Play all audios:
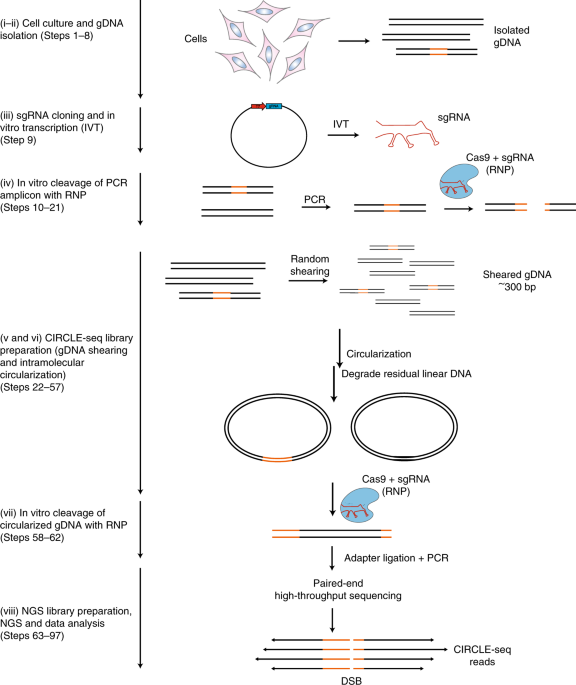
ABSTRACT Circularization for in vitro reporting of cleavage effects by sequencing (CIRCLE-seq) is a sensitive and unbiased method for defining the genome-wide activity (on-target and
off-target) of CRISPR–Cas9 nucleases by selective sequencing of nuclease-cleaved genomic DNA (gDNA). Here, we describe a detailed experimental and analytical protocol for CIRCLE-seq. The
principle of our method is to generate a library of circularized gDNA with minimized numbers of free ends. Highly purified gDNA circles are treated with CRISPR–Cas9 ribonucleoprotein
complexes, and nuclease-linearized DNA fragments are then ligated to adapters for high-throughput sequencing. The primary advantages of CIRCLE-seq as compared with other in vitro methods for
defining genome-wide genome editing activity are (i) high enrichment for sequencing nuclease-cleaved gDNA/low background, enabling sensitive detection with low sequencing depth
requirements; and (ii) the fact that paired-end reads can contain complete information on individual nuclease cleavage sites, enabling use of CIRCLE-seq in species without high-quality
reference genomes. The entire protocol can be completed in 2 weeks, including time for gRNA cloning, sequence verification, in vitro transcription, library preparation, and sequencing.
Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54
other Nature Portfolio journals Get Nature+, our best-value online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and
online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes
which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY
OTHERS TRACKING-SEQ REVEALS THE HETEROGENEITY OF OFF-TARGET EFFECTS IN CRISPR–CAS9-MEDIATED GENOME EDITING Article 02 July 2024 DEFINING GENOME-WIDE CRISPR–CAS GENOME-EDITING NUCLEASE
ACTIVITY WITH GUIDE-SEQ Article 12 November 2021 BID-SEQ FOR TRANSCRIPTOME-WIDE QUANTITATIVE SEQUENCING OF MRNA PSEUDOURIDINE AT BASE RESOLUTION Article 15 November 2023 REFERENCES * Silva,
G. et al. Meganucleases and other tools for targeted genome engineering: perspectives and challenges for gene therapy. _Curr. Gene Ther._ 11, 11–27 (2011). Article CAS PubMed Google
Scholar * Urnov, F. D., Rebar, E. J., Holmes, M. C., Zhang, H. S. & Gregory, P. D. Genome editing with engineered zinc finger nucleases. _Nature_ 11, 636–646 (2010). CAS Google Scholar
* Cermak, T. et al. Efficient design and assembly of custom TALEN and other TAL effector based constructs for DNA targeting. _Nucleic Acids Res_. 39, e82 (2011). Article CAS PubMed
Google Scholar * Sander, J. D. & Joung, J. K. CRISPR-Cas systems for editing, regulating and targeting genomes. _Nat. Biotechnol._ 32, 347–355 (2014). Article CAS PubMed Google
Scholar * Doudna, J. A. & Charpentier, E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. _Science_ 346, 1258096 (2014). Article PubMed Google Scholar *
Rouet, P., Smih, F. & Jasin, M. Introduction of double-strand breaks into the genome of mouse cells by expression of a rare-cutting endonuclease. _Mol. Cell. Biol._ 14, 8096–8106 (1994).
Article CAS PubMed Google Scholar * Fu, Y. et al. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. _Nat. Biotechnol._ 31, 822–826 (2013). Article
CAS PubMed Google Scholar * Hsu, P. D. et al. DNA targeting specificity of RNA-guided Cas9 nucleases. _Nat. Biotechnol._ 31, 827–832 (2013). Article CAS PubMed Google Scholar * Tsai,
S. Q. et al. CIRCLE-seq: a highly sensitive in vitro screen for genome-wide CRISPR-Cas9 nuclease off-targets. _Nat. Methods_ 14, 607–614 (2017). Article CAS PubMed Google Scholar * Tsai,
S. Q. & Joung, J. K. Defining and improving the genome-wide specificities of CRISPR-Cas9 nucleases. _Nat. Rev. Genet._ 17, 300 (2016). Article CAS Google Scholar * Wang, X. et al.
Unbiased detection of off-target cleavage by CRISPR-Cas9 and TALENs using integrase-defective lentiviral vectors. _Nat. Biotechnol._ 33, 175–178 (2015). Article CAS Google Scholar * Tsai,
S. Q. et al. GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. _Nat. Biotechnol._ 33, 187–197 (2015). Article CAS Google Scholar * Kleinstiver, B.
P. et al. Genome-wide specificities of CRISPR-Cas Cpf1 nucleases in human cells. _Nat. Biotechnol._ 34, 869–874 (2016). Article CAS PubMed Google Scholar * Gabriel, R. et al. An unbiased
genome-wide analysis of zinc-finger nuclease specificity. _Nat. Biotechnol._ 29, 816–823 (2011). Article CAS Google Scholar * Frock, R. L. et al. Genome-wide detection of DNA
double-stranded breaks induced by engineered nucleases. _Nat. Biotechnol._ 33, 179–186 (2015). Article CAS Google Scholar * Hu, J. et al. Detecting DNA double-stranded breaks in mammalian
genomes by linear amplification-mediated high-throughput genome-wide translocation sequencing. _Nat. Protoc._ 11, 853–871 (2016). Article CAS PubMed Google Scholar * Crosetto, N. et al.
Nucleotide-resolution DNA double-strand break mapping by next-generation sequencing. _Nat. Methods_ 10, 361–365 (2013). Article CAS PubMed Google Scholar * Yan, W. X. et al. BLISS is a
versatile and quantitative method for genome-wide profiling of DNA double-strand breaks. _Nat. Commun._ 8, 15058 (2017). Article CAS PubMed Google Scholar * Leansing, S. V. et al.
DSBCapture: in situ capture and sequencing of DNA breaks. _Nat. Methods_ 13, 855–857 (2016). Article Google Scholar * Canela, A. et al. DNA breaks and end resection measured genome-wide by
end-sequencing. _Mol. Cell_ 63, 898–911 (2016). Article CAS Google Scholar * Kim, D. et al. Digenome-seq: genome-wide profiling of CRISPR-Cas9 off-target effects in human cells. _Nat.
Methods_ 12, 237–243 (2015). Article CAS Google Scholar * Kim, D. et al. Genome-wide analysis reveals specificities of Cpf1 endonucleases in human cells. _Nat. Biotechnol._ 34, 863–868
(2016). Article CAS Google Scholar * Cameron, P. et al. Mapping the genomic landscape of CRISPR-Cas9 cleavage. _Nat. Methods_ 14, 600–606 (2017). Article CAS Google Scholar * Fisher,
S. et al. A scalable, fully automated process for construction of sequence-ready human exome targeted capture libraries. _Genome Biol._ 12, R1 (2011). Article PubMed Google Scholar *
Robin, J. D. et al. Comparison of DNA quantification methods for next generation sequencing. _Sci. Rep._ 6, 24067 (2016). Article CAS PubMed Google Scholar * Fu, Y. et al. Improving
CRISPR-Cas nuclease specificity using truncated guide RNAs. _Nat. Biotechnol._ 32, 279–284 (2014). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank N.
Malinin for helpful comments and suggestions on the manuscript. This work was supported by St. Jude Children’s Research Hospital and ALSAC, St. Jude Children’s Research Hospital
Collaborative Research Consortium on Novel Gene Therapies for Sickle Cell Disease (SCD), the Doris Duke Charitable Foundation (2017093), National Institutes of Health (NIH) grant U01HL145793
(to S.Q.T.), an NIH Director’s Pioneer Award (DP1GM105378) (to J.K.J.), NIH grants R35GM118158 and NIH R01GM107427 (to J.K.J.), and the Desmond and Ann Heathwood MGH Research Scholar Award
(to J.K.J.). AUTHOR INFORMATION Author notes * Nhu T. Nguyen Present address: Cutaneous Biology Research Center, Department of Dermatology, Massachusetts General Hospital and Harvard Medical
School, Boston, MA, USA AUTHORS AND AFFILIATIONS * Department of Hematology, St. Jude Children’s Research Hospital, Memphis, TN, USA Cicera R. Lazzarotto, Xing Tang & Shengdar Q. Tsai *
Molecular Pathology Unit, Center for Cancer Research, and Center for Computational and Integrative Biology, Massachusetts General Hospital, Charlestown, MA, USA Nhu T. Nguyen, Jose
Malagon-Lopez, Jimmy A. Guo, Martin J. Aryee & J. Keith Joung * Department of Pathology, Harvard Medical School, Boston, MA, USA Jose Malagon-Lopez, Martin J. Aryee & J. Keith Joung
* Department of Biostatistics, Harvard T.H. Chan School of Public Health, Boston, MA, USA Jose Malagon-Lopez & Martin J. Aryee Authors * Cicera R. Lazzarotto View author publications You
can also search for this author inPubMed Google Scholar * Nhu T. Nguyen View author publications You can also search for this author inPubMed Google Scholar * Xing Tang View author
publications You can also search for this author inPubMed Google Scholar * Jose Malagon-Lopez View author publications You can also search for this author inPubMed Google Scholar * Jimmy A.
Guo View author publications You can also search for this author inPubMed Google Scholar * Martin J. Aryee View author publications You can also search for this author inPubMed Google
Scholar * J. Keith Joung View author publications You can also search for this author inPubMed Google Scholar * Shengdar Q. Tsai View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS C.R.L. and S.Q.T. wrote the manuscript with input from all authors. N.T.N. and S.Q.T. developed the original experimental protocol in the J.K.J. lab.
C.R.L. in the S.Q.T. lab and J.A.G. in the J.K.J. lab further optimized the protocol. X.T., J.M.-L., M.J.A. and S.Q.T. contributed to the CIRCLE-seq software analysis pipeline. C.R.L.
performed experiments and data analysis. CORRESPONDING AUTHOR Correspondence to Shengdar Q. Tsai. ETHICS DECLARATIONS COMPETING INTERESTS J.K.J. has financial interests in Beam Therapeutics,
Blink Therapeutics, Editas Medicine, Encadia, Monitor Biotechnologies (formerly Beacon Genomics), Pairwise Plants, Poseida Therapeutics and Transposagen Biopharmaceuticals. S.Q.T. and
M.J.A. have financial interests in Monitor Biotechnologies. M.J.A. and J.K.J.’s interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in
accordance with their conflict of interest policies. J.K.J. and S.Q.T. are co-inventors on a patent describing the CIRCLE-seq method that has been licensed to Monitor Biotechnologies. J.K.J.
is a member of the Board of Directors of the American Society of Gene & Cell Therapy. The remaining authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE:
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RELATED LINKS KEY REFERENCES USING THIS PROTOCOL 1. Tsai, S. Q. et al.
_Nat. Methods_ 14, 607–614 (2017): https://doi.org/10.1038/nmeth.4278 2. Akcakaya, P. et al. _Nature_ 561, 416–419 (2018): https://doi.org/10.1038/s41586-018-0500-9 RIGHTS AND PERMISSIONS
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Lazzarotto, C.R., Nguyen, N.T., Tang, X. _et al._ Defining CRISPR–Cas9 genome-wide nuclease activities with CIRCLE-seq. _Nat
Protoc_ 13, 2615–2642 (2018). https://doi.org/10.1038/s41596-018-0055-0 Download citation * Published: 19 October 2018 * Issue Date: November 2018 * DOI:
https://doi.org/10.1038/s41596-018-0055-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative