Play all audios:
ABSTRACT The chromosome conformation capture method and its derivatives, such as circularized chromosome conformation capture, carbon copy chromosome conformation capture, high-throughput
chromosome conformation capture and capture high-throughput chromosome conformation capture, have pioneered our understanding of the principles of chromosome folding in the nucleus. These
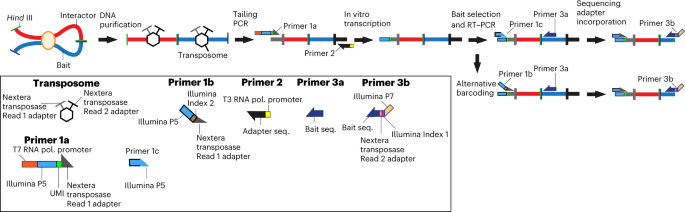
technical advances, however, cannot precisely quantitate interaction frequency in very small input samples. Here we describe a protocol for the Nodewalk assay, which is based on converting
chromosome conformation capture DNA samples to RNA and subsequently to cDNA using strategically placed primers. This pipeline enables the quantitative analyses of chromatin fiber
interactions without compromising its sensitivity down to <300 cells, making it suitable for MiSeq analyses of higher-order chromatin structures in biopsies, circulating tumor cells and
transitional cell states, for example. Importantly, the quality of the Nodewalk sample can be assessed before sequencing to avoid unnecessary costs. Moreover, it enables analyses from
hundreds of different restriction enzyme fragment viewpoints within the same initial small input sample to uncover complex, genome-wide networks. Following optimization of the different
steps, the entire protocol can be completed within 2 weeks. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS
OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn
more Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to
full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our
FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS CAPTURE-C: A MODULAR AND FLEXIBLE APPROACH FOR HIGH-RESOLUTION CHROMOSOME CONFORMATION CAPTURE Article 04 February 2022
SYSTEMATIC EVALUATION OF CHROMOSOME CONFORMATION CAPTURE ASSAYS Article Open access 03 September 2021 ORCHESTRATING CHROMOSOME CONFORMATION CAPTURE ANALYSIS WITH BIOCONDUCTOR Article Open
access 05 February 2024 DATA AVAILABILITY All processed Nodewalk data have been deposited in the Gene Expression Omnibus (GSE76049). The ChIP and DamId-seq data were retrieved from the Gene
Expression Omnibus as follows: cLADs (GSE22428), H3K9me2 (GSE58534) and H3K4me1 (GSM1240111). Source data for Figs. 6, 7, 8 and 3, 4 are available online in refs. 24,25, respectively. The
images in Fig. 2 represent unprocessed original Bioanalyzer files. CODE AVAILABILITY The analysis pipeline is available at https://github.com/Anita-Rolf-lab/Nodewalk. REFERENCES * Hansen, A.
S., Cattoglio, C., Darzacq, X. & Tjian, R. Recent evidence that TADs and chromatin loops are dynamic structures. _Nucleus_ 9, 20–32 (2018). Article CAS PubMed Google Scholar *
Lieberman-Aiden, E. et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. _Science_ 326, 289–293 (2009). Article CAS PubMed PubMed
Central Google Scholar * Dixon, J. R. et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. _Nature_ 485, 376–380 (2012). Article CAS PubMed
PubMed Central Google Scholar * Brant, L. et al. Exploiting native forces to capture chromosome conformation in mammalian cell nuclei. _Mol. Syst. Biol._ 12, 891 (2016). Article PubMed
PubMed Central Google Scholar * Beagrie, R. A. et al. Complex multi-enhancer contacts captured by genome architecture mapping. _Nature_ 543, 519–524 (2017). Article CAS PubMed PubMed
Central Google Scholar * Bintu, B. et al. Super-resolution chromatin tracing reveals domains and cooperative interactions in single cells. _Science_ 362, eaau1783 (2018). Article PubMed
PubMed Central Google Scholar * Quinodoz, S. A. et al. Higher-order inter-chromosomal hubs shape 3D genome organization in the nucleus. _Cell_ 174, 744–757 e724 (2018). Article CAS
PubMed PubMed Central Google Scholar * Tan, L., Xing, D., Chang, C. H., Li, H. & Xie, X. S. Three-dimensional genome structures of single diploid human cells. _Science_ 361, 924–928
(2018). Article CAS PubMed PubMed Central Google Scholar * Ulianov, S. V. et al. Order and stochasticity in the folding of individual Drosophila genomes. _Nat. Commun._ 12, 41 (2021).
Article CAS PubMed PubMed Central Google Scholar * Chang, L. H., Ghosh, S. & Noordermeer, D. TADs and their borders: free movement or building a wall? _J. Mol. Biol._ 432, 643–652
(2020). Article CAS PubMed Google Scholar * Lupianez, D. G. et al. Disruptions of topological chromatin domains cause pathogenic rewiring of gene–enhancer interactions. _Cell_ 161,
1012–1025 (2015). Article CAS PubMed PubMed Central Google Scholar * Osterwalder, M. et al. Enhancer redundancy provides phenotypic robustness in mammalian development. _Nature_ 554,
239–243 (2018). Article CAS PubMed PubMed Central Google Scholar * Darrow, E. M. et al. Deletion of DXZ4 on the human inactive X chromosome alters higher-order genome architecture.
_Proc. Natl Acad. Sci. USA_ 113, E4504–E4512 (2016). Article CAS PubMed PubMed Central Google Scholar * Ay, F. et al. Identifying multi-locus chromatin contacts in human cells using
tethered multiple 3C. _BMC Genomics_ 16, 121 (2015). Article PubMed PubMed Central Google Scholar * Olivares-Chauvet, P. et al. Capturing pairwise and multi-way chromosomal conformations
using chromosomal walks. _Nature_ 540, 296–300 (2016). Article CAS PubMed Google Scholar * de Laat, W. & Grosveld, F. Spatial organization of gene expression: the active chromatin
hub. _Chromosome Res._ 11, 447–459 (2003). Article PubMed Google Scholar * Allahyar, A. et al. Enhancer hubs and loop collisions identified from single-allele topologies. _Nat. Genet._
50, 1151–1160 (2018). Article CAS PubMed Google Scholar * Oudelaar, A. M. et al. Single-allele chromatin interactions identify regulatory hubs in dynamic compartmentalized domains. _Nat.
Genet._ 50, 1744–1751 (2018). Article CAS PubMed PubMed Central Google Scholar * Fudenberg, G. et al. Formation of chromosomal domains by loop extrusion. _Cell Rep._ 15, 2038–2049
(2016). Article CAS PubMed PubMed Central Google Scholar * Zhao, Z. et al. Circular chromosome conformation capture (4C) uncovers extensive networks of epigenetically regulated intra-
and interchromosomal interactions. _Nat. Genet._ 38, 1341–1347 (2006). Article CAS PubMed Google Scholar * Zhao, H. et al. PARP1- and CTCF-mediated interactions between active and
repressed chromatin at the lamina promote oscillating transcription. _Mol. Cell_ 59, 984–997 (2015). Article CAS PubMed Google Scholar * Gondor, A., Rougier, C. & Ohlsson, R.
High-resolution circular chromosome conformation capture assay. _Nat. Protoc._ 3, 303–313 (2008). Article PubMed Google Scholar * Paulsen, J. et al. Long-range interactions between
topologically associating domains shape the four-dimensional genome during differentiation. _Nat. Genet._ 51, 835–843 (2019). Article CAS PubMed Google Scholar * Sumida, N. et al. MYC as
a driver of stochastic chromatin networks: implications for the fitness of cancer cells. _Nucleic Acids Res._ 48, 10867–10876 (2020). Article CAS PubMed PubMed Central Google Scholar *
Scholz, B. A. et al. WNT signaling and AHCTF1 promote oncogenic MYC expression through super-enhancer-mediated gene gating. _Nat. Genet._ 51, 1723–1731 (2019). Article CAS PubMed Google
Scholar * Chachoua, I. et al. Canonical WNT signaling-dependent gating of MYC requires a noncanonical CTCF function at a distal binding site. _Nat. Commun._ 13, 204 (2022). Article CAS
PubMed PubMed Central Google Scholar * Dekker, J., Rippe, K., Dekker, M. & Kleckner, N. Capturing chromosome conformation. _Science_ 295, 1306–1311 (2002). Article CAS PubMed
Google Scholar * Timp, W. et al. Large hypomethylated blocks as a universal defining epigenetic alteration in human solid tumors. _Genome Med._ 6, 61 (2014). Article PubMed PubMed Central
Google Scholar * Lewenshtein, B. Binary codes capable of correcting deletions, insertions, and reversals. _Sov. Phys. Dokl._ 10, 707–710 (1965). Google Scholar * Williams, J. &
Mackinnon, D. P. Resampling and distribution of the product methods for testing indirect effects in complex models. _Struct. Equ. Modeling_ 15, 23–51 (2008). Article PubMed PubMed Central
Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the Swedish Research Council (VR 03108), the Swedish Childhood Cancer Fund (PR2017-0132), the Swedish Cancer
Society (CAN 708), the Lundberg Foundation (2018-0138), Karolinska Institutet, the Novo Nordisk Foundation (NNF16OC0021512), the Cancer Society in Stockholm (2018–2021) and the KA
Wallenberg Foundation (KAW 2017.0077). We thank A. F. Woodbridge for his efforts in the initial development of the Nodewalk pipeline and R. Ohlsson for valuable discussions. AUTHOR
INFORMATION Author notes * Noriyuki Sumida Present address: Bio Systems Design Department, Bio Analytical Systems Product Division, Analytical & Medical Solution Business Group, Hitachi
High Technologies, Hitachinaka, Ibaraki, Japan * These authors contributed equally: Johanna Vestlund, Noriyuki Sumida, Rashid Mehmood. AUTHORS AND AFFILIATIONS * Department of Oncology and
Pathology, Bioclinicum, Karolinska University Hospital, U2, Akademiska Stråket 1, Karolinska Institutet, Stockholm, Sweden Johanna Vestlund, Noriyuki Sumida, Rashid Mehmood, Deeksha
Bhartiya, Shuangyang Wu & Anita Göndör Authors * Johanna Vestlund View author publications You can also search for this author inPubMed Google Scholar * Noriyuki Sumida View author
publications You can also search for this author inPubMed Google Scholar * Rashid Mehmood View author publications You can also search for this author inPubMed Google Scholar * Deeksha
Bhartiya View author publications You can also search for this author inPubMed Google Scholar * Shuangyang Wu View author publications You can also search for this author inPubMed Google
Scholar * Anita Göndör View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS J.V. updated the Nodewalk protocol and contributed to manuscript
writing; N.S. contributed to the invention of the Nodewalk assay, developed the entire protocol and contributed to the development of the analysis pipeline and manuscript writing; R.M. and
D.B. designed the submitted state of pipeline and implemented the Python code; D.B. designed the manual and tested the code; S.W. contributed to the pipeline code integration and A.G.
contributed to the invention of the Nodewalk assay and wrote the manuscript. CORRESPONDING AUTHOR Correspondence to Anita Göndör. ETHICS DECLARATIONS COMPETING INTERESTS D.B. has formed a
bioinformatics company, Genomiki Solution Ltd., that analyses high throughput data, including chromatin fibre interaction maps. PEER REVIEW PEER REVIEW INFORMATION _Nature Protocols_ thanks
Argyris Papantonis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with
regard to jurisdictional claims in published maps and institutional affiliations. RELATED LINKS KEY REFERENCES USING THIS PROTOCOL Sumida, N. et al_. Nucleic Acids Res_. 48, 10867–10876
(2020): https://doi.org/10.1093/nar/gkaa817 Scholz, B. A. et al. _Nat. Genet_. 51, 1723–1731 (2019): https://doi.org/10.1038/s41588-019-0535-3 SUPPLEMENTARY INFORMATION SUPPLEMENTARY
INFORMATION Supplementary Note. REPORTING SUMMARY RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a
publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing
agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Vestlund, J., Sumida, N., Mehmood, R. _et al._ The Nodewalk assay to quantitate chromatin fiber
interactomes in very small cell populations. _Nat Protoc_ 18, 755–782 (2023). https://doi.org/10.1038/s41596-022-00774-8 Download citation * Received: 06 December 2019 * Accepted: 18 August
2022 * Published: 25 November 2022 * Issue Date: March 2023 * DOI: https://doi.org/10.1038/s41596-022-00774-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative