Play all audios:
ABSTRACT Optically controlled nongenetic neuromodulation represents a promising approach for the fundamental study of neural circuits and the clinical treatment of neurological disorders.
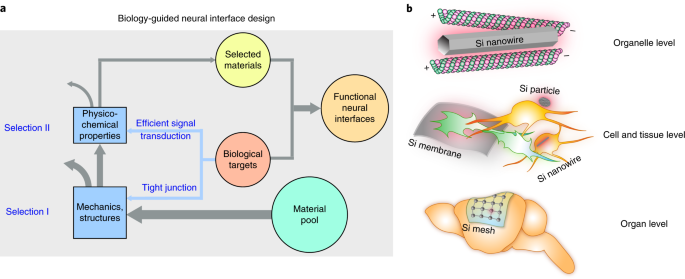
Among the existing material candidates that can transduce light energy into biologically relevant cues, silicon (Si) is particularly advantageous due to its highly tunable electrical and
optical properties, ease of fabrication into multiple forms, ability to absorb a broad spectrum of light, and biocompatibility. This protocol describes a rational design principle for
Si-based structures, general procedures for material synthesis and device fabrication, a universal method for evaluating material photoresponses, detailed illustrations of all
instrumentation used, and demonstrations of optically controlled nongenetic modulation of cellular calcium dynamics, neuronal excitability, neurotransmitter release from mouse brain slices,
and brain activity in the mouse brain in vivo using the aforementioned Si materials. The entire procedure takes ~4–8 d in the hands of an experienced graduate student, depending on the
specific biological targets. We anticipate that our approach can also be adapted in the future to study other systems, such as cardiovascular tissues and microbial communities. Access
through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other
Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and online
access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which
are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS
BIORESORBABLE THIN-FILM SILICON DIODES FOR THE OPTOELECTRONIC EXCITATION AND INHIBITION OF NEURAL ACTIVITIES Article 05 September 2022 NEURAL MODULATION WITH PHOTOTHERMALLY ACTIVE
NANOMATERIALS Article 31 January 2023 IMPLANTABLE NANOPHOTONIC NEURAL PROBES FOR INTEGRATED PATTERNED PHOTOSTIMULATION AND ELECTROPHYSIOLOGICAL RECORDING Article Open access 05 April 2025
DATA AVAILABILITY The authors declare that all data supporting the findings of this study are available within the original papers. Other supporting data are available upon reasonable
request to the corresponding authors. REFERENCES * Ashkan, K., Rogers, P., Bergman, H. & Ughratdar, I. Insights into the mechanisms of deep brain stimulation. _Nat. Rev. Neurol._ 13,
548–554 (2017). Article Google Scholar * Famm, K., Litt, B., Tracey, K. J., Boyden, E. S. & Slaoui, M. Drug discovery: a jump-start for electroceuticals. _Nature_ 496, 159–161 (2013).
Article CAS Google Scholar * Wichmann, T. & Delong, M. R. Deep brain stimulation for neurologic and neuropsychiatric disorders. _Neuron_ 52, 197–204 (2006). Article CAS Google
Scholar * Fisher, R. S. & Velasco, A. L. Electrical brain stimulation for epilepsy. _Nat. Rev. Neurol._ 10, 261–270 (2014). Article Google Scholar * Jefferys, J. G. Nonsynaptic
modulation of neuronal activity in the brain: electric currents and extracellular ions. _Physiol. Rev._ 75, 689–723 (1995). Article CAS Google Scholar * Fregni, F. & Pascual-Leone, A.
Technology insight: noninvasive brain stimulation in neurology—perspectives on the therapeutic potential of rTMS and tDCS. _Nat. Clin. Pract. Neurol._ 3, 383–393 (2007). Article Google
Scholar * Perlmutter, J. S. & Mink, J. W. Deep brain stimulation. _Annu. Rev. Neurosci._ 29, 229–257 (2006). Article CAS Google Scholar * Kringelbach, M. L., Jenkinson, N., Owen, S.
L. & Aziz, T. Z. Translational principles of deep brain stimulation. _Nat. Rev. Neurosci._ 8, 623–635 (2007). Article CAS Google Scholar * Chen, R., Canales, A. & Anikeeva, P.
Neural recording and modulation technologies. _Nat. Rev. Mater._ 2, 16093 (2017). Article CAS Google Scholar * Lacour, S. P., Courtine, G. & Guck, J. Materials and technologies for
soft implantable neuroprostheses. _Nat. Rev. Mater._ 1, 16063 (2016). Article CAS Google Scholar * Salatino, J. W., Ludwig, K. A., Kozai, T. D. Y. & Purcell, E. K. Glial responses to
implanted electrodes in the brain. _Nat. Biomed. Eng._ 1, 862–877 (2017). Article Google Scholar * Jeong, J. W. et al. Soft materials in neuroengineering for hard problems in neuroscience.
_Neuron_ 86, 175–186 (2015). Article CAS Google Scholar * Minev, I. R. et al. Electronic dura mater for long-term multimodal neural interfaces. _Science_ 347, 159–163 (2015). Article
CAS Google Scholar * Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. _Nat. Neurosci._
8, 1263–1268 (2005). Article CAS Google Scholar * Zhang, F. et al. The microbial opsin family of optogenetic tools. _Cell_ 147, 1446–1457 (2011). Article CAS Google Scholar * Zhang, F.
et al. Multimodal fast optical interrogation of neural circuitry. _Nature_ 446, 633–639 (2007). Article CAS Google Scholar * Yizhar, O., Fenno, L. E., Davidson, T. J., Mogri, M. &
Deisseroth, K. Optogenetics in neural systems. _Neuron_ 71, 9–34 (2011). Article CAS Google Scholar * Bareket, L. et al. Semiconductor nanorod-carbon nanotube biomimetic films for
wire-free photostimulation of blind retinas. _Nano Lett._ 14, 6685–6692 (2014). Article CAS Google Scholar * Carvalho-de-Souza, J. L. et al. Photosensitivity of neurons enabled by
cell-targeted gold nanoparticles. _Neuron_ 86, 207–217 (2015). Article CAS Google Scholar * Ghezzi, D. et al. A polymer optoelectronic interface restores light sensitivity in blind rat
retinas. _Nat. Photonics_ 7, 400–406 (2013). Article CAS Google Scholar * Maya-Vetencourt, J. F. et al. A fully organic retinal prosthesis restores vision in a rat model of degenerative
blindness. _Nat. Mater._ 16, 681–689 (2017). Article CAS Google Scholar * Kim, D. H. et al. Stretchable and foldable silicon integrated circuits. _Science_ 320, 507–511 (2008). Article
CAS Google Scholar * Patolsky, F. et al. Detection, stimulation, and inhibition of neuronal signals with high-density nanowire transistor arrays. _Science_ 313, 1100–1104 (2006). Article
CAS Google Scholar * Tian, B. et al. Three-dimensional, flexible nanoscale field-effect transistors as localized bioprobes. _Science_ 329, 830–834 (2010). Article CAS Google Scholar *
Tian, B. et al. Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. _Nat. Mater._ 11, 986–994 (2012). Article CAS Google Scholar * Jiang, Y. & Tian, B. Inorganic
semiconductor biointerfaces. _Nat. Rev. Mater._ 3, 473–490 (2018). Article Google Scholar * Wang, W., Wu, S., Reinhardt, K., Lu, Y. & Chen, S. Broadband light absorption enhancement in
thin-film silicon solar cells. _Nano Lett._ 10, 2012–2018 (2010). Article CAS Google Scholar * Wang, K. X., Yu, Z., Liu, V., Cui, Y. & Fan, S. Absorption enhancement in ultrathin
crystalline silicon solar cells with antireflection and light-trapping nanocone gratings. _Nano Lett._ 12, 1616–1619 (2012). Article CAS Google Scholar * Hong, G., Antaris, A. L. &
Dai, H. Near-infrared fluorophores for biomedical imaging. _Nat. Biomed. Eng._ 1, 0010 (2017). Article Google Scholar * Yun, S. H. & Kwok, S. J. J. Light in diagnosis, therapy and
surgery. _Nat. Biomed. Eng._ 1, 0008 (2017). Article Google Scholar * Tian, B. et al. Coaxial silicon nanowires as solar cells and nanoelectronic power sources. _Nature_ 449, 885–889
(2007). Article CAS Google Scholar * Walter, M. G. et al. Solar water splitting cells. _Chem. Rev._ 110, 6446–6473 (2010). Article CAS Google Scholar * Hochbaum, A. I. et al. Enhanced
thermoelectric performance of rough silicon nanowires. _Nature_ 451, 163–167 (2008). Article CAS Google Scholar * Roder, P. B., Smith, B. E., Davis, E. J. & Pauzauskie, P. J.
Photothermal heating of nanowires. _J. Phys. Chem. C_ 118, 1407–1416 (2014). Article CAS Google Scholar * Jiang, Y. et al. Heterogeneous silicon mesostructures for lipid-supported
bioelectric interfaces. _Nat. Mater._ 15, 1023–1030 (2016). Article CAS Google Scholar * Parameswaran, R. et al. Photoelectrochemical modulation of neuronal activity with free-standing
coaxial silicon nanowires. _Nat. Nanotechnol._ 13, 260–266 (2018). Article CAS Google Scholar * Jiang, Y. et al. Rational design of silicon structures for optically controlled multiscale
biointerfaces. _Nat. Biomed. Eng._ 2, 508–521 (2018). Article Google Scholar * Verkhratsky, A., Krishtal, O. A. & Petersen, O. H. From Galvani to patch clamp: the development of
electrophysiology. _Pflügers Arch._ 453, 233–247 (2006). Article CAS Google Scholar * Sakmann, B. & Neher, E. _Single-Channel Recording_ 2nd edn (Springer, New York, 1995). * Hai, A.,
Shappir, J. & Spira, M. E. Long-term, multisite, parallel, in-cell recording and stimulation by an array of extracellular microelectrodes. _J. Neurophysiol._ 104, 559–568 (2010).
Article CAS Google Scholar * Spira, M. E. & Hai, A. Multi-electrode array technologies for neuroscience and cardiology. _Nat. Nanotechnol._ 8, 83–94 (2013). Article CAS Google
Scholar * Robinson, J. T. et al. Vertical nanowire electrode arrays as a scalable platform for intracellular interfacing to neuronal circuits. _Nat. Nanotechnol._ 7, 180–184 (2012). Article
CAS Google Scholar * Ghezzi, D. et al. A hybrid bioorganic interface for neuronal photoactivation. _Nat. Commun._ 2, 166 (2011). Article Google Scholar * Chen, R., Romero, G.,
Christiansen, M. G., Mohr, A. & Anikeeva, P. Wireless magnetothermal deep brain stimulation. _Science_ 347, 1477–1480 (2015). Article CAS Google Scholar * Dobson, J. Remote control of
cellular behaviour with magnetic nanoparticles. _Nat. Nanotechnol._ 3, 139–143 (2008). Article CAS Google Scholar * Huang, H., Delikanli, S., Zeng, H., Ferkey, D. M. & Pralle, A.
Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. _Nat. Nanotechnol._ 5, 602–606 (2010). Article CAS Google Scholar * Legon, W. et al.
Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. _Nat. Neurosci._ 17, 322–329 (2014). Article CAS Google Scholar * Marino, A. et al.
Piezoelectric nanoparticle-assisted wireless neuronal stimulation. _ACS Nano_ 9, 7678–7689 (2015). Article CAS Google Scholar * Tufail, Y. et al. Transcranial pulsed ultrasound stimulates
intact brain circuits. _Neuron_ 66, 681–694 (2010). Article CAS Google Scholar * Tufail, Y., Yoshihiro, A., Pati, S., Li, M. M. & Tyler, W. J. Ultrasonic neuromodulation by brain
stimulation with transcranial ultrasound. _Nat. Protoc._ 6, 1453–1470 (2011). Article CAS Google Scholar * Tyler, W. J., Lani, S. W. & Hwang, G. M. Ultrasonic modulation of neural
circuit activity. _Curr. Opin. Neurobiol._ 50, 222–231 (2018). Article CAS Google Scholar * Tyler, W. J. et al. Remote excitation of neuronal circuits using low-intensity, low-frequency
ultrasound. _PLoS ONE_ 3, e3511 (2008). Article Google Scholar * Montgomery, K. L., Iyer, S. M., Christensen, A. J., Deisseroth, K. & Delp, S. L. Beyond the brain: optogenetic control
in the spinal cord and peripheral nervous system. _Sci. Transl. Med._ 8, 337rv5 (2016). Article Google Scholar * Maimon, B. E., Sparks, K., Srinivasan, S., Zorzos, A. N. & Herr, H. M.
Spectrally distinct channelrhodopsins for two-colour optogenetic peripheral nerve stimulation. _Nat. Biomed. Eng._ 2, 485–496 (2018). Article Google Scholar * Klapoetke, N. C. et al.
Independent optical excitation of distinct neural populations. _Nat. Methods_ 11, 338–346 (2014). Article CAS Google Scholar * Lorach, H. et al. Photovoltaic restoration of sight with
high visual acuity. _Nat. Med._ 21, 476–482 (2015). Article CAS Google Scholar * Mandel, Y. et al. Cortical responses elicited by photovoltaic subretinal prostheses exhibit similarities
to visually evoked potentials. _Nat. Commun._ 4, 1980 (2013). Article Google Scholar * Mathieson, K. et al. Photovoltaic retinal prosthesis with high pixel density. _Nat. Photonics_ 6,
391–397 (2012). Article CAS Google Scholar * Lugo, K., Miao, X., Rieke, F. & Lin, L. Y. Remote switching of cellular activity and cell signaling using light in conjunction with
quantum dots. _Biomed. Opt. Express_ 3, 447–454 (2012). Article CAS Google Scholar * Pappas, T. C. et al. Nanoscale engineering of a cellular interface with semiconductor nanoparticle
films for photoelectric stimulation of neurons. _Nano Lett._ 7, 513–519 (2007). Article CAS Google Scholar * Rand, D. et al. Direct electrical neurostimulation with organic pigment
photocapacitors. _Adv. Mater._ 30, e1707292 (2018). Article Google Scholar * Sytnyk, M. et al. Cellular interfaces with hydrogen-bonded organic semiconductor hierarchical nanocrystals.
_Nat. Commun._ 8, 91 (2017). Article Google Scholar * Sato, T., Shapiro, M. G. & Tsao, D. Y. Ultrasonic neuromodulation causes widespread cortical activation via an indirect auditory
mechanism. _Neuron_ 98, 1031–1041 (2018). Article CAS Google Scholar * Guo, H. et al. Ultrasound produces extensive brain activation via a cochlear pathway. _Neuron_ 98, 1020–1030 (2018).
Article CAS Google Scholar * Yoo, S., Hong, S., Choi, Y., Park, J. H. & Nam, Y. Photothermal inhibition of neural activity with near-infrared-sensitive nanotransducers. _ACS Nano_ 8,
8040–8049 (2014). Article CAS Google Scholar * Rogers, J. A., Lagally, M. G. & Nuzzo, R. G. Synthesis, assembly and applications of semiconductor nanomembranes. _Nature_ 477, 45–53
(2011). Article CAS Google Scholar * Patolsky, F., Zheng, G. & Lieber, C. M. Fabrication of silicon nanowire devices for ultrasensitive, label-free, real-time detection of biological
and chemical species. _Nat. Protoc._ 1, 1711–1724 (2006). Article CAS Google Scholar * Zhao, D. et al. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom
pores. _Science_ 279, 548–552 (1998). Article CAS Google Scholar * Kleitz, F., Choi, S. H. & Ryoo, R. Cubic Ia3d large mesoporous silica: synthesis and replication to platinum
nanowires, carbon nanorods and carbon nanotubes. _Chem. Commun_. 2003, 2136-2137 (2003). * Fang, Y. et al. Texturing silicon nanowires for highly localized optical modulation of cellular
dynamics. _Nano Lett._ 18, 4487–4492 (2018). Article CAS Google Scholar * Merrill, D. R., Bikson, M. & Jefferys, J. G. Electrical stimulation of excitable tissue: design of
efficacious and safe protocols. _J. Neurosci. Methods_ 141, 171–198 (2005). Article Google Scholar * Luo, Z. et al. Atomic gold-enabled three-dimensional lithography for silicon
mesostructures. _Science_ 348, 1451–1455 (2015). Article CAS Google Scholar * Melli, G. & Hoke, A. Dorsal root ganglia sensory neuronal cultures: a tool for drug discovery for
peripheral neuropathies. _Expert Opin. Drug. Discov._ 4, 1035–1045 (2009). Article CAS Google Scholar * Oesterle, A. with Sutter Instrument Company. Pipette cookbook 2018: P-97 &
P-1000 micropipette pullers. https://www.sutter.com/PDFs/pipette_cookbook.pdf (2018). * Molecular Devices. The Axon™ guide: a guide to electrophysiology and biophysics laboratory techniques.
https://mdc.custhelp.com/euf/assets/content/Axon%20Guide%203rd%20edition.pdf (2012). * Molleman, A. _Patch Clamping: An Introductory Guide to Patch Clamp Electrophysiology_ (John Wiley
& Sons, Chichester, UK, 2003). * Rueda, A. G. Whole cell patch clamp recordings for characterizing neuronal electrical properties of iPSC-derive neurons.
https://docs.axolbio.com/wp-content/uploads/patch-clamp-protocol-final.pdf (2018). * Hamill, O. P., Marty, A., Neher, E., Sakmann, B. & Sigworth, F. J. Improved patch-clamp techniques
for high-resolution current recording from cells and cell-free membrane patches. _Pflügers Arch._ 391, 85–100 (1981). Article CAS Google Scholar * Edwards, F. A., Konnerth, A., Sakmann,
B. & Takahashi, T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. _Pflügers Arch._ 414, 600–612 (1989). Article CAS Google
Scholar * Stuart, G. J., Dodt, H. U. & Sakmann, B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. _Pflügers Arch._ 423,
511–518 (1993). Article CAS Google Scholar * Suter, B. A. et al. Ephus: multipurpose data acquisition software for neuroscience experiments. _Front. Neural Circuits_ 4, 100 (2010).
Article Google Scholar * Mostany, R. & Portera-Cailliau, C. A craniotomy surgery procedure for chronic brain imaging. _J. Vis. Exp_. 2008, e680 (2008). * Tang, J. et al. Nanowire
arrays restore vision in blind mice. _Nat. Commun._ 9, 786 (2018). Article Google Scholar * Savchenko, A. et al. Graphene biointerfaces for optical stimulation of cells. _Sci. Adv._ 4,
eaat0351 (2018). Article Google Scholar * Yao, J., Liu, B. & Qin, F. Rapid temperature jump by infrared diode laser irradiation for patch-clamp studies. _Biophys. J._ 96, 3611–3619
(2009). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the Air Force Office of Scientific Research (AFOSR FA9550-18-1-0503), the US Army
Research Office (W911NF-18-1-0042), the US Office of Naval Research (N000141612530, N000141612958), the National Science Foundation (NSF MRSEC, DMR 1420709), the Searle Scholars Foundation,
the National Institutes of Health (NIH NS101488, NS061963, GM030376, R21-EY023430, R21-EY027101), an MSTP Training Grant (T32GM007281), and the Paul and Daisy Soros Foundation. Atom-probe
tomography was performed at the Northwestern University Center for Atom-Probe Tomography (NUCAPT), whose atom-probe tomography equipment was purchased and upgraded with funding from NSF-MRI
(DMR-0420532) and ONR-DURIP (N00014-0400798, N00014-0610539, N00014-0910781) grants. NUCAPT is a Research Facility at the Materials Research Center of Northwestern University, supported by
the National Science Foundation’s MRSEC program (grant DMR-1121262). Instrumentation at NUCAPT was further upgraded by the Initiative for Sustainability and Energy at Northwestern (ISEN).
This work made use of the Japan Electron Optics Laboratory (JEOL) JEM-ARM200CF and JEOL JEM-3010 TEM in the Electron Microscopy Service of the Research Resources Center at the University of
Illinois at Chicago (UIC). The acquisition of the UIC JEOL JEM-ARM200CF was supported by an MRI-R2 grant from the National Science Foundation (DMR-0959470). AUTHOR INFORMATION Author notes *
These authors contributed equally: Yuanwen Jiang, Ramya Parameswaran, Xiaojian Li, João L. Carvalho-de-Souza. AUTHORS AND AFFILIATIONS * Department of Chemistry, The University of Chicago,
Chicago, IL, USA Yuanwen Jiang & Bozhi Tian * The James Franck Institute, The University of Chicago, Chicago, IL, USA Yuanwen Jiang, Xiang Gao & Bozhi Tian * The Graduate Program in
Biophysical Sciences, The University of Chicago, Chicago, IL, USA Ramya Parameswaran * Department of Physiology, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA
Xiaojian Li & Gordon M. G. Shepherd * Department of Biochemistry and Molecular Biology, The University of Chicago, Chicago, IL, USA João L. Carvalho-de-Souza & Francisco Bezanilla *
Insitute for Molecular Engineering, The University of Chicago, Chicago, IL, USA Lingyuan Meng * Institute for Biophysical Dynamics, The University of Chicago, Chicago, IL, USA Francisco
Bezanilla & Bozhi Tian * Centro Interdisciplinario de Neurociencia de Valparaíso, Facultad de Ciencias, Universidad de Valparaíso, Valparaíso, Chile Francisco Bezanilla Authors * Yuanwen
Jiang View author publications You can also search for this author inPubMed Google Scholar * Ramya Parameswaran View author publications You can also search for this author inPubMed Google
Scholar * Xiaojian Li View author publications You can also search for this author inPubMed Google Scholar * João L. Carvalho-de-Souza View author publications You can also search for this
author inPubMed Google Scholar * Xiang Gao View author publications You can also search for this author inPubMed Google Scholar * Lingyuan Meng View author publications You can also search
for this author inPubMed Google Scholar * Francisco Bezanilla View author publications You can also search for this author inPubMed Google Scholar * Gordon M. G. Shepherd View author
publications You can also search for this author inPubMed Google Scholar * Bozhi Tian View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Y.J.,
R.P., X.L., J.L.C.-d.-S., F.B., G.M.G.S., and B.T. developed the protocol. Y.J., R.P., X.L., and J.L.C.-d.-S. performed the experiments. Y.J., R.P., X.L., and B.T. wrote the manuscript with
input from J.L.C.-d.-S., X.G., L.M., F.B., and G.M.G.S. CORRESPONDING AUTHORS Correspondence to Yuanwen Jiang or Bozhi Tian. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no
competing interests. ADDITIONAL INFORMATION JOURNAL PEER REVIEW INFORMATION: _Nature Protocols_ thanks Tal Dvir and other anonymous reviewer(s) for their contribution to the peer review of
this work. PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RELATED LINKS KEY REFERENCES USING THIS
PROTOCOL Jiang, Y. et al. _Nat. Mater_. 15, 1023–1030 (2016): https://doi.org/10.1038/nmat4673 Parameswaran, R. et al. _Nat. Nanotechnol_. 13, 260–266 (2018):
https://doi.org/10.1038/s41565-017-0041-7 Jiang, Y. et al. _Nat. Biomed. Eng_. 2, 508–521 (2018): https://doi.org/10.1038/s41551-018-0230-1 SUPPLEMENTARY INFORMATION REPORTING SUMMARY
SUPPLEMENTARY DATA 1 Mask design for the Si mesh structure SUPPLEMENTARY DATA 2 Mask design for the SU-8 pillar structure RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Jiang, Y., Parameswaran, R., Li, X. _et al._ Nongenetic optical neuromodulation with silicon-based materials. _Nat Protoc_ 14, 1339–1376 (2019).
https://doi.org/10.1038/s41596-019-0135-9 Download citation * Received: 10 September 2018 * Accepted: 10 January 2019 * Published: 12 April 2019 * Issue Date: May 2019 * DOI:
https://doi.org/10.1038/s41596-019-0135-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative