h, using p62 as a marker for aggresomes/aggregated proteins. Images were acquired on an LSM880
confocal microscope at ×63 objective and 2× manual zoom. All scale bars = 10 µm. (B) Immunoblot analysis PDIA3 and PDIA6 knockdown efficiency for the cells used in A and Fig. 6e. All data
are representative of three independent measurements. Source data EXTENDED DATA FIG. 10 RECOMMENDATIONS FOR BEST PRACTICES. Suggested steps to facilitate delineation of on- versus off-target
activities of covalent fragments. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figs. 1–30, supporting data of Supplementary Figs. 2–11, 14–16, 21, 23–26 (uncropped and
unprocessed western blots), Supplementary Tables 6–10 and Supplementary Note. REPORTING SUMMARY SUPPLEMENTARY TABLE 1 MS datasets corresponding to Fig. 1. SUPPLEMENTARY TABLE 2 MS datasets
corresponding to Fig. 3. SUPPLEMENTARY TABLE 3 MS datasets corresponding to Fig. 4. SUPPLEMENTARY TABLE 4 MS datasets corresponding to Fig. 5. SUPPLEMENTARY TABLE 5 MS datasets corresponding
to Fig. 6. SUPPLEMENTARY TABLE 11 Annotation of all files uploaded to the PRIDE. SUPPLEMENTARY DATA 1 Raw data corresponding to data shown in Supplementary Fig. 2b. SUPPLEMENTARY DATA 2 Raw
data corresponding to data shown in Supplementary Fig. 11b. SOURCE DATA SOURCE DATA FIG. 1 Uncropped blots. SOURCE DATA FIG. 2 Uncropped blots. SOURCE DATA FIG. 2 Raw data corresponding to
data shown in Fig. 2e. SOURCE DATA FIG. 3 Uncropped blots. SOURCE DATA FIG. 4 Uncropped blots. SOURCE DATA FIG. 4 Raw data corresponding to data shown in Fig. 4k. SOURCE DATA FIG. 5
Uncropped blots. SOURCE DATA FIG. 5 Raw data corresponding to data shown in Fig. 5h. SOURCE DATA FIG. 6 Raw data corresponding to data shown in Fig. 6c. SOURCE DATA EXTENDED DATA FIG. 2
Uncropped blots. SOURCE DATA EXTENDED DATA FIG. 3 Uncropped blots. SOURCE DATA EXTENDED DATA FIG. 4 Uncropped blots. SOURCE DATA EXTENDED DATA FIG. 7 Source data. SOURCE DATA EXTENDED DATA
FIG. 7 Uncropped blots. SOURCE DATA EXTENDED DATA FIG. 9 Uncropped blots. RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to
this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the
terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Julio, A.R., Shikwana, F., Truong, C. _et al._ Delineating
cysteine-reactive compound modulation of cellular proteostasis processes. _Nat Chem Biol_ 21, 693–705 (2025). https://doi.org/10.1038/s41589-024-01760-9 Download citation * Received: 16
November 2023 * Accepted: 23 September 2024 * Published: 24 October 2024 * Issue Date: May 2025 * DOI: https://doi.org/10.1038/s41589-024-01760-9 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative