Play all audios:
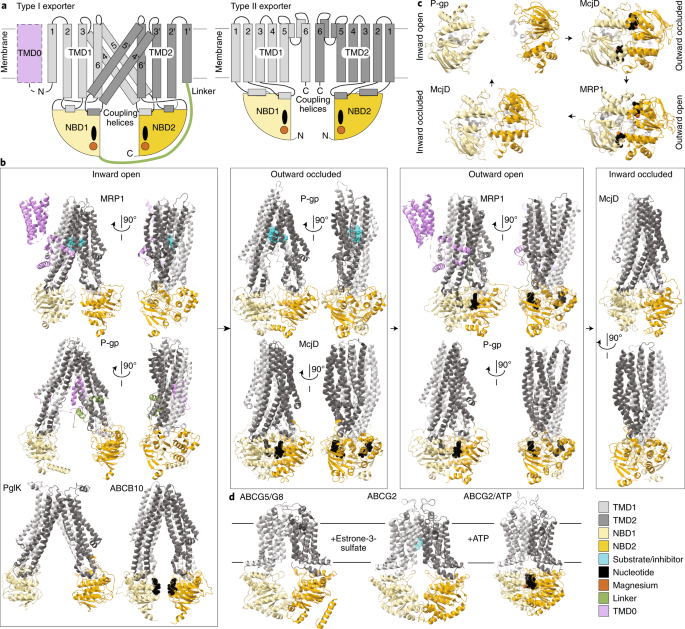
ABSTRACT Much structural information has been amassed on ATP-binding cassette (ABC) transporters, including hundreds of structures of isolated domains and an increasing array of full-length
transporters. The structures capture different steps in the transport cycle and have aided in the design and interpretation of computational simulations and biophysics experiments. These
data provide a maturing, although still incomplete, elucidation of the protein dynamics and mechanisms of substrate selection and transit through the transporters. We present an updated view
of the classical alternating-access mechanism as it applies to eukaryotic ABC transporters, focusing on type I exporters. Our model helps frame the progress in, and remaining questions
about, transporter energetics, how substrates are selected and how ATP is consumed to perform work at the molecular scale. Many human ABC transporters are associated with disease; we
highlight progress in understanding their pharmacology through the lens of structural biology and describe how this knowledge suggests approaches to pharmacologically targeting these
transporters. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access
Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print
issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to
local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT
BEING VIEWED BY OTHERS ASYMMETRIC DRUG BINDING IN AN ATP-LOADED INWARD-FACING STATE OF AN ABC TRANSPORTER Article 20 December 2021 THE NET ELECTROSTATIC POTENTIAL AND HYDRATION OF ABCG2
AFFECT SUBSTRATE TRANSPORT Article Open access 18 August 2023 ON THE INTERPLAY BETWEEN LIPIDS AND ASYMMETRIC DYNAMICS OF AN NBS DEGENERATE ABC TRANSPORTER Article Open access 03 February
2023 REFERENCES * Ford, R. C. & Beis, K. Learning the ABCs one at a time: structure and mechanism of ABC transporters. _Biochem. Soc. Trans._ 47, 23–36 (2019). Article CAS PubMed
Google Scholar * Cui, J. & Davidson, A. L. ABC solute importers in bacteria. _Essays Biochem._ 50, 85–99 (2011). Article CAS PubMed Google Scholar * Davidson, A. L., Dassa, E.,
Orelle, C. & Chen, J. Structure, function, and evolution of bacterial ATP-binding cassette systems. _Microbiol. Mol. Biol. Rev._ 72, 317–64 (2008). Article CAS PubMed PubMed Central
Google Scholar * Decottignies, A. & Goffeau, A. Complete inventory of the yeast ABC proteins. _Nat. Genet._ 15, 137–145 (1997). Article CAS PubMed Google Scholar * Vasiliou, V.,
Vasiliou, K. & Nebert, D. W. Human ATP-binding cassette (ABC) transporter family. _Hum. Genom._ 3, 281–290 (2009). Article CAS Google Scholar * Garcia, O., Bouige, P., Forestier, C.
& Dassa, E. Inventory and comparative analysis of rice and _Arabidopsis_ ATP-binding cassette (ABC) systems. _J. Mol. Biol._ 343, 249–265 (2004). Article CAS PubMed Google Scholar *
Quazi, F., Lenevich, S. & Molday, R. S. ABCA4 is an _N_-retinylidene-phosphatidylethanolamine and phosphatidylethanolamine importer. _Nat. Commun._ 3, 925 (2012). Article PubMed CAS
Google Scholar * Xiong, J., Feng, J., Yuan, D., Zhou, J. & Miao, W. Tracing the structural evolution of eukaryotic ATP binding cassette transporter superfamily. _Sci. Rep._ 5, 16724
(2015). Article CAS PubMed PubMed Central Google Scholar * Theodoulou, F. L. & Kerr, I. D. ABC transporter research: going strong 40 years on. _Biochem. Soc. Trans._ 43, 1033–1040
(2015). Article CAS PubMed PubMed Central Google Scholar * Riordan, J. R. et al. Amplification of P-glycoprotein genes in multidrug-resistant mammalian cell lines. _Nature_ 316, 817–819
(1985). Article CAS PubMed Google Scholar * Robey, R. W. et al. Revisiting the role of ABC transporters in multidrug-resistant cancer. _Nat. Rev. Cancer_ 18, 452–464 (2018). Article
CAS PubMed PubMed Central Google Scholar * Cavalheiro, M., Pais, P., Galocha, M. & Teixeira, M. C. Host-pathogen interactions mediated by MDR transporters in fungi: as pleiotropic as
it gets! _Genes (Basel)_ 9, E332 (2018). Article CAS Google Scholar * Du, D. et al. Multidrug efflux pumps: structure, function and regulation. _Nat. Rev. Microbiol._ 16, 523–539 (2018).
Article CAS PubMed Google Scholar * Moitra, K. & Dean, M. Evolution of ABC transporters by gene duplication and their role in human disease. _Biol. Chem._ 392, 29–37 (2011). Article
CAS PubMed Google Scholar * Gaudet, R. & Wiley, D. C. Structure of the ABC ATPase domain of human TAP1, the transporter associated with antigen processing. _EMBO J._ 20, 4964–4972
(2001). Article CAS PubMed PubMed Central Google Scholar * Hung, L. W. et al. Crystal structure of the ATP-binding subunit of an ABC transporter. _Nature_ 396, 703–707 (1998). Article
CAS PubMed Google Scholar * Chen, J., Lu, G., Lin, J., Davidson, A. L. & Quiocho, F. A. A tweezers-like motion of the ATP-binding cassette dimer in an ABC transport cycle. _Mol. Cell_
12, 651–661 (2003). Article CAS PubMed Google Scholar * Scapin, G., Potter, C. S. & Carragher, B. Cryo-EM for small molecules discovery, design, understanding, and application.
_Cell Chem. Biol._ 25, 1318–1325 (2018). Article CAS PubMed PubMed Central Google Scholar * Frank, J. Advances in the field of single-particle cryo-electron microscopy over the last
decade. _Nat. Protoc._ 12, 209–212 (2017). Article CAS PubMed PubMed Central Google Scholar * Jardetzky, O. Simple allosteric model for membrane pumps. _Nature_ 211, 969–970 (1966).
Article CAS PubMed Google Scholar * Wen, P. C., Verhalen, B., Wilkens, S., Mchaourab, H. S. & Tajkhorshid, E. On the origin of large flexibility of P-glycoprotein in the
inward-facing state. _J. Biol. Chem._ 288, 19211–19220 (2013). Article CAS PubMed PubMed Central Google Scholar * Jin, M. S., Oldham, M. L., Zhang, Q. & Chen, J. Crystal structure
of the multidrug transporter P-glycoprotein from _Caenorhabditis elegans_. _Nature_ 490, 566–569 (2012). Article CAS PubMed PubMed Central Google Scholar * Li, J., Jaimes, K. F. &
Aller, S. G. Refined structures of mouse P-glycoprotein. _Protein Sci._ 23, 34–46 (2014). Article PubMed CAS Google Scholar * Lee, J. Y., Yang, J. G., Zhitnitsky, D., Lewinson, O. &
Rees, D. C. Structural basis for heavy metal detoxification by an Atm1-type ABC exporter. _Science_ 343, 1133–1136 (2014). Article CAS PubMed PubMed Central Google Scholar * Esser, L.
et al. Structures of the multidrug transporter P-glycoprotein reveal asymmetric ATP binding and the mechanism of polyspecificity. _J. Biol. Chem._ 292, 446–461 (2017). Article CAS PubMed
Google Scholar * Alam, A. et al. Structure of a zosuquidar and UIC2-bound human-mouse chimeric ABCB1. _Proc. Natl Acad. Sci. USA_ 115, E1973–E1982 (2018). Article CAS PubMed PubMed
Central Google Scholar * Johnson, Z. L. & Chen, J. ATP binding enables substrate release from multidrug resistance protein 1. _Cell_ 172, 81–89.e10 (2018). Article CAS PubMed Google
Scholar * Dawson, R. J. & Locher, K. P. Structure of the multidrug ABC transporter Sav1866 from _Staphylococcus aureus_ in complex with AMP-PNP. _FEBS Lett._ 581, 935–938 (2007).
Article CAS PubMed Google Scholar * Barth, K. et al. Conformational coupling and trans-inhibition in the human antigen transporter ortholog TmrAB resolved with dipolar EPR spectroscopy.
_J. Am. Chem. Soc._ 140, 4527–4533 (2018). Article CAS PubMed Google Scholar * Kim, Y. & Chen, J. Molecular structure of human P-glycoprotein in the ATP-bound, outward-facing
conformation. _Science_ 359, 915–919 (2018). Article CAS PubMed Google Scholar * Hutter, C. A. J. et al. The extracellular gate shapes the energy profile of an ABC exporter. _Nat.
Commun._ 10, 2260 (2019). Article PubMed PubMed Central CAS Google Scholar * Kodan, A. et al. Structural basis for gating mechanisms of a eukaryotic P-glycoprotein homolog. _Proc. Natl
Acad. Sci. USA_ 111, 4049–4054 (2014). Article CAS PubMed PubMed Central Google Scholar * Bountra, K. et al. Structural basis for antibacterial peptide self-immunity by the bacterial
ABC transporter McjD. _EMBO J._ 36, 3062–3079 (2017). Article CAS PubMed PubMed Central Google Scholar * Choudhury, H. G. et al. Structure of an antibacterial peptide ATP-binding
cassette transporter in a novel outward occluded state. _Proc. Natl Acad. Sci. USA_ 111, 9145–9150 (2014). Article CAS PubMed PubMed Central Google Scholar * Alam, A., Kowal, J.,
Broude, E., Roninson, I. & Locher, K. P. Structural insight into substrate and inhibitor discrimination by human P-glycoprotein. _Science_ 363, 753–756 (2019). Article CAS PubMed
PubMed Central Google Scholar * Loo, T. W., Bartlett, M. C. & Clarke, D. M. Drug binding in human P-glycoprotein causes conformational changes in both nucleotide-binding domains. _J.
Biol. Chem._ 278, 1575–1578 (2003). Article CAS PubMed Google Scholar * Johnson, Z. L. & Chen, J. Structural basis of substrate recognition by the multidrug resistance protein MRP1.
_Cell_ 168, 1075–1085.e9 (2017). Article CAS PubMed Google Scholar * Penczek, P. A., Kimmel, M. & Spahn, C. M. Identifying conformational states of macromolecules by eigen-analysis
of resampled cryo-EM images. _Structure_ 19, 1582–1590 (2011). Article CAS PubMed PubMed Central Google Scholar * Loveland, A. B. & Korostelev, A. A. Structural dynamics of protein
S1 on the 70S ribosome visualized by ensemble cryo-EM. _Methods_ 137, 55–66 (2018). Article CAS PubMed Google Scholar * Frank, G. A. et al. Cryo-EM analysis of the conformational
landscape of human P-glycoprotein (ABCB1) during its catalytic cycle. _Mol. Pharmacol._ 90, 35–41 (2016). Article CAS PubMed PubMed Central Google Scholar * Harpole, T. J. &
Delemotte, L. Conformational landscapes of membrane proteins delineated by enhanced sampling molecular dynamics simulations. _Biochim. Biophys. Acta_ 1860, 909–926 (2018). Article CAS
Google Scholar * Marinelli, F. & Faraldo-Gómez, J. D. Ensemble-biased metadynamics: a molecular simulation method to sample experimental distributions. _Biophys. J._ 108, 2779–2782
(2015). Article CAS PubMed PubMed Central Google Scholar * Lefèvre, F. & Boutry, M. Towards identification of the substrates of ATP-binding cassette transporters. _Plant Physiol._
178, 18–39 (2018). PubMed PubMed Central Google Scholar * Dean, M., Rzhetsky, A. & Allikmets, R. The human ATP-binding cassette (ABC) transporter superfamily. _Genome Res._ 11,
1156–1166 (2001). Article CAS PubMed Google Scholar * Cole, S. P. Multidrug resistance protein 1 (MRP1, ABCC1), a “multitasking” ATP-binding cassette (ABC) transporter. _J. Biol. Chem._
289, 30880–30888 (2014). Article CAS PubMed PubMed Central Google Scholar * Litman, T., Druley, T. E., Stein, W. D. & Bates, S. E. From MDR to MXR: new understanding of multidrug
resistance systems, their properties and clinical significance. _Cell. Mol. Life Sci._ 58, 931–959 (2001). Article CAS PubMed Google Scholar * Pedersen, J. M. et al. Substrate and method
dependent inhibition of three ABC-transporters (MDR1, BCRP, and MRP2). _Eur. J. Pharm. Sci._ 103, 70–76 (2017). Article CAS PubMed Google Scholar * Loo, T. W., Bartlett, M. C. &
Clarke, D. M. Transmembrane segment 7 of human P-glycoprotein forms part of the drug-binding pocket. _Biochem. J._ 399, 351–359 (2006). Article CAS PubMed PubMed Central Google Scholar
* Loo, T. W., Bartlett, M. C. & Clarke, D. M. Simultaneous binding of two different drugs in the binding pocket of the human multidrug resistance P-glycoprotein. _J. Biol. Chem._ 278,
39706–39710 (2003). Article CAS PubMed Google Scholar * Loo, T. W. & Clarke, D. M. Identification of residues within the drug-binding domain of the human multidrug resistance
P-glycoprotein by cysteine-scanning mutagenesis and reaction with dibromobimane. _J. Biol. Chem._ 275, 39272–39278 (2000). Article CAS PubMed Google Scholar * Loo, T. W. & Clarke, D.
M. Thiol-reactive drug substrates of human P-glycoprotein label the same sites to activate ATPase activity in membranes or dodecyl maltoside detergent micelles. _Biochem. Biophys. Res.
Commun._ 488, 573–577 (2017). Article CAS PubMed Google Scholar * Aller, S. G. et al. Structure of P-glycoprotein reveals a molecular basis for poly specific drug binding. _Science_ 323,
1718–1722 (2009). Article CAS PubMed PubMed Central Google Scholar * Nicklisch, S. C. et al. Global marine pollutants inhibit P-glycoprotein: environmental levels, inhibitory effects,
and cocrystal structure. _Sci. Adv._ 2, e1600001 (2016). Article PubMed PubMed Central CAS Google Scholar * Oldham, M. L., Grigorieff, N. & Chen, J. Structure of the transporter
associated with antigen processing trapped by herpes simplex virus. _eLife_ 5, e21829 (2016). Article PubMed PubMed Central Google Scholar * Lehnert, E. & Tampé, R. Structure and
dynamics of antigenic peptides in complex with TAP. _Front. Immunol._ 8, 10 (2017). PubMed PubMed Central Google Scholar * Koopmann, J. O., Post, M., Neefjes, J. J., Hämmerling, G. J.
& Momburg, F. Translocation of long peptides by transporters associated with antigen processing (TAP). _Eur. J. Immunol._ 26, 1720–1728 (1996). Article CAS PubMed Google Scholar *
Ritz, U. et al. Impaired transporter associated with antigen processing (TAP) function attributable to a single amino acid alteration in the peptide TAP subunit TAP1. _J. Immunol._ 170,
941–946 (2003). Article CAS PubMed Google Scholar * Armandola, E. A. et al. A point mutation in the human transporter associated with antigen processing (TAP2) alters the peptide
transport specificity. _Eur. J. Immunol._ 26, 1748–1755 (1996). Article CAS PubMed Google Scholar * Baldauf, C., Schrodt, S., Herget, M., Koch, J. & Tampé, R. Single residue within
the antigen translocation complex TAP controls the epitope repertoire by stabilizing a receptive conformation. _Proc. Natl Acad. Sci. USA_ 107, 9135–9140 (2010). Article CAS PubMed PubMed
Central Google Scholar * Geng, J., Pogozheva, I. D., Mosberg, H. I. & Raghavan, M. Use of functional polymorphisms to elucidate the peptide binding site of TAP complexes. _J.
Immunol._ 195, 3436–3448 (2015). Article CAS PubMed Google Scholar * Deverson, E. V. et al. Functional analysis by site-directed mutagenesis of the complex polymorphism in rat
transporter associated with antigen processing. _J. Immunol._ 160, 2767–2779 (1998). CAS PubMed Google Scholar * Lehnert, E. et al. Antigenic peptide recognition on the human ABC
transporter TAP resolved by DNP-enhanced solid-state NMR spectroscopy. _J. Am. Chem. Soc._ 138, 13967–13974 (2016). Article CAS PubMed Google Scholar * Nöll, A. et al. Crystal structure
and mechanistic basis of a functional homolog of the antigen transporter TAP. _Proc. Natl Acad. Sci. USA_ 114, E438–E447 (2017). Article PubMed PubMed Central CAS Google Scholar * Mi,
W. et al. Structural basis of MsbA-mediated lipopolysaccharide transport. _Nature_ 549, 233–237 (2017). Article CAS PubMed PubMed Central Google Scholar * Loo, T. W., Bartlett, M. C.
& Clarke, D. M. Substrate-induced conformational changes in the transmembrane segments of human P-glycoprotein. Direct evidence for the substrate-induced fit mechanism for drug binding.
_J. Biol. Chem._ 278, 13603–13606 (2003). Article CAS PubMed Google Scholar * Spadaccini, R., Kaur, H., Becker-Baldus, J. & Glaubitz, C. The effect of drug binding on specific sites
in transmembrane helices 4 and 6 of the ABC exporter MsbA studied by DNP-enhanced solid-state NMR. _Biochim. Biophys. Acta_ 1860, 833–840 (2018). Article CAS Google Scholar * Szewczyk, P.
et al. Snapshots of ligand entry, malleable binding and induced helical movement in P-glycoprotein. _Acta Crystallogr. D. Biol. Crystallogr._ 71, 732–741 (2015). Article CAS PubMed
PubMed Central Google Scholar * Perez, C. et al. Structure and mechanism of an active lipid-linked oligosaccharide flippase. _Nature_ 524, 433–438 (2015). Article CAS PubMed Google
Scholar * Szöllősi, D., Rose-Sperling, D., Hellmich, U. A. & Stockner, T. Comparison of mechanistic transport cycle models of ABC exporters. _Biochim. Biophys. Acta_ 1860, 818–832
(2018). Article CAS Google Scholar * Moeller, A. et al. Distinct conformational spectrum of homologous multidrug ABC transporters. _Structure_ 23, 450–460 (2015). Article CAS PubMed
PubMed Central Google Scholar * Dastvan, R., Mishra, S., Peskova, Y. B., Nakamoto, R. K. & Mchaourab, H. S. Mechanism of allosteric modulation of P-glycoprotein by transport substrates
and inhibitors. _Science_ 364, 689–692 (2019). Article CAS PubMed PubMed Central Google Scholar * Gu, R. X. et al. Conformational changes in the antibacterial peptide ATP binding
cassette transporter McjD revealed by molecular dynamics simulations. _Biochemistry_ 54, 5989–5998 (2015). Article CAS PubMed Google Scholar * Grossmann, N. et al. Mechanistic
determinants of the directionality and energetics of active export by a heterodimeric ABC transporter. _Nat. Commun._ 5, 5419 (2014). Article CAS PubMed Google Scholar * Csanády, L.,
Vergani, P. & Gadsby, D. C. Structure, gating, and regulation of the Cftr anion channel. _Physiol. Rev._ 99, 707–738 (2019). Article PubMed CAS Google Scholar * Pan, L. & Aller,
S. G. Allosteric role of substrate occupancy toward the alignment of P-glycoprotein nucleotide binding domains. _Sci. Rep._ 8, 14643 (2018). Article PubMed PubMed Central CAS Google
Scholar * Procko, E., Ferrin-O’Connell, I., Ng, S. L. & Gaudet, R. Distinct structural and functional properties of the ATPase sites in an asymmetric ABC transporter. _Mol. Cell_ 24,
51–62 (2006). Article CAS PubMed Google Scholar * Mishra, S. et al. Conformational dynamics of the nucleotide binding domains and the power stroke of a heterodimeric ABC transporter.
_eLife_ 3, e02740 (2014). Article PubMed PubMed Central Google Scholar * Collauto, A., Mishra, S., Litvinov, A., McHaourab, H. S. & Goldfarb, D. Direct spectroscopic detection of ATP
turnover reveals mechanistic divergence of ABC exporters. _Structure_ 25, 1264–1274.e3 (2017). Article CAS PubMed PubMed Central Google Scholar * Verhalen, B. et al. Energy
transduction and alternating access of the mammalian ABC transporter P-glycoprotein. _Nature_ 543, 738–741 (2017). Article CAS PubMed PubMed Central Google Scholar * Borst, P. &
Elferink, R. O. Mammalian ABC transporters in health and disease. _Annu. Rev. Biochem._ 71, 537–592 (2002). Article CAS PubMed Google Scholar * Procko, E. & Gaudet, R. Antigen
processing and presentation: TAPping into ABC transporters. _Curr. Opin. Immunol._ 21, 84–91 (2009). Article CAS PubMed Google Scholar * Eggensperger, S. & Tampé, R. The transporter
associated with antigen processing: a key player in adaptive immunity. _Biol. Chem._ 396, 1059–1072 (2015). Article CAS PubMed Google Scholar * Palmeira, A., Sousa, E., Vasconcelos, M.
H. & Pinto, M. M. Three decades of P-gp inhibitors: skimming through several generations and scaffolds. _Curr. Med. Chem._ 19, 1946–2025 (2012). Article CAS PubMed Google Scholar *
Ma, J. & Biggin, P. C. Substrate versus inhibitor dynamics of P-glycoprotein. _Proteins_ 81, 1653–1668 (2013). Article CAS PubMed Google Scholar * Dawson, R. J. & Locher, K. P.
Structure of a bacterial multidrug ABC transporter. _Nature_ 443, 180–185 (2006). Article CAS PubMed Google Scholar * Perez, C. et al. Structural basis of inhibition of lipid-linked
oligosaccharide flippase PglK by a conformational nanobody. _Sci. Rep._ 7, 46641 (2017). Article CAS PubMed PubMed Central Google Scholar * Ho, H. et al. Structural basis for dual-mode
inhibition of the ABC transporter MsbA. _Nature_ 557, 196–201 (2018). Article CAS PubMed Google Scholar * Praest, P., Liaci, A.M., Forster, F. & Wiertz, E. New insights into the
structure of the MHC class I peptide-loading complex and mechanisms of TAP inhibition by viral immune evasion proteins. _Mol. Immunol_. https://doi.org/10.1016/j.molimm.2018.03.020 (2018). *
Herbring, V., Bäucker, A., Trowitzsch, S. & Tampé, R. A dual inhibition mechanism of herpesviral ICP47 arresting a conformationally thermostable TAP complex. _Sci. Rep._ 6, 36907
(2016). Article CAS PubMed PubMed Central Google Scholar * Oldham, M. L. et al. A mechanism of viral immune evasion revealed by cryo-EM analysis of the TAP transporter. _Nature_ 529,
537–540 (2016). Article CAS PubMed PubMed Central Google Scholar * Parreira, B. et al. Persistence of the ABCC6 genes and the emergence of the bony skeleton in vertebrates. _Sci. Rep._
8, 6027 (2018). Article PubMed PubMed Central CAS Google Scholar * Lee, J. Y. et al. Crystal structure of the human sterol transporter ABCG5/ABCG8. _Nature_ 533, 561–564 (2016). Article
CAS PubMed PubMed Central Google Scholar * Taylor, N. M. I. et al. Structure of the human multidrug transporter ABCG2. _Nature_ 546, 504–509 (2017). Article CAS PubMed Google
Scholar * Borghi, L., Kang, J., Ko, D., Lee, Y. & Martinoia, E. The role of ABCG-type ABC transporters in phytohormone transport. _Biochem. Soc. Trans._ 43, 924–930 (2015). Article CAS
PubMed PubMed Central Google Scholar * Luo, Y. L. et al. Tissue expression pattern of ABCG transporter indicates functional roles in reproduction of _Toxocara canis_. _Parasitol. Res._
117, 775–782 (2018). Article PubMed Google Scholar * Manolaridis, I. et al. Cryo-EM structures of a human ABCG2 mutant trapped in ATP-bound and substrate-bound states. _Nature_ 563,
426–430 (2018). Article CAS PubMed PubMed Central Google Scholar * Jackson, S. M. et al. Structural basis of small-molecule inhibition of human multidrug transporter ABCG2. _Nat.
Struct. Mol. Biol._ 25, 333–340 (2018). Article CAS PubMed Google Scholar * Qian, H. et al. Structure of the human lipid exporter ABCA1. _Cell_ 169, 1228–1239.e10 (2017). Article CAS
PubMed Google Scholar * Lee, J. Y. & Parks, J. S. ATP-binding cassette transporter AI and its role in HDL formation. _Curr. Opin. Lipidol._ 16, 19–25 (2005). Article PubMed Google
Scholar * Smith, P. C. et al. ATP binding to the motor domain from an ABC transporter drives formation of a nucleotide sandwich dimer. _Mol. Cell_ 10, 139–149 (2002). Article CAS PubMed
PubMed Central Google Scholar * Kodan, A. et al. Inward- and outward-facing X-ray crystal structures of homodimeric P-glycoprotein CmABCB1. _Nat. Commun._ 10, 88 (2019). Article PubMed
PubMed Central CAS Google Scholar * Molinski, S. V. et al. Comprehensive mapping of cystic fibrosis mutations to CFTR protein identifies mutation clusters and molecular docking predicts
corrector binding site. _Proteins_ 86, 833–843 (2018). Article CAS PubMed Google Scholar * de Wet, H. & Proks, P. Molecular action of sulphonylureas on KATP channels: a real
partnership between drugs and nucleotides. _Biochem. Soc. Trans._ 43, 901–907 (2015). Article PubMed PubMed Central CAS Google Scholar * Vergani, P., Lockless, S. W., Nairn, A. C. &
Gadsby, D. C. CFTR channel opening by ATP-driven tight dimerization of its nucleotide-binding domains. _Nature_ 433, 876–880 (2005). Article CAS PubMed PubMed Central Google Scholar *
Zhang, Z. & Chen, J. Atomic structure of the cystic fibrosis transmembrane conductance regulator. _Cell_ 167, 1586–1597.e9 (2016). Article CAS PubMed Google Scholar * Zhang, Z., Liu,
F. & Chen, J. Conformational changes in CFTR upon phosphorylation and ATP binding. _Cell_ 170, 483–491.e8 (2017). Article CAS PubMed Google Scholar * Liu, F., Zhang, Z., Csanady,
L., Gadsby, D. C. & Chen, J. Molecular structure of the human CFTR ion channel. _Cell_ 169, 85–95.e8 (2017). Article CAS PubMed Google Scholar * Zhang, Z., Liu, F. & Chen, J.
Molecular structure of the ATP-bound, phosphorylated human CFTR. _Proc. Natl Acad. Sci. USA_ 115, 12757–12762 (2018). Article CAS PubMed PubMed Central Google Scholar * Tordai, H.,
Leveles, I. & Hegedűs, T. Molecular dynamics of the cryo-EM CFTR structure. _Biochem. Biophys. Res. Commun._ 491, 986–993 (2017). Article CAS PubMed Google Scholar * Veit, G. et al.
From CFTR biology toward combinatorial pharmacotherapy: expanded classification of cystic fibrosis mutations. _Mol. Biol. Cell_ 27, 424–433 (2016). Article CAS PubMed PubMed Central
Google Scholar * Jih, K. Y. & Hwang, T. C. Vx-770 potentiates CFTR function by promoting decoupling between the gating cycle and ATP hydrolysis cycle. _Proc. Natl Acad. Sci. USA_ 110,
4404–4409 (2013). Article CAS PubMed PubMed Central Google Scholar * Yu, H. et al. Ivacaftor potentiation of multiple CFTR channels with gating mutations. _J. Cyst. Fibros._ 11, 237–245
(2012). Article CAS PubMed Google Scholar * Li, N. et al. Structure of a pancreatic ATP-sensitive potassium channel. _Cell_ 168, 101–110.e10 (2017). Article CAS PubMed Google Scholar
* Martin, G. M., Kandasamy, B., DiMaio, F., Yoshioka, C. & Shyng, S. L. Anti-diabetic drug binding site in a mammalian KATP channel revealed by Cryo-EM. _eLife_ 6, e31054 (2017).
Article PubMed PubMed Central Google Scholar * Martin, G. M. et al. Cryo-EM structure of the ATP-sensitive potassium channel illuminates mechanisms of assembly and gating. _eLife_ 6,
e24149 (2017). Article PubMed PubMed Central Google Scholar * Lee, K. P. K., Chen, J. & MacKinnon, R. Molecular structure of human KATP in complex with ATP and ADP. _eLife_ 6, e32481
(2017). Article PubMed PubMed Central Google Scholar * Nichols, C. G. KATP channels as molecular sensors of cellular metabolism. _Nature_ 440, 470–476 (2006). Article CAS PubMed
Google Scholar * Vedovato, N., Ashcroft, F. M. & Puljung, M. C. The nucleotide-binding sites of sur1: a mechanistic model. _Biophys. J._ 109, 2452–2460 (2015). Article CAS PubMed
PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank A. Murray and members of the laboratories of R.G. and A. Murray for insightful discussions. This work was funded
in part by NIH grant R01GM120996 (to R.G.). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Molecular and Cellular Biology, Harvard University, Cambridge, MA, USA Sriram Srikant
& Rachelle Gaudet Authors * Sriram Srikant View author publications You can also search for this author inPubMed Google Scholar * Rachelle Gaudet View author publications You can also
search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Rachelle Gaudet. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests.
ADDITIONAL INFORMATION PEER REVIEW INFORMATION: Katarzyna Marcinkiewicz was the primary editor on this article and managed its editorial process and peer review in collaboration with the
rest of the editorial team. PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION Supplementary Table 1 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Srikant, S., Gaudet, R. Mechanics and pharmacology of
substrate selection and transport by eukaryotic ABC exporters. _Nat Struct Mol Biol_ 26, 792–801 (2019). https://doi.org/10.1038/s41594-019-0280-4 Download citation * Received: 29 December
2018 * Accepted: 17 July 2019 * Published: 26 August 2019 * Issue Date: September 2019 * DOI: https://doi.org/10.1038/s41594-019-0280-4 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative