Play all audios:
ABSTRACT Bacteria use surface-exposed, proteinaceous fibers called pili for diverse behaviors, including horizontal gene transfer, surface sensing, motility, and pathogenicity. Visualization
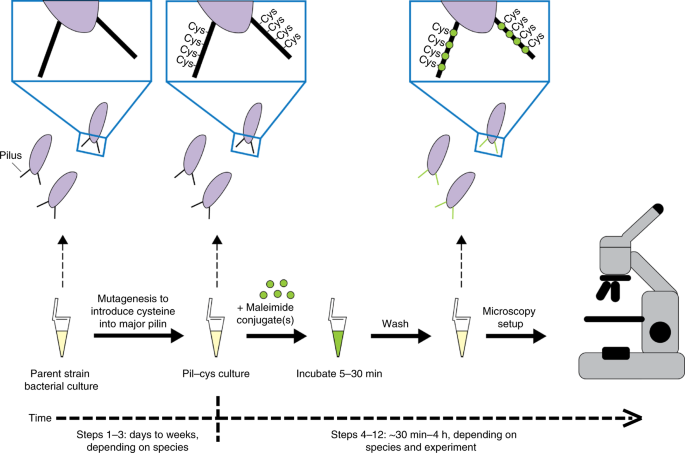
of these filamentous nanomachines and their activity in live cells has proven challenging, largely due to their small size. Here, we describe a broadly applicable method for labeling and
imaging pili and other surface-exposed nanomachines in live cells. This technique uses a combination of genetics and maleimide-based click chemistry in which a cysteine substitution is made
in the major pilin subunit for subsequent labeling with thiol-reactive maleimide dyes. Large maleimide-conjugated molecules can also be used to physically interfere with the dynamic activity
of filamentous nanomachines. We describe parameters for selecting cysteine substitution positions, optimized labeling conditions for epifluorescence imaging of pilus fibers, and methods for
impeding pilus activity. After cysteine knock-in strains have been generated, this protocol can be completed within 30 min to a few hours, depending on the species and the experiment of
choice. Visualization of extracellular nanomachines such as pili using this approach can provide a more comprehensive understanding of the role played by these structures in distinct
bacterial behaviors. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution
Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12
print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be
subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR
CONTENT BEING VIEWED BY OTHERS ADHESION PILUS RETRACTION POWERS TWITCHING MOTILITY IN THE THERMOACIDOPHILIC CRENARCHAEON _SULFOLOBUS ACIDOCALDARIUS_ Article Open access 14 June 2024
_ACINETOBACTER BAYLYI_ REGULATES TYPE IV PILUS SYNTHESIS BY EMPLOYING TWO EXTENSION MOTORS AND A MOTOR PROTEIN INHIBITOR Article Open access 18 June 2021 LIVE-PAINT ALLOWS SUPER-RESOLUTION
MICROSCOPY INSIDE LIVING CELLS USING REVERSIBLE PEPTIDE-PROTEIN INTERACTIONS Article Open access 20 August 2020 DATA AVAILABILITY The data that support this study are available from the
corresponding author upon reasonable request. REFERENCES * Green, E. R. & Mecsas, J. Bacterial secretion systems: an overview. in _Virulence Mechanisms of Bacterial Pathogens_ 5th edn
(eds Kudva, I. T. et al.) 215–239 (2016). * Kearns, D. B. A field guide to bacterial swarming motility. _Nat. Rev. Microbiol._ 8, 634–644 (2010). Article CAS Google Scholar * Pelicic, V.
et al. Type IV pili: e pluribus unum? _Microbiology_ 68, 827–837 (2003). Google Scholar * Berry, J.-L. & Pelicic, V. Exceptionally widespread nanomachines composed of type IV pilins:
the prokaryotic Swiss Army knives. _FEMS Microbiol. Rev._ 39, 134–154 (2015). Article CAS Google Scholar * Blair, K. M., Turner, L., Winkelman, J. T., Berg, H. C. & Kearns, D. B. A
molecular clutch disables flagella in the _Bacillus subtilis_ biofilm. _Science_ 320, 1636–1638 (2008). Article CAS Google Scholar * Ellison, C. K. et al. Obstruction of pilus retraction
stimulates bacterial surface sensing. _Science_ 358, 535–538 (2017). Article CAS Google Scholar * Paradis, G. et al. Variability in bacterial flagella re-growth patterns after breakage.
_Sci. Rep._ 7, 1282 (2017). Article Google Scholar * Ellison, C. K. et al. Retraction of DNA-bound type IV competence pili initiates DNA uptake during natural transformation in _Vibrio
cholerae_. _Nat. Microbiol._ 3, 773–780 (2018). Article CAS Google Scholar * Berne, C. et al. Feedback regulation of _Caulobacter crescentus_ holdfast synthesis by flagellum assembly via
the holdfast inhibitor HfiA. _Mol. Microbiol._ 110, 219–238 (2018). Article CAS Google Scholar * Cairns, L. S. et al. FlgN is required for flagellum-based motility by _Bacillus subtilis_.
_J. Bacteriol._ 196, 2216–2226 (2014). Article Google Scholar * Turner, L., Stern, A. S. & Berg, H. C. Growth of flagellar filaments of _Escherichia coli_ is independent of filament
length. _J. Bacteriol._ 194, 2437–2442 (2012). Article CAS Google Scholar * Dietrich, M., Mollenkopf, H., So, M. & Friedrich, A. Pilin regulation in the _pilT_ mutant of _Neisseria
gonorrhoeae_ strain MS11. _FEMS Microbiol. Lett._ 296, 248–256 (2009). Article CAS Google Scholar * Blocker, A., Komoriya, K. & Aizawa, S.-I. Type III secretion systems and bacterial
flagella: insights into their function from structural similarities. _Proc. Natl. Acad. Sci. USA_ 100, 3027–3030 (2003). Article CAS Google Scholar * Craig, L. et al. Type IV pilus
structure by cryo-electron microscopy and crystallography: implications for pilus assembly and functions. _Mol. Cell_ 23, 651–662 (2006). Article CAS Google Scholar * Chang, Y.-W. et al.
Architecture of the type IVa pilus machine. _Science_ 351, aad2001 (2016). Article Google Scholar * Wang, F. et al. Cryoelectron microscopy reconstructions of the _Pseudomonas aeruginosa_
and _Neisseria gonorrhoeae_ type IV pili at sub-nanometer resolution. _Structure_ 25, 1423–1435.e4 (2017). Article CAS Google Scholar * Mahmoud, K. K. & Koval, S. F. Characterization
of type IV pili in the life cycle of the predator bacterium _Bdellovibrio_. _Microbiology_ 156, 1040–1051 (2010). Article CAS Google Scholar * Seitz, P. & Blokesch, M. DNA-uptake
machinery of naturally competent _Vibrio cholerae_. _Proc. Natl. Acad. Sci. USA_ 110, 17987–17992 (2013). Article CAS Google Scholar * Imhaus, A.-F. & Duménil, G. The number of
_Neisseria meningitidis_ type IV pili determines host cell interaction. _EMBO J._ 33, 1767–1783 (2014). Article CAS Google Scholar * Skerker, J. M. & Berg, H. C. Direct observation of
extension and retraction of type IV pili. _Proc. Natl. Acad. Sci. USA_ 98, 6901–6904 (2001). Article CAS Google Scholar * Skerker, J. M. & Shapiro, L. Identification and cell cycle
control of a novel pilus system in _Caulobacter crescentus_. _EMBO J._ 19, 3223–3234 (2000). Article CAS Google Scholar * Bernard, C. S., Bordi, C., Termine, E., Filloux, A. & de
Bentzmann, S. Organization and PprB-dependent control of the Pseudomonas aeruginosa tad Locus, involved in Flp pilus biology. _J. Bacteriol._ 191, 1961–1973 (2009). Article CAS Google
Scholar * Turner, L. & Berg, H. C. Labeling bacterial flagella with fluorescent dyes. _Methods Mol. Biol._ 1729, 71–76 (2018). Article CAS Google Scholar * Giltner, C. L., Nguyen, Y.
& Burrows, L. L. Type IV pilin proteins: versatile molecular modules. _Microbiol. Mol. Biol. Rev._ 76, 740–772 (2012). Article CAS Google Scholar * Craig, L., Pique, M. E. &
Tainer, J. A. Type IV pilus structure and bacterial pathogenicity. _Nat. Rev. Microbiol._ 2, 363–378 (2004). Article CAS Google Scholar * Petersen, B. et al. A generic method for
assignment of reliability scores applied to solvent accessibility predictions. _BMC Struct. Biol._ 9, 51 (2009). Article Google Scholar * Ng, D. et al. The _Vibrio cholerae_ minor pilin
TcpB initiates assembly and retraction of the toxin-coregulated pilus. _PLOS Pathog._ 12, e1006109 (2016). Article Google Scholar * Jones, C. J. et al. C-di-GMP regulates motile to sessile
transition by modulating MshA pili biogenesis and near-surface motility behavior in _Vibrio cholerae_. _PLoS Pathog._ 11, e1005068 (2015). Article Google Scholar * Burrows, L. L.
Twitching motility: type IV pili in action. _Annu. Rev. Microbiol._ 66, 493–520 (2012). Article CAS Google Scholar * Marks, M. E. et al. The genetic basis of laboratory adaptation in
_Caulobacter crescentus_. _J. Bacteriol._ 192, 3678–3688 (2010). Article CAS Google Scholar * Miller, V. L., DiRita, V. J. & Mekalanos, J. J. Identification of toxS, a regulatory gene
whose product enhances toxR-mediated activation of the cholera toxin promoter. _J. Bacteriol._ 171, 1288–1293 (1989). Article CAS Google Scholar * Ried, J. L. & Collmer, A. An
nptI-sacB-sacR cartridge for constructing directed, unmarked mutations in gram-negative bacteria by marker exchange-eviction mutagenesis. _Gene_ 57, 239–246 (1987). Article CAS Google
Scholar * Dalia, A. B., McDonough, E. & Camilli, A. Multiplex genome editing by natural transformation. _Proc. Natl. Acad. Sci. USA_ 111, 8937–8942 (2014). Article CAS Google Scholar
* Dalia, T. N. et al. Enhancing multiplex genome editing by natural transformation (MuGENT) via inactivation of ssDNA exonucleases. _Nucleic Acids Res._ 45, 7527–7537 (2017). Article CAS
Google Scholar * Dalia, A. B. Natural cotransformation and multiplex genome editing by natural transformation (MuGENT) of _Vibrio cholerae_. _Methods Mol. Biol._ 1839, 53–64 (2018).
Article Google Scholar * POINDEXTER, J. S. Biological properties and classification of the _Caulobacter_ group. _Bacteriol. Rev._ 28, 231–295 (1964). CAS PubMed PubMed Central Google
Scholar * Hepp, C. & Maier, B. Kinetics of DNA uptake during transformation provide evidence for a translocation ratchet mechanism. _Proc. Natl. Acad. Sci. USA_ 113, 12467–12472 (2016).
Article CAS Google Scholar * Pönisch, W. et al. Pili mediated intercellular forces shape heterogeneous bacterial microcolonies prior to multicellular differentiation. _Sci. Rep._ 8,
16567 (2018). Article Google Scholar * Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M. N. & Sternberg, M. J. E. The Phyre2 web portal for protein modeling, prediction and analysis.
_Nat. Protoc._ 10, 845–858 (2015). Article CAS Google Scholar * Craig, L. et al. Type IV pilin structure and assembly. _Mol. Cell_ 11, 1139–1150 (2003). Article CAS Google Scholar *
Pettersen, E. F. et al. UCSF Chimera? A visualization system for exploratory research and analysis. _J. Comput. Chem._ 25, 1605–1612 (2004). Article CAS Google Scholar Download references
ACKNOWLEDGEMENTS We thank M. D. Koch, A. M. Randich, and M. Jacq for critical feedback on the manuscript. This work was supported by grant R35GM122556 from the National Institutes of Health
and by a Canada 150 Research Chair in Bacterial Cell Biology to Y.V.B., by grants R35GM12867 and AI118863 from the National Institutes of Health to A.B.D., and by National Science
Foundation Fellowship 1342962 to C.K.E. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Biology, Indiana University, Bloomington, IN, USA Courtney K. Ellison, Triana N. Dalia,
Ankur B. Dalia & Yves V. Brun * Département de Microbiologie, Infectiologie et Immunologie, Université de Montréal, Montreal, Quebec, Canada Yves V. Brun Authors * Courtney K. Ellison
View author publications You can also search for this author inPubMed Google Scholar * Triana N. Dalia View author publications You can also search for this author inPubMed Google Scholar *
Ankur B. Dalia View author publications You can also search for this author inPubMed Google Scholar * Yves V. Brun View author publications You can also search for this author inPubMed
Google Scholar CONTRIBUTIONS C.K.E. and Y.V.B conceived the study. C.K.E. and T.N.D. performed the experiments. C.K.E., A.B.D., and Y.V.B. analyzed the data. C.K.E. wrote the manuscript with
help from A.B.D. and Y.V.B. CORRESPONDING AUTHOR Correspondence to Yves V. Brun. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION
PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RELATED LINKS KEY REFERENCES USING THIS PROTOCOL
Ellison, C. K. et al. _Science_ 358, 535–538 (2017): http://science.sciencemag.org/content/358/6362/535 Ellison, C. K. et al. _Nat. Microbiol_. 3, 773–780 (2018):
https://www.nature.com/articles/s41564-018-0174-y SUPPLEMENTARY INFORMATION REPORTING SUMMARY RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ellison,
C.K., Dalia, T.N., Dalia, A.B. _et al._ Real-time microscopy and physical perturbation of bacterial pili using maleimide-conjugated molecules. _Nat Protoc_ 14, 1803–1819 (2019).
https://doi.org/10.1038/s41596-019-0162-6 Download citation * Received: 30 November 2018 * Accepted: 05 March 2019 * Published: 26 April 2019 * Issue Date: June 2019 * DOI:
https://doi.org/10.1038/s41596-019-0162-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative