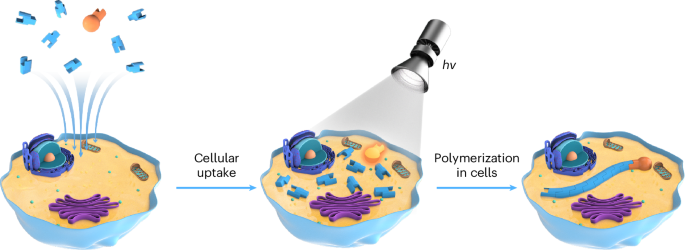
Play all audios:
ABSTRACT The synthesis of synthetic intracellular polymers offers groundbreaking possibilities in cellular biology and medical research, allowing for novel experiments in drug delivery,
bioimaging and targeted cancer therapies. These macromolecules, composed of biocompatible monomers, are pivotal in manipulating cellular functions and pathways due to their bioavailability,
cytocompatibility and distinct chemical properties. This protocol details two innovative methods for intracellular polymerization. The first one uses
2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone (Irgacure 2959) as a photoinitiator for free radical polymerization under UV light (365 nm, 5 mW/cm2). The second method employs
photoinduced electron transfer-reversible addition–fragmentation chain-transfer polymerization with visible light (470 nm, 100 mW/cm2). We further elaborate on isolating these intracellular
polymers by streptavidin/biotin interaction or immobilized metal ion affinity chromatography for polymers tagged with biotin or histidine. The entire process, from polymerization to
isolation, takes ~48 h. Moreover, the intracellular polymers thus generated demonstrate significant potential in enhancing actin polymerization, in bioimaging applications and as a novel
avenue in cancer treatment strategies. The protocol extends to animal models, providing a comprehensive approach from cellular to systemic applications. Users are advised to have a basic
understanding of organic synthesis and cell biology techniques. KEY POINTS * Light-driven free radical and photoinduced electron transfer-reversible addition–fragmentation chain-transfer
polymerization synthesis of structurally defined cellular polymers from biocompatible monomers in the presence of light-sensitive catalysts can modulate cell function and is a potential
anticancer therapy. * The protocol includes guidelines for the design of biotin and/or histidine-tagged polymers and streptavidin- and/or immobilized metal affinity chromatography-based
strategies for their isolation from cell lysate for downstream analysis. Access through your institution Buy or subscribe This is a preview of subscription content, access via your
institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel
any time Learn more Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink *
Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional
subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS NANOCOMPARTMENT-CONFINED POLYMERIZATION IN LIVING SYSTEMS Article Open access 26 August 2023
APPLICATIONS OF SYNTHETIC POLYMERS DIRECTED TOWARD LIVING CELLS Article 20 June 2024 INTRINSICALLY FLUORESCENT POLYUREAS TOWARD CONFORMATION-ASSISTED METAMORPHOSIS, DISCOLORATION AND
INTRACELLULAR DRUG DELIVERY Article Open access 05 August 2022 DATA AVAILABILITY The data that support the findings of this study are available from the corresponding author upon reasonable
request. REFERENCES * Liu, J. & Liu, B. Living cell-mediated in situ polymerization for biomedical applications. _Prog. Polym. Sci._ 129, 101545 (2022). Article CAS Google Scholar *
Wu, D. et al. Polymerization in living organisms. _Chem. Soc. Rev._ 52, 2911–2945 (2023). Article CAS PubMed Google Scholar * Laskar, P., Varghese, O. P. & Shastri, V. P. Advances in
intracellular and on-surface polymerization in living cells: implications for nanobiomedicines. _Adv. Biomed. Res._ 3, 2200174 (2023). CAS Google Scholar * Dergham, M., Lin, S. &
Geng, J. Supramolecular self‐assembly in living cells. _Angew. Chem. Int. Ed._ 61, e202114267 (2022). Article CAS Google Scholar * Streu, C. & Meggers, E. Ruthenium-induced
allylcarbamate cleavage in living cells. _Angew. Chem. Int. Ed._ 45, 5645 (2006). Article CAS Google Scholar * Yusop, R. M., Unciti-Broceta, A., Johansson, E. M., Sánchez-Martín, R. M.
& Bradley, M. Palladium-mediated intracellular chemistry. _Nat. Chem._ 3, 239–243 (2011). Article CAS PubMed Google Scholar * Tomás-Gamasa, M., Martínez-Calvo, M., Couceiro, J. R.
& Mascareñas, J. L. Transition metal catalysis in the mitochondria of living cells. _Nat. Commun._ 7, 12538 (2016). Article PubMed PubMed Central Google Scholar * Devaraj, N. K.,
Thurber, G. M., Keliher, E. J., Marinelli, B. & Weissleder, R. Reactive polymer enables efficient in vivo bioorthogonal chemistry. _Proc. Natl Acad. Sci. USA_ 109, 4762–4767 (2012).
Article CAS PubMed PubMed Central Google Scholar * Wang, J., Wang, X., Fan, X. & Chen, P. R. Unleashing the power of bond cleavage chemistry in living systems. _ACS Cent. Sci._ 7,
929–943 (2021). Article CAS PubMed PubMed Central Google Scholar * Jasinski, F., Zetterlund, P. B., Braun, A. M. & Chemtob, A. Photopolymerization in dispersed systems. _Prog.
Polym. Sci._ 84, 47–88 (2018). Article CAS Google Scholar * Noè, C., Hakkarainen, M. & Sangermano, M. Cationic UV curing of epoxidized biobased resins. _Polymers_ 13, 89 (2021).
Article Google Scholar * Yuan, Y., Li, C., Zhang, R., Liu, R. & Liu, J. Low volume shrinkage photopolymerization system using hydrogen-bond-based monomers. _Prog. Org. Coat._ 137,
105308 (2019). Article CAS Google Scholar * Khudyakov, I. V., Legg, J. C., Purvis, M. B. & Overton, B. J. Kinetics of photopolymerization of acrylates with functionality of 1–6. _Ind.
Eng. Chem. Res._ 38, 3353–3359 (1999). Article CAS Google Scholar * Xiao, P. et al. Visible light sensitive photoinitiating systems: recent progress in cationic and radical
photopolymerization reactions under soft conditions. _Prog. Polym. Sci._ 41, 32–66 (2015). Article CAS Google Scholar * Bonardi, A. et al. High performance near-infrared (NIR)
photoinitiating systems operating under low light intensity and in the presence of oxygen. _Macromolecules_ 51, 1314–1324 (2018). Article CAS Google Scholar * Zhao, H. et al.
Spatiotemporal control of polymer brush formation through photoinduced radical polymerization regulated by DMD light modulation. _Lab Chip_ 19, 2651–2662 (2019). Article CAS PubMed Google
Scholar * Xi, W. et al. Spatial and temporal control of thiol-Michael addition via photocaged superbase in photopatterning and two-stage polymer networks formation. _Macromolecules_ 47,
6159–6165 (2014). Article CAS PubMed PubMed Central Google Scholar * Lang, M., Hirner, S., Wiesbrock, F. & Fuchs, P. A review on modeling cure kinetics and mechanisms of
photopolymerization. _Polymers_ 14, 2074 (2022). Article CAS PubMed PubMed Central Google Scholar * Geng, J. et al. Radical polymerization inside living cells. _Nat. Chem._ 11, 578–586
(2019). Article CAS PubMed Google Scholar * Zhang, Y. et al. Controlled intracellular polymerization for cancer treatment. _JACS Au._ 2, 579–589 (2022). Article CAS PubMed PubMed
Central Google Scholar * Rinnerthaler, M., Bischof, J., Streubel, M. K., Trost, A. & Richter, K. Oxidative stress in aging human skin. _Biomolecules_ 5, 545–589 (2015). Article CAS
PubMed PubMed Central Google Scholar * Xu, J., Shanmugam, S., Duong, H. T. & Boyer, C. Organo-photocatalysts for photoinduced electron transfer-reversible addition–fragmentation chain
transfer (PET–RAFT) polymerization. _Polym. Chem._ 6, 5615–5624 (2015). Article CAS Google Scholar * Xu, Y., Chen, C., Hellwarth, P. B. & Bao, X. Biomaterials for stem cell
engineering and biomanufacturing. _Bioact. Mater._ 4, 366–379 (2019). PubMed PubMed Central Google Scholar * Murakami, T. et al. ALS/FTD mutation-induced phase transition of FUS liquid
droplets and reversible hydrogels into irreversible hydrogels impairs RNP granule function. _Neuron_ 88, 678–690 (2015). Article CAS PubMed PubMed Central Google Scholar * Shen, Q.,
Huang, Y., Bai, H., Lv, F. & Wang, S. Polymer materials synthesized through cell-mediated polymerization strategies for regulation of biological functions. _Acc. Mater. Res._ 4, 57–70
(2022). Article Google Scholar * Chen, Y. et al. Nanocompartment-confined polymerization in living systems. _Nat. Commun._ 14, 5229 (2023). Article CAS PubMed PubMed Central Google
Scholar * Hai, Z. et al. γ-Glutamyltranspeptidase-triggered intracellular gadolinium nanoparticle formation enhances the T2-weighted MR contrast of tumor. _Nano Lett._ 19, 2428–2433 (2019).
Article CAS PubMed Google Scholar * Shen, Q. et al. Intracellular radical polymerization of paclitaxel-bearing acrylamide for self-inflicted apoptosis of cancer cells. _ACS Mater.
Lett._ 3, 1307–1314 (2021). Article CAS Google Scholar * Li, L.-L. et al. Intracellular construction of topology-controlled polypeptide nanostructures with diverse biological functions.
_Nat. Commun._ 8, 1276 (2017). Article PubMed PubMed Central Google Scholar * Liu, C. et al. Intracellular hyperbranched polymerization for circumventing cancer drug resistance. _ACS
Nano_ 17, 11905–11913 (2023). Article CAS PubMed Google Scholar * Marino, A. et al. Nanostructured Brownian surfaces prepared through two-photon polymerization: investigation of stem
cell response. _ACS Nano_ 8, 11869–11882 (2014). Article CAS PubMed Google Scholar * Han, S. B., Kim, J. K., Lee, G. & Kim, D. H. Mechanical properties of materials for stem cell
differentiation. _Adv. Biosyst._ 4, 2000247 (2020). Article Google Scholar * Ghosh, P. & De, P. Modulation of amyloid protein fibrillation by synthetic polymers: Recent advances in the
context of neurodegenerative diseases. _ACS Appl. Bio Mater._ 3, 6598–6625 (2020). Article CAS PubMed Google Scholar * Sundelacruz, S. & Kaplan, D. L. Stem cell-and scaffold-based
tissue engineering approaches to osteochondral regenerative medicine. _Semin. Cell Dev. Biol._ 20, 646–655 (2009). Article CAS PubMed PubMed Central Google Scholar * Liang, G., Ren, H.
& Rao, J. A biocompatible condensation reaction for controlled assembly of nanostructures in living cells. _Nat. Chem._ 2, 54–60 (2010). Article CAS PubMed PubMed Central Google
Scholar * Rong, L. H., Caldona, E. B. & Advincula, R. C. PET–RAFT polymerization under flow chemistry and surface‐initiated reactions. _Polym. Int._ 72, 145–157 (2023). Article CAS
Google Scholar * Parkatzidis, K., Wang, H. S., Truong, N. P. & Anastasaki, A. Recent developments and future challenges in controlled radical polymerization. _Chem_ 6, 1575–1588 (2020).
Article CAS Google Scholar * Xie, W., Zhao, L., Wei, Y. & Yuan, J. Advances in enzyme-catalysis-mediated RAFT polymerization. _Cell Rep. Phys. Sci._ 2, 100487 (2021). Article CAS
Google Scholar * Wu, J., Lin, J. & Huang, P. Harnessing abiotic organic chemistry in living systems for biomedical applications. _Chem. Soc. Rev._ 52, 3973–3990 (2023). Article CAS
PubMed Google Scholar * Joshi, T. & Nebhani, L. Light-regulated growth of polymer chains from the surface of RAFT agent primed mesoporous silica nanoparticles. _Surf. Interfaces_ 29,
101764 (2022). Article CAS Google Scholar * Dufrêne, Y. F. Atomic force microscopy and chemical force microscopy of microbial cells. _Nat. Protoc._ 3, 1132–1138 (2008). Article PubMed
Google Scholar * Stribbling, S. M. & Ryan, A. J. The cell-line-derived subcutaneous tumor model in preclinical cancer research. _Nat. Protoc._ 17, 2108–2128 (2022). Article CAS PubMed
Google Scholar * Heise, C. C., Williams, A., Olesch, J. & Kirn, D. H. Efficacy of a replication-competent adenovirus (ONYX-015) following intratumoral injection: intratumoral spread
and distribution effects. _Cancer Gene Ther._ 6, 499–504 (1999). Article CAS PubMed Google Scholar * Watanabe, S. et al. Transplantation of intestinal organoids into a mouse model of
colitis. _Nat. Protoc._ 17, 649–671 (2022). Article CAS PubMed Google Scholar * Martí, M. et al. Characterization of pluripotent stem cells. _Nat. Protoc._ 8, 223–253 (2013). Article
PubMed Google Scholar * Kaiser, E., Colescott, R. L., Bossinger, C. D. & CookMartí, P. I. Color test for detection of free terminal amino groups in the solid-phase synthesis of
peptides. _Anal. Biochem._ 34, 595–598 (1970). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS The authors thank the National Natural Science Foundation of China
(22071263), the Guangdong Province Zhujiang Talents Program (2019QN01Y127) and the Shenzhen Fundamental Research Program (JCYJ20200109110215774). We thank M. Galluzzi for the AFM
measurement. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China Mohamed Abdelrahim, Quan Gao, Yichuan
Zhang, Qi Xing & Jin Geng * School of Pharmacy, Henan University, Kaifeng, China Yichuan Zhang * Center for Molecular Metabolism, School of Environmental and Biological Engineering,
Nanjing University of Science and Technology, Nanjing, China Weishuo Li * Precision Healthcare University Research Institute, Queen Mary University of London, London, UK Mark Bradley Authors
* Mohamed Abdelrahim View author publications You can also search for this author inPubMed Google Scholar * Quan Gao View author publications You can also search for this author inPubMed
Google Scholar * Yichuan Zhang View author publications You can also search for this author inPubMed Google Scholar * Weishuo Li View author publications You can also search for this author
inPubMed Google Scholar * Qi Xing View author publications You can also search for this author inPubMed Google Scholar * Mark Bradley View author publications You can also search for this
author inPubMed Google Scholar * Jin Geng View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.A. and Q.G. contributed equally to the work.
All authors contributed extensively to the work presented and wrote the manuscript. CORRESPONDING AUTHORS Correspondence to Mark Bradley or Jin Geng. ETHICS DECLARATIONS COMPETING INTERESTS
The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Protocols_ thanks Greg Qiao, Dayong Yang and the other, anonymous, reviewer(s) for their contribution
to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. RELATED LINKS KEY REFERENCES USING THIS PROTOCOL Geng, J. et al. _Nat. Chem_. 11, 578–586 (2019): https://doi.org/10.1038/s41557-019-0240-y Zhang, Y. et al. _JACS Au_ 2,
579–589 (2022): https://doi.org/10.1021/jacsau.1c00373 EXTENDED DATA EXTENDED DATA FIG. 1 CONCENTRATION CALIBRATIONS OF DMA, HPMA, CA-CTA, AND EOSIN Y AND 1H NMR OF ISOLATED POLYMER. A,
UV-vis spectral representation of DMA at concentrations of 1, 5, 10, 20, and 50 mM. B, UV-vis spectra showcasing HPMA at concentrations of 2, 5, 10, 20, and 50 mM. C, UV-vis spectra of
CA-CTA at concentrations of 0.02, 0.1, 0.2, 0.5, and 1.0 mM. D, UV-vis spectral plots of Eosin Y at concentrations of 0.002, 0.01, 0.02, 0.05, and 0.1 mM. E, The graphical depiction of
cellular uptake data. This data was quantified utilizing UV-vis spectroscopy. F, G, 1H NMR spectra (recorded in D2O) of isolated polymers. F, Displays the 1H NMR spectrum for His-PDMA. The
upper spectrum represents the polymer when polymerized in PBS, whereas the lower spectrum is for the polymerized variant in cells. G, Showcases the 1H NMR spectrum for His-PHPMA. As with
(f), the upper graph pertains to the polymer processed in PBS, and the lower represents the cell-polymerized version. For both (f) and (g), the polymer isolated from PBS underwent a dialysis
process against water with a molecular weight cut-off (MWCO) of 1,000. On the other hand, the polymers derived from cell lysates were isolated using magnetic beads and then released at a
temperature of 60 °C. Panels a–e reproduced with permission from ref. 20, American Chemical Society. EXTENDED DATA FIG. 2 CHARACTERIZATIONS OF HIS-POLYMERS ISOLATED FROM HELA CELLS. PET-RAFT
polymerization was conducted within cells utilizing a hexa-histidine-tagged CTA known as His-CTA (for detailed polymerization cocktail compositions, refer to Table 1). Polymers synthesized
intracellularly were procured through dialysis (with a molecular weight cut-off, MWCO, of 1,000 Da) and IMAC isolation. The following polymers were characterized using 1H NMR (in d6-DMSO):
A, His-PHPMA-1. B, His-PHPMA-2. C, His-PDMA-3. D, His-PDMA-1. E, His-PDMA-2. F, His-PDMA-3. G, GPC traces for isolated polymers: His-PHPMA-1, His-PHPMA-2, and His-PHPMA-3. H, GPC traces for
isolated polymers: His-PDMA-1, His-PDMA-2, and His-PDMA-3. Figure adapted with permission from ref. 20, American Chemical Society. EXTENDED DATA FIG. 3 EXPLORATION OF INTRACELLULAR RETENTION
TIME OF MONOMERS, CTAS, AND EOSIN Y. Cells were exposed to PC1, supplemented with YFMA (1.0 µM) and RF-CTA (100 µM), for a duration of 4 h. Then, cells were washed and subjected to either a
10-minute illumination (+hv) or left without illumination (-hv). Representative confocal microscopy images are presented here to show the cellular state immediately after (0 h) and 24 hours
post-illumination. The columns, from left to right, depict the cellular uptake and distribution of Eosin Y, monomers, CTAs, and a merged view, respectively. The top two rows showcase cells
without illumination (- hν) and the bottom two rows present cells with illumination (+ hν). Scale bar = 50 µm. Figure reproduced with permission from ref. 20, American Chemical Society.
EXTENDED DATA FIG. 4 INTRACELLULAR POLYMERIZATION INDUCES CELL DEATH. A, Viability assessment of HeLa, 4T1, and MDA-MB-231 cells post-intracellular polymerization using the CCK-8 assay.
Cells underwent treatment with PC1 for a duration of 4 hours, were subsequently washed, and then illuminated for 10 minutes. This was followed by an incubation period of 24 hours. The
results are expressed as the mean ± SD, based on 6 independent samples per group. For statistical analysis, one-way ANOVA with Dunnett post-test was utilized, comparing to the untreated
cells. Significance levels are indicated as follows: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, with indicating non-significance. B, Evaluation of apoptosis and
necroptosis levels induced by intracellular polymerization, determined using flow cytometry. The cells undergoing apoptosis are Annexin-V-positive and PI-negative, while those undergoing
necroptosis are indicated in the graph. Numerical analysis regarding the number of cells evaluated and the absolute numbers or percentages of the relevant cell populations within post-sort
fractions is provided in the figure. C, Quantitative representation of apoptosis and necroptosis levels from flow cytometry data presented in panel b. Data are presented as percentages of
cells undergoing apoptosis and necroptosis. Significance levels are indicated as: *P < 0.05, **P < 0.01, ****P < 0.0001, ns (not significant). D, E, Analysis of crucial biological
markers using techniques such as immunoblotting and immunoprecipitation assays. The displayed bands correspond to markers like PARP, C-PARP, p53, BCL-2, BAX, AIF, and γ-H2AX. The molecular
weight (in kDa) of each protein is indicated on the right. Figure adapted with permission from ref. 20, American Chemical Society. Source data EXTENDED DATA FIG. 5 INTRACELLULAR
POLYMERIZATION INHIBITED PROLIFERATION. A, Colony growth assay depicting the impact of intracellular polymerization on the proliferative ability of HeLa cells. Left panel shows cells treated
with PBS in the absence of light, while the right panel displays cells under similar conditions but with light exposure. The scale bar is indicative of 1 cm. B, Representation of cell cycle
phases for HeLa cells subjected to treatments with or without intracellular polymerization. The four groups depicted are cells treated with PBS in the absence and presence of light, and
cells treated with PC1 in the absence and presence of light. The percentages of cells in the G0/G1, S, and G2/M phases of the cell cycle are presented. Data were based on n = 6 samples. C,
Immunoblotting assays highlight the expression levels of key cell cycle regulators, specifically Cyclin B1 and Cyclin E1, in HeLa cells under different treatment conditions. GAPDH serves as
the loading control, and the molecular weight (in kDa) of each protein is denoted on the right. Statistical evaluations were executed using one-way ANOVA accompanied by a Dunnett post-test
in comparison with the untreated control groups. Levels of significance are denoted as: *P < 0.05, ***P < 0.001, ****P < 0.0001, ns (not significant). Figure reproduced with
permission from ref. 20, American Chemical Society. Source data EXTENDED DATA FIG. 6 ANALYSIS OF CELL MOBILITY AND PHYSIOLOGICAL CHANGES INDUCED BY INTRACELLULAR POLYMERIZATION. A,
Representative wound-healing assay images capture cell mobilities across different treatment conditions and time points. Each image denotes the percentage of the area not covered by cells in
the wound area at the indicated time post-treatment. B, Quantitative representation of the wound-healing assay in A, presenting the normalized gap percentage over the incubation periods of
0, 24, 48, and 72 hours. Data are from n = 3 samples per condition. C, Immunoblotting assays showing the expression levels of cell-motility-associated proteins E-cadherin, Snail, and
Vimentin. GAPDH is used as the loading control, with the molecular weight (in kDa) for each protein mentioned on the right. D, Illustration of the viscosity p’obe’s molecular structure,
which is activated at an excitation/emission wavelength (_λ_ex/em) of 640/660 nm. The increase in viscosity corresponds to a higher fluorescence intensity. E, Flow cytometric analysis
detailing cellular viscosity across various treatment groups. The measurements employ a Cy5-based viscosity probe, with the fluorescence intensities depicted on the x-axis. F, Atomic force
microscopy results indicating the stiffness of cells subjected to different treatments. In all figures, data points represent individual measurements with mean values shown. Statistical
analysis was performed using one-way ANOVA with a Dunnett post-test compared to untreated control groups, ****P < 0.0001, ns (not significant). Panels a–e reproduced with permission from
ref. 20, American Chemical Society. Source data EXTENDED DATA FIG. 7 IN VIVO EVALUATION OF INTRACELLULAR POLYMERIZATION FOR CANCER TREATMENT. A–C, Tumor size progression in BALB/c nude mice
bearing different tumor models: HeLa (a), MDA-MB-231 (b), and 4T1 (c) over a specified number of days. Mice were subjected to different treatment regimens, and data are representative of n =
5 mice per group. Error bars represent for standard deviations. D, Histological analyses of tumor sections stained with hematoxylin/eosin (H&E), TUNEL, and Ki67. Each staining method
illuminates different features within the tumor tissue, including cellular morphology, apoptosis, and proliferation, respectively. Red arrowheads highlight areas of interest. E, F,
Quantitative evaluations of the tumor sections. The number of TUNEL-positive cells (E) and Ki67-positive cells (F) are represented. Cell numbers were quantified from six random areas within
the tissue, with each experiment conducted with n = 6 samples. Statistical evaluations were executed using one-way ANOVA accompanied by a Dunnett post-test in comparison with the untreated
control groups. Levels of significance are denoted as: *P < 0.05, ***P < 0.001, ****P < 0.0001, ns (not significant). G, Visual representation of the xenografted tumors at day 14
post various treatments, showcasing the size reduction in response to intracellular polymerization. H, Comprehensive hematoxylin and eosin (H&E) stained lung sections taken from mice on
day 19 post-treatment. These illustrations offer insights into potential metastatic spread, with metastatic sites demarcated by black dashed lines. Scale notations: For H&E images in
(d), the scale bar corresponds to 1 mm, whereas for the TUNEL and Ki67 images, the scale bar represents 200 μm and 2 mm in (h). Panels b–h reproduced with permission from ref. 20, American
Chemical Society. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figs. 1 and 2 and Table 1. REPORTING SUMMARY SOURCE DATA SOURCE DATA EXTENDED DATA FIG. 4 Unprocessed
western blots. SOURCE DATA EXTENDED DATA FIG. 5 Unprocessed western blots. SOURCE DATA EXTENDED DATA FIG. 6 Unprocessed western blots. RIGHTS AND PERMISSIONS Springer Nature or its licensor
(e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted
manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Abdelrahim,
M., Gao, Q., Zhang, Y. _et al._ Light-mediated intracellular polymerization. _Nat Protoc_ 19, 1984–2025 (2024). https://doi.org/10.1038/s41596-024-00970-8 Download citation * Received: 20
April 2023 * Accepted: 24 December 2023 * Published: 21 March 2024 * Issue Date: July 2024 * DOI: https://doi.org/10.1038/s41596-024-00970-8 SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative