Play all audios:
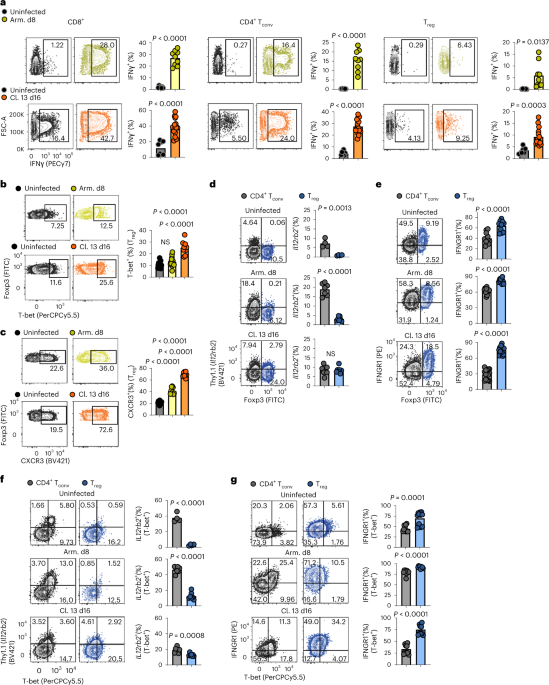
ABSTRACT Regulatory T (Treg) cells are an immunosuppressive population that are required to maintain peripheral tolerance and prevent tissue damage from immunopathology, via
anti-inflammatory cytokines, inhibitor receptors and metabolic disruption. Here we show that Treg cells acquire an effector-like state, yet remain stable and functional, when exposed to
interferon gamma (IFNγ) during infection with lymphocytic choriomeningitis and influenza A virus. Treg cell-restricted deletion of the IFNγ receptor (encoded by _Ifngr1_), but not the
interleukin 12 (IL12) receptor (encoded by _Il12rb2_), prevented TH1-like polarization (decreased expression of T-bet, CXC motif chemokine receptor 3 and IFNγ) and promoted TH2-like
polarization (increased expression of GATA-3, CCR4 and IL4). TH1-like Treg cells limited CD8+ T cell effector function, proliferation and memory formation during acute and chronic infection.
These findings provide fundamental insights into how Treg cells sense inflammatory cues from the environment (such as IFNγ) during viral infection to provide guidance to the effector immune
response. This regulatory circuit prevents prolonged immunoinflammatory responses and shapes the quality and quantity of the memory T cell response. Access through your institution Buy or
subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get
Nature+, our best-value online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and online access $209.00 per year only
$17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout
ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS PD-L1–PD-1 INTERACTIONS LIMIT
EFFECTOR REGULATORY T CELL POPULATIONS AT HOMEOSTASIS AND DURING INFECTION Article 18 April 2022 TRANSCRIPTION TIPPING POINTS FOR T FOLLICULAR HELPER CELL AND T-HELPER 1 CELL FATE
COMMITMENT Article Open access 30 September 2020 AIOLOS REPRESSES CD4+ T CELL CYTOTOXIC PROGRAMMING VIA RECIPROCAL REGULATION OF TFH TRANSCRIPTION FACTORS AND IL-2 SENSITIVITY Article Open
access 24 March 2023 DATA AVAILABILITY The RNA sequencing data that support the findings of this study have been deposited in the GEO under the accession code GSE223210. All other data are
present in the article and Supplementary files or from the corresponding author upon reasonable request. Source data files are present. Source data are provided with this paper. REFERENCES *
Veiga-Parga, T., Sehrawat, S. & Rouse, B. T. Role of regulatory T cells during virus infection. _Immunol. Rev._ 255, 182–196 (2013). Article PubMed PubMed Central Google Scholar *
Punkosdy, G. A. et al. Regulatory T-cell expansion during chronic viral infection is dependent on endogenous retroviral superantigens. _Proc. Natl Acad. Sci. USA_ 108, 3677–3682 (2011).
Article CAS PubMed PubMed Central Google Scholar * Cabrera, R. et al. An immunomodulatory role for CD4+CD25+ regulatory T lymphocytes in hepatitis C virus infection. _Hepatology_ 40,
1062–1071 (2004). Article CAS PubMed Google Scholar * Betts, R. J., Ho, A. W. S. & Kemeny, D. M. Partial depletion of natural CD4+CD25+ regulatory T cells with anti-CD25 antibody
does not alter the course of acute influenza A virus infection. _PLoS ONE_ 6, e27849 (2011). Article CAS PubMed PubMed Central Google Scholar * Penaloza-MacMaster, P. et al. Interplay
between regulatory T cells and PD-1 in modulating T cell exhaustion and viral control during chronic LCMV infection. _J. Exp. Med._ 211, 1905–1918 (2014). Article CAS PubMed PubMed
Central Google Scholar * Saravia, J., Chapman, N. M. & Chi, H. Helper T cell differentiation. _Cell Mol. Immunol._ 16, 634–643 (2019). Article CAS PubMed PubMed Central Google
Scholar * Zhu, J. et al. The transcription factor T-bet is induced by multiple pathways and prevents an endogenous Th2 cell program during Th1 cell responses. _Immunity_ 37, 660–673 (2012).
Article CAS PubMed PubMed Central Google Scholar * Lazarevic, V. et al. T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORγt. _Nat.
Immunol._ 12, 96–104 (2011). Article CAS PubMed Google Scholar * Verma, N. D. et al. Interleukin-12 (IL-12p70) promotes induction of highly potent Th1-like CD4+CD25+ T regulatory cells
that inhibit allograft rejection in unmodified recipients. _Front. Immunol._ 5, 190 (2014). Article PubMed PubMed Central Google Scholar * Cousens, L. P., Orange, J. S., Su, H. C. &
Biron, C. A. Interferon-alpha/beta inhibition of interleukin 12 and interferon-gamma production in vitro and endogenously during viral infection. _Proc. Natl Acad. Sci. USA_ 94, 634–639
(1997). Article CAS PubMed PubMed Central Google Scholar * Byrnes, A. A. et al. Type I interferons and IL-12: convergence and cross-regulation among mediators of cellular immunity.
_Eur. J. Immunol._ 31, 2026–2034 (2001). Article CAS PubMed Google Scholar * Levine, A. G. et al. Stability and function of regulatory T cells expressing the transcription factor T-bet.
_Nature_ 546, 421–425 (2017). Article CAS PubMed PubMed Central Google Scholar * Overacre-Delgoffe, A. E. et al. Interferon-γ drives treg fragility to promote anti-tumor immunity.
_Cell_ 169, 1130–1141 (2017). Article CAS PubMed PubMed Central Google Scholar * Rubtsov, Y. P. et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental
interfaces. _Immunity_ 28, 546–558 (2008). Article CAS PubMed Google Scholar * Smeltz, R. B., Chen, J., Ehrhardt, R. & Shevach, E. M. Role of IFN-gamma in Th1 differentiation:
IFN-gamma regulates IL-18R alpha expression by preventing the negative effects of IL-4 and by inducing/maintaining IL-12 receptor beta 2 expression. _J. Immunol._ 168, 6165–6172 (2002).
Article CAS PubMed Google Scholar * Naka, T. et al. SOCS-1/SSI-1-deficient NKT cells participate in severe hepatitis through dysregulated cross-talk inhibition of IFN-gamma and IL-4
signaling in vivo. _Immunity_ 14, 535–545 (2001). Article CAS PubMed Google Scholar * Lee, S. H. et al. Identifying the initiating events of anti-Listeria responses using mice with
conditional loss of IFN-γ receptor subunit 1 (IFNGR1). _J. Immunol._ 191, 4223–4234 (2013). Article CAS PubMed Google Scholar * Yang, X., Bam, M., Becker, W., Nagarkatti, P. S. &
Nagarkatti, M. Long noncoding RNA AW112010 promotes the differentiation of inflammatory T cells by suppressing IL-10 expression through histone demethylation. _J. Immunol._ 205, 987–993
(2020). Article CAS PubMed Google Scholar * Marshall, H. D. et al. Differential expression of Ly6C and T-bet distinguish effector and memory Th1 CD4+ cell properties during viral
infection. _Immunity_ 35, 633–646 (2011). Article CAS PubMed PubMed Central Google Scholar * Heninger, A.-K. et al. IL-7 abrogates suppressive activity of human CD4+CD25+FOXP3+
regulatory T cells and allows expansion of alloreactive and autoreactive T cells. _J. Immunol._ 189, 5649–5658 (2012). Article CAS PubMed Google Scholar * Wei, G. et al. Genome-wide
analyses of transcription factor GATA3-mediated gene regulation in distinct T cell types. _Immunity_ 35, 299–311 (2011). Article CAS PubMed PubMed Central Google Scholar * Schiering, C.
et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. _Nature_ 513, 564–568 (2014). Article CAS PubMed PubMed Central Google Scholar * Stubbington, M. J. et
al. An atlas of mouse CD4+ T cell transcriptomes. _Biol. Direct_ 10, 14 (2015). Article PubMed PubMed Central Google Scholar * Briggs, S. F. & Reijo Pera, R. A. X chromosome
inactivation: recent advances and a look forward. _Curr. Opin. Genet. Dev._ 28, 78–82 (2014). Article CAS PubMed PubMed Central Google Scholar * Rubtsov, Y. P. et al. Stability of the
regulatory T cell lineage in vivo. _Science_ 329, 1667–1671 (2010). Article CAS PubMed PubMed Central Google Scholar * Baazim, H. et al. CD8+ T cells induce cachexia during chronic
viral infection. _Nat. Immunol._ 20, 701–710 (2019). Article CAS PubMed PubMed Central Google Scholar * Zinkernagel, R. M. et al. T cell-mediated hepatitis in mice infected with
lymphocytic choriomeningitis virus. Liver cell destruction by H-2 class I-restricted virus-specific cytotoxic T cells as a physiological correlate of the 51Cr-release assay? _J. Exp. Med._
164, 1075–1092 (1986). Article CAS PubMed Google Scholar * Zajac, A. J. et al. Viral immune evasion due to persistence of activated T cells without effector function. _J. Exp. Med._ 188,
2205–2213 (1998). Article CAS PubMed PubMed Central Google Scholar * Wherry, E. J., Blattman, J. N., Murali-Krishna, K., van der Most, R. & Ahmed, R. Viral persistence alters CD8
T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. _J. Virol._ 77, 4911–4927 (2003). Article CAS PubMed PubMed Central Google Scholar
* Liu, C. et al. Treg cells promote the SREBP1-dependent metabolic fitness of tumor-promoting macrophages via repression of CD8+ T cell-derived interferon-γ. _Immunity_ 51, 381–397 (2019).
Article CAS PubMed PubMed Central Google Scholar * Ferreira, C. et al. Type 1 Treg cells promote the generation of CD8+ tissue-resident memory T cells. _Nat. Immunol._ 21, 766–776
(2020). Article CAS PubMed Google Scholar * Pace, L. et al. Regulatory T cells increase the avidity of primary CD8+ T cell responses and promote memory. _Science_ 338, 532–536 (2012).
Article CAS PubMed Google Scholar * Bhattacharyya, M. & Penaloza-MacMaster, P. T regulatory cells are critical for the maintenance, anamnestic expansion and protection elicited by
vaccine-induced CD8 T cells. _Immunology_ 151, 340–348 (2017). Article CAS PubMed PubMed Central Google Scholar * Kaech, S. M., Hemby, S., Kersh, E. & Ahmed, R. Molecular and
functional profiling of memory CD8 T cell differentiation. _Cell_ 111, 837–851 (2002). Article CAS PubMed Google Scholar * Franckaert, D. et al. Promiscuous Foxp3-cre activity reveals a
differential requirement for CD28 in Foxp3+ and Foxp3− T cells. _Immunol. Cell Biol._ 93, 417–423 (2015). Article CAS PubMed Google Scholar * Bittner-Eddy, P. D., Fischer, L. A. &
Costalonga, M. Cre-loxP reporter mouse reveals stochastic activity of the Foxp3 promoter. _Front. Immunol._ 10, 2228 (2019). Article CAS PubMed PubMed Central Google Scholar * Martin,
M. D. & Badovinac, V. P. Defining memory CD8 T cell. _Front. Immunol._ 9, 2692 (2018). Article PubMed PubMed Central Google Scholar * Littringer, K. et al. Common features of
regulatory T cell specialization during Th1 responses. _Front. Immunol._ 9, 1344 (2018). Article PubMed PubMed Central Google Scholar * Lynch, E. A., Heijens, C. A. W., Horst, N. F.,
Center, D. M. & Cruikshank, W. W. Cutting edge: IL-16/CD4 preferentially induces Th1 cell migration: requirement of CCR5. _J. Immunol._ 171, 4965–4968 (2003). Article CAS PubMed
Google Scholar * Cipolla, E. M. et al. Heterotypic influenza infections mitigate susceptibility to secondary bacterial infection. _J. Immunol._ 209, 760–771 (2022). Article CAS PubMed
Google Scholar * Koch, M. A. et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. _Nat. Immunol._ 10, 595–602 (2009).
Article CAS PubMed PubMed Central Google Scholar * McFadden, C. et al. Preferential migration of T regulatory cells induced by IL-16. _J. Immunol._ 179, 6439–6445 (2007). Article CAS
PubMed Google Scholar * Oxenius, A., Karrer, U., Zinkernagel, R. M. & Hengartner, H. IL-12 is not required for induction of type 1 cytokine responses in viral infections. _J. Immunol._
162, 965–973 (1999). Article CAS PubMed Google Scholar * Ashour, D. et al. IL-12 from endogenous cDC1, and not vaccine DC, is required for Th1 induction. _JCI Insight_ 5, e135143
(2020). Article PubMed PubMed Central Google Scholar * Cao, X. et al. Interleukin 12 stimulates IFN-gamma-mediated inhibition of tumor-induced regulatory T-cell proliferation and
enhances tumor clearance. _Cancer Res._ 69, 8700–8709 (2009). Article CAS PubMed PubMed Central Google Scholar * Solouki, S. et al. TCR signal strength and antigen affinity regulate
CD8+ memory T cells. _J. Immunol._ 205, 1217–1227 (2020). Article CAS PubMed Google Scholar * Iborra, S. et al. Optimal generation of tissue-resident but not circulating memory T cells
during viral infection requires crosspriming by DNGR-1+ dendritic cells. _Immunity_ 45, 847–860 (2016). Article CAS PubMed PubMed Central Google Scholar * Slütter, B., Pewe, L. L.,
Kaech, S. M. & Harty, J. T. Lung airway-surveilling CXCR3(hi) memory CD8(+) T cells are critical for protection against influenza A virus. _Immunity_ 39, 939–948 (2013). Article PubMed
Google Scholar * Ndure, J. & Flanagan, K. L. Targeting regulatory T cells to improve vaccine immunogenicity in early life. _Front. Microbiol._ 5, 477 (2014). Article PubMed PubMed
Central Google Scholar * Safar, H. A., Mustafa, A. S., Amoudy, H. A. & El-Hashim, A. The effect of adjuvants and delivery systems on Th1, Th2, Th17 and Treg cytokine responses in mice
immunized with _Mycobacterium tuberculosis_-specific proteins. _PLoS ONE_ 15, e0228381 (2020). Article CAS PubMed PubMed Central Google Scholar * Welsh, R. M. & Seedhom, M. O.
Lymphocytic choriomeningitis virus (LCMV): propagation, quantitation, and storage. _Curr. Protoc. Microbiol._ 15, 15A.1 (2008). Google Scholar * Robinson, K. M. et al. Novel protective
mechanism for interleukin-33 at the mucosal barrier during influenza-associated bacterial superinfection. _Mucosal Immunol._ 11, 199–208 (2018). Article CAS PubMed Google Scholar *
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. _Nat. Neurosci._ 13, 133–140 (2010). Article CAS PubMed Google Scholar
* Shen, F. W. et al. Cloning of Ly-5 cDNA. _Proc. Natl Acad. Sci. USA_ 82, 7360–7363 (1985). Article CAS PubMed PubMed Central Google Scholar * Mombaerts, P. et al. RAG-1-deficient
mice have no mature B and T lymphocytes. _Cell_ 68, 869–877 (1992). Article CAS PubMed Google Scholar * Snell, G. D., Cherry, M., McKenzie, I. F. & Bailey, D. W. Ly-4, a new locus
determining a lymphocyte cell-surface alloantigen in mice. _Proc. Natl. Acad. Sci. USA_ 70, 1108–1111 (1973). * Liu, P., Jenkins, N. A. & Copeland, N. G. A highly efficient
recombineering-based method for generating conditional knockout mutations. _Genome Res._ 13, 476–484 (2003). Article CAS PubMed PubMed Central Google Scholar * Liu, C. et al.
Neuropilin-1 is a T cell memory checkpoint limiting long-term antitumor immunity. _Nat. Immunol._ 21, 1010–1021 (2020). Article CAS PubMed PubMed Central Google Scholar * Roederer, M.,
Nozzi, J. L. & Nason, M. C. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. _Cytometry A_ 79, 167–174 (2011). Article PubMed PubMed Central Google
Scholar * Turnis, M. E. et al. Interleukin-35 limits anti-tumor immunity. _Immunity_ 44, 316–329 (2016). Article CAS PubMed PubMed Central Google Scholar * Schreiber, R. D. Measurement
of mouse and human interferon gamma. _Curr. Protoc. Immunol._ 6, 6.8 (2001). Google Scholar * Buettner, F. et al. Computational analysis of cell-to-cell heterogeneity in single-cell
RNA-sequencing data reveals hidden subpopulations of cells. _Nat. Biotechnol._ 33, 155–160 (2015). Article CAS PubMed Google Scholar * He, Z., Brazovskaja, A., Ebert, S., Camp, J. G.
& Treutlein, B. CSS: cluster similarity spectrum integration of single-cell genomics data. _Genome Biol._ 21, 224 (2020). Article PubMed PubMed Central Google Scholar * Korotkevich,
G. et al. Fast gene set enrichment analysis. Preprint at _bioRxiv_ https://doi.org/10.1101/060012 (2019). * Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach
for interpreting genome-wide expression profiles. _Proc. Natl Acad. Sci. USA_ 102, 15545–15550 (2005). Article CAS PubMed PubMed Central Google Scholar * Yu, G., Wang, L.-G., Han, Y.
& He, Q.-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. _OMICS_ 16, 284–287 (2012). Article CAS PubMed PubMed Central Google Scholar * Wu, T.
et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. _Innovation_ 2, 100141 (2021). CAS PubMed PubMed Central Google Scholar * Gu, Z., Eils, R. &
Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. _Bioinformatics_ 32, 2847–2849 (2016). Article CAS PubMed Google Scholar Download
references ACKNOWLEDGEMENTS We thank everyone in the Vignali Lab (Vignali-lab.com; @Vignali_Lab) for all their constructive comments and advice. We thank R. Ahmed (Emory University) for
providing parental LCMV viral stocks and protocols, S. Kaech (Yale University) for providing LM-GP33 stocks and protocols, L. D’Cruz and A. Piccirillo (University of Pittsburgh) for
providing LM-OVA stocks and protocols, S. Canna, E. Rapp, P. Tsoukas and H. Nieves-Rosado (University of Pittsburgh) for growing up LCMV viral stocks, C. Workman for intravenous injections,
E. Brunazzi for maintenance of mouse colonies, R. Dadey for helping with flow cytometry staining on LCMV-infected samples, C. Liu for aiding in flow cytometry panel design, C. Cardello for
conducting libraries for scRNA-seq, H. Yano for constructing SPICE plots, D. Normolle for calculating statistics for the weight loss curves, L. Andrews for weighing mice and conducting
intravenous injections, L. Rigatti for scoring livers, the University of Pittsburgh Biospecimen Core for histology, the University of Pittsburgh Innovative Technologies Development Core for
developing new mouse strains, the University of Pittsburgh Center for Research Computing (HTC cluster) for next-generation sequencing and the University of Pittsburgh Flow Core for FACS and
flow cytometry. This work was supported by the National Institutes of Health (F32 CA247004-01 and T32 CA082084 to A.M.G.D.; P01 AI108545, R35 CA263850 and R01 CA203689 to D.A.A.V.; R01
DK130897, R21 CA259636, P30 DK120531 and P30 CA047904 to M.M.; R01 HL107380 to J.F.A.; and R01 CA206517 and R01 AI138504 to L.P.K.). This work benefited from a SPECIAL ORDER BD LSR FORTESSA
(funded by NIH 1S10 OD011925-01) used in the UPSOM Unified Flow Core and the University of Pittsburgh Center for Research Computing (funded by NIH S10OD028483). AUTHOR INFORMATION AUTHORS
AND AFFILIATIONS * Department of Immunology, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA Angela M. Gocher-Demske, Jian Cui, Andrea L. Szymczak-Workman, Kate M. Vignali,
Julianna N. Latini, Gwen P. Pieklo, Jesse C. Kimball, Lyndsay Avery, Lee Hedden, Marlies Meisel, Lawrence P. Kane, Creg J. Workman & Dario A. A. Vignali * Tumor Microenvironment Center,
University of Pittsburgh Cancer Institute, Pittsburgh, PA, USA Angela M. Gocher-Demske, Jian Cui, Kate M. Vignali, Julianna N. Latini, Gwen P. Pieklo, Jesse C. Kimball, Creg J. Workman &
Dario A. A. Vignali * Program in Infectious Diseases and Microbiology, University of Pittsburgh School of Public Health, Pittsburgh, PA, USA Lyndsay Avery, Brydie R. Huckestein & John
F. Alcorn * Department of Pediatrics, UPMC Children’s Hospital of Pittsburgh, Pittsburgh, PA, USA Ellyse M. Cipolla * Program in Microbiology and Immunology, University of Pittsburgh School
of Medicine, Pittsburgh, PA, USA Ellyse M. Cipolla & Brydie R. Huckestein * Cancer Immunology and Immunotherapy Program, UPMC Hillman Cancer Center, Pittsburgh, PA, USA Marlies Meisel,
Lawrence P. Kane & Dario A. A. Vignali Authors * Angela M. Gocher-Demske View author publications You can also search for this author inPubMed Google Scholar * Jian Cui View author
publications You can also search for this author inPubMed Google Scholar * Andrea L. Szymczak-Workman View author publications You can also search for this author inPubMed Google Scholar *
Kate M. Vignali View author publications You can also search for this author inPubMed Google Scholar * Julianna N. Latini View author publications You can also search for this author
inPubMed Google Scholar * Gwen P. Pieklo View author publications You can also search for this author inPubMed Google Scholar * Jesse C. Kimball View author publications You can also search
for this author inPubMed Google Scholar * Lyndsay Avery View author publications You can also search for this author inPubMed Google Scholar * Ellyse M. Cipolla View author publications You
can also search for this author inPubMed Google Scholar * Brydie R. Huckestein View author publications You can also search for this author inPubMed Google Scholar * Lee Hedden View author
publications You can also search for this author inPubMed Google Scholar * Marlies Meisel View author publications You can also search for this author inPubMed Google Scholar * John F.
Alcorn View author publications You can also search for this author inPubMed Google Scholar * Lawrence P. Kane View author publications You can also search for this author inPubMed Google
Scholar * Creg J. Workman View author publications You can also search for this author inPubMed Google Scholar * Dario A. A. Vignali View author publications You can also search for this
author inPubMed Google Scholar CONTRIBUTIONS D.A.A.V. conceived, directed and obtained funding for the project. A.M.G.D., C.J.W. and D.A.A.V. conceptualized, designed and analyzed the
experiments and wrote the manuscript. A.M.G.D. performed the majority of the experiments. J.C. performed the analysis of the scRNA-seq dataset. A.L.S.W., K.M.V. and C.J.W. generated new
mouse strains. J.N.L., G.P.P and J.C.K. helped with processing the tissues before flow cytometry and FACS. J.N.L. helped with LM-GP33 experiments and plaque assays. G.P.P. validated mouse
strains, performed qPCRs and isolated splenic T cells. J.C.K. performed qPCRs and isolated splenic T cells. L.A.A. and L.P.K. helped with the LCMV and LM-GP33 model and L.A.A. conducted some
Arm. experiments. E.M.S. and B.R.H. infected mice with IAV and helped in necroscopy. L.H. and M.M. performed the ALT and AST activity assay. J.F.A. and C.J.W. contributed to experimental
design and interpretation of the data. J.F.A. scored infected lungs. All authors provided feedback and approved the manuscript. CORRESPONDING AUTHOR Correspondence to Dario A. A. Vignali.
ETHICS DECLARATIONS COMPETING INTERESTS D.A.A.V. is a cofounder and stockholder for Novasenta, Potenza, Tizona and Trishula, is a stockholder for Oncorus, Werewolf and Apeximmune, holds
patents licensed and royalties in BMS and Novasenta, serves on a scientific advisory board for Tizona, Werewolf, F-Star, Bicara, Apeximmune and T7/Imreg Bio and is a consultant for BMS,
Incyte, G1 Therapeutics, Inzen Therapeutics, Regeneron and Avidity Partners. All the other authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Immunology_
thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: N. Bernard managed the editorial process and peer review in collaboration with
the rest of the editorial team of this article. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. EXTENDED DATA EXTENDED DATA FIG. 1 IFNΓ AND T-BET EXPRESSION IN TREG CELLS DURING ACUTE AND CHRONIC INFECTION. A, Gating strategy for all flow cytometric
experiments. B-F, _Foxp3_Cre-YFP mice remained uninfected (_n_ = 5) or were infected (_n_ = 6) with Arm. or Cl. 13. B, C, Expression of _Ifng_ (B) and _Tbx21_ (C) mRNA by purified splenic
Treg cells were determined by qPCR after ex vivo stimulation with anti-CD3/anti-CD28 (Arm.) or PMA and Ionomycin (Cl. 13). C, Linear regression of _Tbx21_ and _Ifng_ co_-_expression. D,
_Foxp3_Cre-YFP mice (_n_ = 14) were infected with Arm. and stimulated ex vivo with anti-CD3 and expression of T-bet and IFNγ was measured by flow cytometry. Linear regression of T-bet and
IFNγ co-expression. E, F, _Foxp3_Cre-YFP mice remained uninfected or were infected with Arm. or Cl. 13. Expression of _Ifng_ (E) (_n_ = 15, 5 independent experiments, Uninfected; _n_ = 12,
Arm.; _n_ = 17 Cl. 13) and _Tbx21_ (F) (_n_ = 16, 5 independent experiments, Uninfected; _n_ = 12, Arm.; _n_ = 14, Cl. 13) mRNA by sorted splenic CD4+ Tconv cells and Treg cells, without
stimulation, were determined by qPCR. B-F, Data are presented as mean values and represent biologically independent mice and (B-D and as indicated) or 3 (E, F) or 5 (as indicated)
independent experiments. Statistical significance was determined by multiple unpaired Student’s _t_-test relative to Uninfected (B, C), or by simple linear regression (D) or by a two-way
ANOVA with multiple comparisons and multiple unpaired _t_-tests relative to CD4+ Tconv cells (E, F) (_P_ values indicated when significant); NS, not significant. Source data EXTENDED DATA
FIG. 2 TH1 SIGNATURES IN TREG CELLS AND CD4+ TCONV CELLS DURING ACUTE AND CHRONIC INFECTION. A, Diagram of _Il12rb2_.Thy1.1 L/LhNGR targeted mouse. B, C, _Il12rb2_.Thy1.1 L/LhNGR mice (_n_ =
11) were infected with Arm. and splenic Thy1.1_–_ and Thy1.1+ CD4+ Tconv. cells purified and expression of _Il12rb2_ by Thy1.1_–_ and Thy1.1+ CD4+ Tconv. cells was determined by qPCR (B).
Thy1.1_–_ and Thy1.1+ CD4+ Tconv. cells were serum starved and treated with PBS or IL-12, and induction of pSTAT4 was measured by flow cytometry (C). D, E, _Foxp3_Cre-YFP mice (_n_ = 16, 5
independent experiments, Uninfected; _n_ = 12, Arm.; _n_ = 14, Cl. 13) remained uninfected or were infected with Arm. or Cl. 13, expression of _Il12rb2_ mRNA (D) and _Ifngr1_ mRNA (E) by
sorted splenic CD4+ Tconv cells and Treg cells, without stimulation, were measured by qPCR. F-I, Expression of IFNGR1 (F) (_n_ = 7, 2 independent experiments, Uninfected; n = 9, 2
independent experiments, Arm.; n = 15, Cl. 13), T-bet (G) (_n_ = 7, Uninfected; _n_ = 11, Arm.; _n_ = 8, Cl. 13), CXCR3 (H) (_n_ = 11, Uninfected; _n_ = 9, 2 independent experiments, Arm.;
_n_ = 15, Cl. 13), T-bet (_n_ = 7, 2 independent experiments, Uninfected; _n_ = 11, 2 independent experiments, Arm.; _n_ = 16, Cl. 13), GATA-3 (_n_ = 6, 2 independent experiments,
Uninfected; _n_ = 9, 2 independent experiments, Arm.; _n_ = 17, Cl. 13), and RORγt (_n_ = 3, 2 independent experiments, Uninfected; _n_ = 11, 2 independent experiments, Arm.; _n_ = 8, 2
independent experiments, Cl. 13), (I) by splenic CD4+ Tconv cells and Treg cells were determined flow cytometry. B-I, Data are presented as mean values and represent biologically independent
mice and 2 (G, and as indicated) or 3 (B-F, H, I) or 5 (as indicated) independent experiments. Statistical significance was determined by an unpaired two-tailed Student’s _t_-test relative
to Thy1.1– (B) or CD4+ Tconv cells (F-H), or by a two-way AVOVA with multiple comparisons (C), or by a two-way ANOVA with multiple comparisons (D-E) and multiple unpaired _t_-tests relative
to CD4+ Tconv cells (D, E, I) (_P_ values indicated when significant); NS, not significant. Source data EXTENDED DATA FIG. 3 TRANSCRIPTOMIC ANALYSIS OF _IFNGR1_-DEFICIENT TREG CELLS VERSES
CONTROL, DURING CHRONIC INFECTION. A, Expression of the deleted region of _Ifngr1_, normalized to the intact region of _Ifngr1_, from uninfected _Ifngr1_L/L_Foxp3_Cre-YFP (_n_ = 5) compared
to _Foxp3_Cre-YFP mice (_n_ = 6). Thy1.2– cells, CD8+ T cells, CD4+ Tconv cells and Treg cells were sorted, gDNA was isolated and qPCR was performed. B, Expression of IFNGR1 in Treg cells
from uninfected _Foxp3_Cre-YFP (_n_ = 7) and _Ifngr1_L/L_Foxp3_Cre-YFP (_n_ = 5) mice was measured by flow cytometry. C, D, _Foxp3_Cre-YFP mice remained uninfected (_n_ = 15, 5 independent
experiments, control; _n_ = 10, 4 independent experiments, _Ifngr1_-deficient) or were infected with Arm. (_n_ = 9, 2 independent experiments, control; _n_ = 8, 2 independent experiments,
_Ifngr1_-deficient) or Cl. 13 (_n_ = 15, control; _n_ = 14 (C), _n_ = 15 (D), _Ifngr1_-deficient) and percentage of Foxp3+ of CD4+ T cells (C), number of Treg cells (D, upper) and CD4+ Tconv
cells (D, lower) per spleen, were determined by flow cytometry. E-G, scRNA-seq of splenic Treg cells from uninfected (_n_ = 3) or Cl. 13 infected (_n_ = 5) (d16) _Foxp3_Cre-YFP or
_Ifngr1_L/L_Foxp3_Cre-YFP mice. E, Distribution of cells amongst clusters. F, Top 50 ranked significant differentially expressed genes from Treg cells in _Foxp3_Cre-YFP and
_Ifngr1_L/L_Foxp3_Cre-YFP mice infected with Cl. 13. G, GSEA overview illustrating pathways upregulated in the control Treg cell gene set compared to _Ifngr1_-deficient Treg cells during Cl.
13 infection. H, _Foxp3_Cre-YFP or _Ifngr1_L/L_Foxp3_Cre-YFP mice remained uninfected and the expression of T-bet (_n_ = 7), GATA-3 (_n_ = 6, control; _n_ = 5, _Ifngr1_-deficient) and RORγt
(_n_ = 3, control; _n_ = 4, _Ifngr1_-deficient) by splenic Treg cells were measured by flow cytometry. A-H, Data are presented as mean values and represents biologically independent mice
and 2 (A, B, E-H and as indicated) or 3 (C, D) or 5 (as indicated) independent experiments. Statistical significance was determined by an unpaired two-tailed Student’s _t_-test (B), or by
multiple unpaired _t_-tests (A, C, D, H), relative to _Foxp3_Cre-YFP mice. Adjusted _P_ values were determined by one-way ANOVA relative to _Foxp3_Cre-YFP mice (E) or by Wilcoxon rank-sum
test (F) or Kolmogorov-Smirnov test (G) relative to infected _Foxp3_Cre-YFP mice (_P_ values indicated when significant); NS, not significant. Source data EXTENDED DATA FIG. 4 INTRINSIC
EFFECTS OF _IFNGR1_ DELETION FROM TREG CELLS USING _IFNGR1_L/L_FOXP3_CRE-YFP_FOXP3_CRE-ERT2-GFP HETEROZYGOUS MICE. A-E, Heterozygous _Foxp3_Cre-YFP._Foxp3_Cre-ERT2-GFP (Cre Het) (_n_ = 5,
Uninfected; _n_ = 12, Cl. 13) and _Ifngr1_L/L_Foxp3_Cre-YFP._Foxp3_Cre-ERT2-GFP (L/L Het) (_n_ = 10, Uninfected; _n_ = 19, Cl. 13) female mice remained uninfected (2 independent experiments)
or were infected with Cl. 13 (3 independent experiments). A, Percent expression of GFP+ and YFP+ by splenic CD4+ T cells were determined by flow cytometry. B-E, Expression of the CXCR3 (B),
CCR4 (C), CD127 (D) and KLRG1 (E) in splenic Treg cells from indicated mice were measured by flow cytometry. F-H, Heterozygous _Foxp3_Cre-YFP._Foxp3_Cre-ERT2-GFP (Cre Het) and
_Ifngr1_L/L_Foxp3_Cre-YFP._Foxp3_Cre-ERT2-GFP (L/L Het) female mice were infected with Cl. 13. F, Gating strategy for IFNGR1_–_ and IFNGR1+ splenic Treg cells (G, H) from indicated mice. G,
H, Expression of T-bet (G) (_n_ = 10, Cre HET; _n_ = 15, L/L HET) and GATA-3 (H) (_n_ = 10, Cre HET; _n_ = 12, L/L HET) by IFNGR1_–_ and IFNGR1+ splenic Treg cells from indicated mice, were
measured by flow cytometry. A-H, Data are presented as mean values and represent biologically independent mice and 2 (as indicated) or 3 (A-E, G, H and as indicated) independent experiments.
Statistical significance was determined by a two-way AVOVA with multiple comparisons (A, G, H) or by one-way AVOVA with multiple comparisons (B-E) (_P_ values indicated when significant);
NS, not significant. Source data EXTENDED DATA FIG. 5 TREG CELLS MAINTAIN STABILITY DURING CHRONIC INFECTION. A, DE of genes induced (upper), or repressed (lower), by Foxp3 in splenic Treg
cells of _Foxp3_Cre-YFP mice compared to _Ifngr1_L/L_Foxp3_Cre-YFP mice, that remained uninfected (_n_ = 3) or were infected with Cl. 13 (_n_ = 5) was measured by scRNA-seq. B, C
_Foxp3_Cre-ERT2-GFP_Rosa26_LSL.tdTom mice were treated with tamoxifen for 7d and then remained uninfected or were infected with Cl. 13. B, Experimental schema. C, Representative flow
cytometry plot of tdTom and GFP co-expression in splenic CD4+ T cells at steady state (_n_ = 7) or at 16 (_n_ = 5, 2 independent experiments) and 35 (_n_ = 11, 2 independent experiments) dpi
with Cl. 13. D-F, _Foxp3_Cre-YFP or _Ifngr1_L/L_Foxp3_Cre-YFP mice were infected with Cl. 13 and levels of Foxp3 (D) (_n_ = 9, control; _n_ = 10, _Ifngr1_-deficient) and percentage of Nrp1
(E) (_n_ = 9, control; _n_ = 8, _Ifngr1_-deficient) CD25 (F, left) (_n_ = 11) and level of CD25 (F, right) (_n_ = 11) by splenic Treg cells were determined by flow cytometry. A, C-F, Data
are presented as mean values and represent biologically independent mice and 2 (A, D-F and as indicated) or 3 (C) independent experiments. Statistical significance was determined by an
unpaired two-tailed Student’s _t_-test relative to _Foxp3_Cre-YFP mice (D-F). Adjusted _P_ value was determined by Wilcoxon rank-sum test (A) (_P_ value indicated when significant); NS, not
significant. Source data EXTENDED DATA FIG. 6 IFNΓ PROMOTES TH1-LIKE POLARIZATION OF TREG CELLS DURING INFLUENZA A VIRAL INFECTION. A-D, _Foxp3_Cre-YFP or _Ifngr1_L/L_Foxp3_Cre-YFP mice were
infected with IAV. A, B, Percentage of initial body weight over time (A) (numbers of mice used indicated in parentheses) and at day 6 (B) (_n_ = 16, control; _n_ = 17, _Ifngr1_-deficient).
C, D, Expression of T-bet (C) (_n_ = 9, control; _n_ = 7, _Ifngr1_-deficient) and GATA-3 (D) (_n_ = 10, control; _n_ = 8, _Ifngr1_-deficient) by lung Treg cells were measured by flow
cytometry. A-D. Data are presented as mean values and represent biologically independent mice and 2 (A, C, D) or 3 (B) independent experiments. Statistical significance was determined by
two-way ANOVA with multiple comparisons (A) or by an unpaired two-tailed Student’s _t_-test (B-D), all relative to _Foxp3_Cre-YFP mice (_P_ values indicated when significant); NS, not
significant. Source data EXTENDED DATA FIG. 7 _IFNGR1_ DELETION FROM TREG CELLS DOES NOT AFFECT VIRAL LOAD NOR MARKERS ASSOCIATED WITH CD8+ T CELL EXHAUSTION DURING CHRONIC INFECTION. A, B,
_Foxp3_Cre-YFP, _Ifngr1_L/L or _Ifngr1_L/L_Foxp3_Cre-YFP mice remained uninfected or were infected with Cl. 13. A, Liver histological score from uninfected mice (_n_ = 2) or mice infected
with Cl. 13 8 dpi (_n_ = 4, _Foxp3_Cre-YFP; _n_ = 3, _Ifngr1_L/L; _n_ = 3, _Ifngr1_L/L_Foxp3_Cre-YFP). Score determined as: 1= Triaditis, rare sinusoidal lymphocytes; 2= Triaditis, prominent
sinusoidal lymphocytes; 3= Triaditis, prominent sinusoidal lymphocytes, single cell apoptosis. B, Serum AST activity was determined in uninfected mice (_n_ = 9, _Foxp3_Cre-YFP; _n_ = 2, 1
independent experiment, _Ifngr1_L/L; _n_ = 8, _Ifngr1_L/L_Foxp3_Cre-YFP) or mice infected with Cl. 13, 8 dpi (_n_ = 13, _Foxp3_Cre-YFP; _n_ = 14, _Ifngr1_L/L_Foxp3_Cre-YFP) or 16 dpi (_n_ =
10, _Foxp3_Cre-YFP; _n_ = 2, 1 independent experiment, _Ifngr1_L/L; _n_ = 9, _Ifngr1_L/L_Foxp3_Cre-YFP). C, _Foxp3_Cre-YFP or _Ifngr1_L/L_Foxp3_Cre-YFP mice were infected with Cl. 13 and the
viral load in serum d16 (_n_ = 12, control; _n_ = 11, _Ifngr1_-deficient; 2 independent experiments) and d30 (_n_ = 11, control; _n_ = 10, _Ifngr1_-deficient; 2 independent experiments),
kidney (_n_ = 7, control; _n_ = 6, _Ifngr1_-deficient; 1 independent experiment) and liver (_n_ = 5, control; _n_ = 3, _Ifngr1_-deficient; 1 independent experiment) were determined. D,
_Foxp3_Cre-YFP or _Ifngr1_L/L_Foxp3_Cre-YFP mice were infected with Cl. 13 and treated with 200 µg isotype control (_n_ = 3, control; _n_ = 4, _Ifngr1_-deficient) or anti-PDL1 (_n_ = 5,
control; _n_ = 2, _Ifngr1_-deficient) every 3 d from 26-38 dpi and the viral load in kidney was determined. E, F, _Foxp3_Cre-YFP (_n_ = 19) or _Ifngr1_L/L_Foxp3_Cre-YFP (_n_ = 16) mice were
infected with Cl. 13 and IR co-expression (indicated on top) with PD1 (E), and SPICE plots visualizing multiple combinations of IR co-expression (F), by pooled Tetramer+ (GP33, GP276, NP396)
splenic CD8+ T cells were determined by flow cytometry. G-J, _Foxp3_Cre-YFP or _Ifngr1_L/L_Foxp3_Cre-YFP mice were infected with Cl. 13 and treated with isotype control (_n_ = 7) or
anti-PDL1 (_n_ = 6, control; _n_ = 8, _Ifngr1_-deficient) as in (D). G, H, Expression of T-bet by bulk splenic CD8+ T cells (G) and PD1 by pooled Tetramer+ (GP33, GP276, NP396) splenic CD8+
T cells (H) were determined by flow cytometry. I, Percentage of pTex (Tcf1+TIM3–) and tTex (TCF1–TIM3+) splenic CD8+ T cells were determined by flow cytometry. J, Percentage of tTex splenic
GP33+CD8+ T cells was determined by flow cytometry. A-J, Data are presented as mean values and represent biologically independent mice and 1 (A, D, and as indicated), 2 (C, G-J) or 3 (B, E,
F) independent experiments. A-E, G-J, Statistical significance was determined by a two-way ANOVA with multiple comparisons (A, B) or by an unpaired two-tailed Student’s _t_-test relative to
_Foxp3_Cre-YFP mice (C, E) or my multiple unpaired Student’s _t_-tests (D, G-J) (_P_ values indicated when significant); NS, not significant. Source data EXTENDED DATA FIG. 8
_IFNGR1-_DEFICIENT TREG CELLS FROM INFECTED MICE MAINTAIN SUPPRESSIVE FUNCTION OF NAÏVE CD4+ TCONV CELLS IN VITRO AND DO NOT IMPACT TH POLARIZATION OF CD4+ TCONV CELLS IN VIVO. A,B, In vitro
microsuppression assay of splenic uninfected CD4+ Tconv (Tresponders) by splenic Treg cells from _Foxp3_Cre-YFP or _Ifngr1_L/L_Foxp3_Cre-YFP mice infected with LCMV Arm. (A) or Cl. 13 (B)
(numbers of mice used indicated in parentheses). C, In vitro microsuppression assay of splenic CD44hiCD62Llo CD4+ Tconv (Teff) from Arm. infected _Foxp3_Cre-YFP mice by splenic Treg cells
from _Foxp3_Cre-YFP or _Ifngr1_L/L_Foxp3_Cre-YFP mice infected with LCMV Arm. (numbers of mice used indicated in parentheses). D-G, _Foxp3_Cre-YFP or _Ifngr1_L/L_Foxp3_Cre-YFP mice infected
with Cl. 13 and expression of T-bet, GATA-3 and RORγt (D) (_n_ = 8), T-bet and GATA-3 co-expression (E) (_n_ = 8), CXCR3 (F) (_n_ = 8) and CCR4 (G) (_n_ = 8, control; _n_ = 9,
_Ifngr1_-deficient) by splenic CD4+ Tconv cells were measured by flow cytometry. H, Expression of the deleted region of _Ifng_, normalized to the intact region of _Ifng_, from
_Ifng_L/L_Foxp3_Cre-YFP mice (_n_ = 4) compared to _Foxp3_Cre-YFP mice (_n_ = 3). Thy1.2– cells, CD8+ T cells, CD4+ Tconv cells and Treg cells were sorted, gDNA was isolated and qPCR was
performed. A-H Data are presented as mean values and represent biologically independent mice and 1 (H), 2 (B-G) or 3 (A) independent experiments. Statistical significance was determined by
two-way ANOVA (A-C), or by multiple unpaired Student _t_-tests (D, E, H), or by an unpaired two-tailed Student’s _t_-test (F, G), relative to _Foxp3_Cre-YFP mice (_P_ values indicated when
significant); NS, not significant. Source data EXTENDED DATA FIG. 9 TRANSCRIPTOMIC ANALYSIS REVEALS THAT TH1-LIKE TREG CELLS INHIBIT CD8+ T CELL MEMORY DURING LCMV INFECTION. A-C, scRNA-seq
of splenic pooled Tetramer+ (GP33, GP276, NP396) CD8+ T cells from _Foxp3_Cre-YFP or _Ifngr1_L/L_Foxp3_Cre-YFP mice (_n_ = 3), 16 dpi with Cl. 13. A, UMAP embedding Tetramer+ CD8+ T cells
into a two-dimensional space to generate 8 distinct clusters, with categories shown. B, Distribution of cells amongst clusters from individual mice. C, Percentage of cells in the memory
cluster. D, E, _Foxp3_Cre-YFP or _Ifngr1_L/L_Foxp3_Cre-YFP mice were infected with Arm. and the expression of effector CD8+ cells (D) (_n_ = 18, control; _n_ = 16, _Ifngr1_-deficient) and
the ratio of MPECs to SLECs within splenic pooled CD44hiCD62Llo Tetramer+ CD8+ T cells (E) (_n_ = 8) were determined by flow cytometry. F, G, Expression of _Ifngr1_ within the bulk
population (F) and within the memory cluster (G) of the splenic pooled Tetramer+ CD8+ T cells from the scRNA-seq dataset in A-C (_n_ = 3). H-J, _Rag1_–/– mice were reconstituted with
_Ifngr1_–/– (_n_ = 12) or control (WT) (_n_ = 13) Treg cells and infected with Arm. H, I, Frequency of splenic CD44hiCD62Llo (H) and CD44hiCD62Lhi (I, left), and number of CD44hiCD62Lhi (I,
right) pooled Tetramer+ CD8+ T cells were determined by flow cytometry. J, Frequency of (left) and total number of (right) MPECs (upper) and SLECs (lower), and the ratio of MPECs to SLECs,
splenic pooled Tetramer+ CD8+ T cells were determined by flow cytometry. K, heatmap of DE of the IL16/CD4/CCR5 signaling axis in Treg cells from the scRNA-seq dataset in A-C. L-N,
_Foxp3_Cre-YFP and _Ifngr1_L/L_Foxp3_Cre-YFP mice were infected with Arm. and transcripts of the IL16/CD4/CCR5 signaling axis in Treg cells (L) (_n_ = 11) or _Il16_ (M) (_n_ = 11) and _Ccl5_
(N) (_n_ = 6, control; _n_ = 9, _Ifngr1_-deficient) expression in Treg cells and CD44hiCD62Llo CD4+ and CD8+ T cells, were determined by qPCR. A-N, Data are presented as mean values and
represent biologically independent mice and 1 (A-C, F, G), 2 (E, K, N) or 3 (D, H-J, L, M) independent experiments. Statistical significance and _P_ values were determined by an unpaired
two-tailed Student’s _t_-test relative to the _Foxp3_Cre-YFP mice (C-G) or _Rag1_–/– + WT Treg cells (H-J), or by multiple unpaired Student _t_-tests relative to _Foxp3_Cre-YFP mice (L) or
by two-way ANOVA with multiple comparisons. (M,N), Adjusted _P_ values were determined by one-way ANOVA (B) or Wilcoxon rank-sum test (K) relative to infected _Foxp3_Cre-YFP mice (_P_ values
indicated when significant); NS, not significant. Source data EXTENDED DATA FIG. 10 TH1-LIKE TREG CELLS DO NOT IMPACT TISSUE RESIDENT MEMORY CD8+ T DURING IAV INFECTION RECHALLENGE. A-G,
_Foxp3_Cre-YFP or _Ifngr1_L/L_Foxp3_Cre-YFP mice were infected with PR8 only (_n_ = 9, control; _n_ = 8, _Ifngr1_-deficient) or X31 and challenged with PR8 (_n_ = 10, control; _n_ = 9,
_Ifngr1_-deficient). A, Experimental schema. B, Expression of LAP/TGFβ1 by lung Treg cells by percent (upper) and level of expression within LAP/TGFβ1+ Treg cells (lower) were determined by
flow cytometry. C, Percentage (upper), and number (lower), of lung IAV pooled Tetramer+ (PA224 and NP366) CD8+ T were determined by flow cytometry. D, Expression of IFNγ by lung CD8+ T cells
was measured, after in vitro stimulation with pooled IAV peptides (PA224-233 and NP366-372), by flow cytometry. E, Expression of CXCR3 by lung pooled Tetramer+ CD8+ T cells was measured by
flow cytometry. F, Percentage (left), and number (lower), of CD103+CD69+ lung pooled Tetramer+ CD8+ T cells were determined by flow cytometry. G, Blinded histology scores (areas indicated)
of paraffin-embedded hematoxylin and eosin stained, lung tissue sections. B-G, Data are presented as mean values and represent biologically independent mice and 3 independent experiments.
Statistical significance was determined by multiple unpaired Student’s _t_-tests relative to _Foxp3_Cre-YFP mice (_P_ values indicated when significant); NS, not significant. Source data
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Table 1. REPORTING SUMMARY SOURCE DATA SOURCE DATA FIG. 1 Statistical source data. SOURCE DATA FIG. 2 Statistical source
data. SOURCE DATA FIG. 3 Statistical source data. SOURCE DATA FIG. 4 Statistical source data. SOURCE DATA FIG. 5 Statistical source data. SOURCE DATA FIG. 6 Statistical source data. SOURCE
DATA FIG. 7 Statistical source data. SOURCE DATA EXTENDED DATA FIG. 1 Statistical source data. SOURCE DATA EXTENDED DATA FIG. 2 Statistical source data. SOURCE DATA EXTENDED DATA FIG. 3
Statistical source data. SOURCE DATA EXTENDED DATA FIG. 4 Statistical source data. SOURCE DATA EXTENDED DATA FIG. 5 Statistical source data. SOURCE DATA EXTENDED DATA FIG. 6 Statistical
source data. SOURCE DATA EXTENDED DATA FIG 7 Statistical source data. SOURCE DATA EXTENDED DATA FIG. 8 Statistical source data. SOURCE DATA EXTENDED DATA FIG. 9 Statistical source data.
SOURCE DATA EXTENDED DATA FIG. 10 Statistical source data. RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article
under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such
publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Gocher-Demske, A.M., Cui, J., Szymczak-Workman, A.L. _et al._ IFNγ-induction of
TH1-like regulatory T cells controls antiviral responses. _Nat Immunol_ 24, 841–854 (2023). https://doi.org/10.1038/s41590-023-01453-w Download citation * Received: 02 December 2021 *
Accepted: 06 February 2023 * Published: 16 March 2023 * Issue Date: May 2023 * DOI: https://doi.org/10.1038/s41590-023-01453-w SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative