Play all audios:
ABSTRACT _Vitis vinifera_ is widely grown worldwide for making wine and for use as table grapes. Of the existing cultivars, some have a floral and fruity flavour, referred to as a Muscat
flavour. It is well-documented that this flavour originates from a series of terpene compounds, but the mechanism of terpene content differences among the Muscat-type cultivars remains
unclear. Transcript and terpene metabolite profiles were integrated to elucidate the molecular mechanism of this phenomenon. In this research, three genotypes with different aromatic
strengths were investigated by RNA sequencing. A total of 27 fruit samples from three biological replicates were sequenced on Illumina HiSeq2000 at three stages, corresponding to the
veraison; berries had intermediate Brix value and were harvest-ripe. After quality assessment and data clearance, a total of 254.18 Gb of data with more than 97% Q20 bases were obtained,
approximately 9.41 Gb data were generated per sample. These results will provide a valuable dataset for the discovery of the mechanism of terpene biosynthesis. Design Type(s) transcription
profiling design • gene expression analysis objective Measurement Type(s) transcription profiling assay Technology Type(s) RNA sequencing Factor Type(s) cultivar • biological replicate •
developmental stage Sample Characteristic(s) Vitis vinifera • berry Machine-accessible metadata file describing the reported data (ISA-Tab format) SIMILAR CONTENT BEING VIEWED BY OTHERS
DIFFERENTIALLY EXPRESSION ANALYSES IN FRUIT OF CULTIVATED AND WILD SPECIES OF GRAPE AND PEACH Article Open access 03 February 2023 COMPARATIVE TRANSCRIPTOME PROFILING AND MOLECULAR MARKER
DEVELOPMENT FOR OIL PALM FRUIT COLOR Article Open access 15 September 2022 THE COMPARISONS OF EXPRESSION PATTERN REVEAL MOLECULAR REGULATION OF FRUIT METABOLITES IN _S. NIGRUM_ AND _S.
LYCOPERSICUM_ Article Open access 23 March 2022 BACKGROUND & SUMMARY The trait of aroma is one of the most important parameters for the quality of grapes and is the main concern when
consumers buy grape products. For genetic improvement research and breeding, the biosynthesis mechanism of aromatic compounds and their regulation has attracted much attention. Terpenes are
the typical aromatic compounds in Muscat grapes, and they belong to the second metabolites1,2,3,4; they have a low olfactory threshold and can be easily precepted by humans. The terpenes
mainly exist in the pericarp and in the flesh of some cultivars5, with their content being affected by the genotype6,7, developmental stage8,9, environment and management of the
grape10,11,12,13. Terpenes have two forms: the free form, which directly leads to the aromatic flavour, and the glycoside bound form, in which the potential aromatic compounds transfer to
the free form by hydrolysis14,15,16. Biologically, the biosynthesis of terpene compounds in plants are synthesized by two pathways, the methyl-erythritol-4-phosphate pathway (DXP/MEP) in the
plastid and the mevalonate pathway (MVA) in the cytoplasm17, with terpenes located in the mesocarp and pericarp18. Starting from pyruvic acid and 3-phosphate glyceraldehyde, by
1-deoxy-D-xylulose-5-phosphate synthase (DXS), which is the entrance enzyme in the MEP pathway, the two compounds were changed into 1-deoxy-D-xyulose-5-phosphate and, then, through six
enzymatic reactions, were converted into geranyl-diphosphate (GPP). Geranyl-diphosphate was the substrate for all the terpenes. Then, by a series of terpene synthases, the GPP was
synthesized into hemiterpenes (C5), monoterpenes (C10), sesquiterpenes (C15) or diterpenes (C20)19,20,21,22. The genetic mechanism of Muscat flavour in grapevines has been studied through
quantitative trait loci analysis (QTL) in different F1 populations23,24, and in selfing populations, it has been shown that VvDXS is a structural candidate gene for geraniol, nerol, and
linalool concentrations in wine grapes25. Battilana reported that single nucleotide polymorphism (SNP) mutations in VvDXS are the main cause of the Muscat flavour. The substitution of a
lysine with an asparagine at position 284 of the VvDXS amino acid sequence affects the monoterpene content of Muscat flavour and neutral cultivars26. In Muscat grapes, some cultivars have a
very strong flavour, while others have moderate or light flavour. The terpene type and concentration varied among the cultivars. To date, terpene accumulation has been well-documented in
some wine grapes. Terpene accumulation in developing Gewurztraminer grapes has been shown to be correlated with an increase in the transcript abundances of early terpenoid pathway enzymes27.
Some transcription factors involved in controlling terpene biosynthesis have been predicted in the grapevine cultivar Muscat Blanc à Petits Grains through gene co-expression network
analysis28. A Nudix hydrolase was also found to be a component of a terpene synthase-independent pathway, with cytochrome P450 hydroxylases, epoxide hydrolases and glucosyltransferases genes
potentially involved in monoterpene metabolism29. However, there are few reports on the table grape30. In this study, we present the transcriptome analysis of three genotypes of table
grapes. During berry development, 27 samples, in total, were sequenced on the Illumina HiSeq Platform. After quality assessment and data clearance, a total of 254.18 Gb high-quality base
pairs with more than 97% Q20 bases were obtained, and an approximately 9.41 Gb per sample. In the aggregate, a total of 776 million reads were yielded, with an average of 31.66 million reads
per sample. Furthermore, approximately 76.65% of the total reads were uniquely aligned to the grape genome (V2)31. These data will provide useful information for investigating terpene
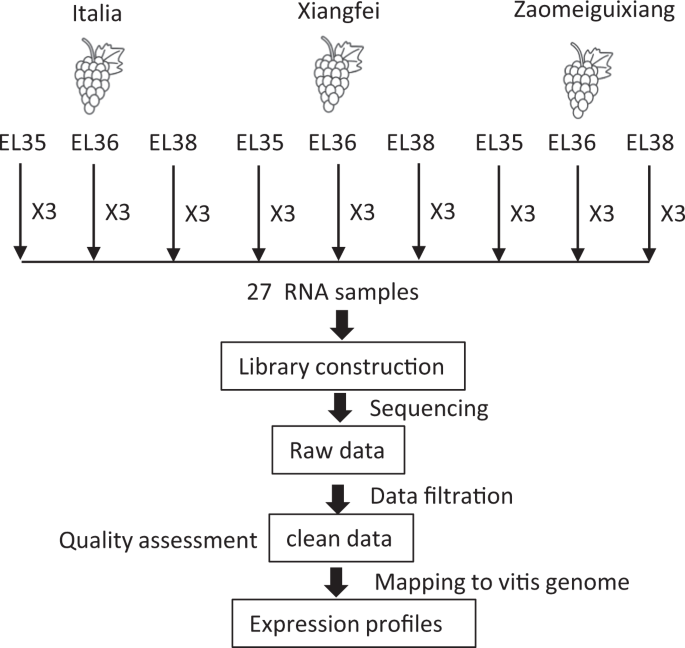
biosynthesis. METHODS OVERVIEW OF THE EXPERIMENTAL DESIGN The berries of three genotypes were collected at three developmental stages. Approximately 300 grape berries were randomly collected
for each replicate, with three replicates harvested for each stage. The experimental design and analysis pipeline are shown in Fig. 1. MATERIALS AND METHODS PLANT MATERIALS Three _V.
vinifera_ cultivars were used for transcript study. ‘Xiangfei’ was registered by our team and has a strong Muscat flavour and a green to golden skin colour, while ‘Italia,’ the famous
mid-late season table grape cultivar that originated in Italy, has a moderate Muscat flavour. ‘Zaomeiguixiang’ has a purple-reddish colour and a strong Muscat flavour. SAMPLING The vines
were grown in the experimental vineyard at the Beijing Academy of Forestry and Pomology Sciences in China (39°58′N and 116°13′E) under a plastic cover and were trained into a two-wire
vertical trellis system with a 2.5-m row space and a 0.75 m plant space. In 2017, berry samples from three vines were harvested at the developmental stages corresponding to EL35, EL36, and
EL3832. The berry begins to colour and soften at EL 35 (about 5% of the berries started to colour and soften), progresses to the complete veraison with an intermediate Brix of EL 36, and
reaches harvest ripeness at EL38. At each stage, three replicates were harvested; approximately 300 grape berries were randomly collected for each replicate. PHYSIOCHEMICAL PARAMETERS Fifty
berries of each replicate were pressed and centrifuged to determine total soluble solids (TSS), pH value and titratable acidity. TSS was measured by a digital refractometer (PAL-1, Atago,
Tokyo, Japan). The pH value was measured by a pH meter (FiveGo F2-Standard, Mettler Toledo, Switzerland). Titratable acidity was analysed by titration with NaOH (0.1M) to the end point of pH
8.2 and expressed as tartaric acid equivalents in accordance with the National Standard of People’s Republic of China (GB/T15038-2006, 2006). The other berries were then frozen in liquid
nitrogen and stored at −80 °C. RNA EXTRACTION AND SEQUENCING The extraction of total RNA from the berries was carried out by a Plant RNA extraction kit (Aidlab Biotechnologies, Beijing,
China). The quality of the RNA was verified by agarose gel electrophoresis, and the concentration was determined by the absorbance ratio (A260/A280, 1.8–2.0) on an Implen P330 nanophotometer
(Implen GmbH, Munich, Germany). The RNA-Seq libraries were constructed from 27 samples according to the methods of Wang33. The enriched mRNA was obtained by using oligo (dT) magnetic beads
then fragmented by 94 °C for 5 min. cDNA was synthesized by Superscript®III Reverse Transcriptase, followed by purification, end repair and dA-tailing and was then ligated with the
sequencing adaptor. Afterwards, PCR amplification was conducted by indexed primers. The constructed library was QC checked by Agilent 2100 Bioanalyzer and ABI StepOnePlus Real-Time PCR
System and then sequenced by Illumina HiSeq2000 platform at BGI Life Tech Co., Ltd. (Shenzhen, China). Low quality reads (more than 20% of the base qualities are lower than 10), reads with
adaptors and reads with unknown bases (N bases more than 5%) were filtered to get clean reads and were stored in FASTQ format. The clean reads were mapped onto the reference grapevine genome
(V2) using Hisat234. DATA RECORDS The RNA-Seq clean data of the 27 samples were deposited at the NCBI Sequence Read Archive with accessions SRP18415235. The files of gene expression level
were deposited in NCBI’s Gene Expression Omnibus (GEO), and are accessible through GEO Series accession number GSE13038636. The information of the differentially expressed genes (DEGs)
between samples were deposited in figshare37. TECHNICAL VALIDATION QUALITY CONTROL The physiochemical parameter of the samples was shown in Table 1. A total of 27 RNA samples were prepared
and sequenced, with the sequencing depth ranging between 22.48 and 33.08 million reads; the Q20 values for the clean reads were above 97%, and the average mapping ratio was 84.72%
(Online-only Table 1). ANALYSIS OF RNA-SEQ DATA After novel transcript detection, novel coding transcripts were merged with reference transcripts to get a complete reference. Clean reads
were mapped to the transcript by using Bowtie238. Gene expression levels were calculated with RSEM39. The distribution of reads based on the detection of read coverage skewness showed good
fragmentation randomness (Fig. 2). The differentially expressed genes (DEGs) between samples were identified by the R package, DESeq240. The DEGs with a |log2ratio| ≥ 1 and a false discovery
rate probability ≤ 0.001 were considered statistically significant. The statistical analyses of DEG are shown in Fig. 3. USAGE NOTES The RNA-Seq fastq.gz files were deposited at Gene
Expression Omnibus and can be downloaded using the fastq-dump tool of the SRA Toolkit (https://www.ncbi.nlm.nih.gov). The V2 reference genome of _V. vinifera_, the annotated file, could be
retrieved at (http://genomes.cribi.unipd.it/grape/). CODE AVAILABILITY SOAPnuke: https://github.com/BGI-flexlab/SOAPnuke. Version: v1.5.2. Parameters: -l 5 -q 0.51 -n 0.55 -i -Q 2–seqType 1.
HISAT2: http://www.ccb.jhu.edu/software/hisat. Version:v2.0.4.Parameters:–phred64–sensitive–no-discordant–no-mixed -I 1 -X 1000. Bowtie2: http://bowtie-bio.sourceforge.net/Bowtie2. Version:
v2.2.5. Parameters: -q–phred64–sensitive–dpad 0–gbar 99999999–mp 1,1–np 1–score-min L,0, −0.1 -I 1 -X 1000–no-mixed–no-discordant -p 1 -k 200. RSEM: http://deweylab.biostat.wisc.edu/RSEM.
Version: v1.2.12. Parameters: default. REFERENCES * Bohlmann, J. & Keeling, C. I. Terpenoid biomaterials. _Plant J._ 54, 656–669 (2008). Article CAS Google Scholar * Magnard, J. L.
_et al_. Plant volatiles. Biosynthesis of monoterpene scent compounds in roses. _Science._ 349, 81–83 (2015). Article ADS CAS Google Scholar * Fenoll, J., Martniez, M. D. A., Hellin, P.
& Flores, P. Changes of free and glycosidically bound monoterpenes and aromatic alcohols in Muscatel and Ruby Seedless table grapes during development. _J. Inter. Des Sciences. De La
Vigne Et Du Vin_ 46, 41–50 (2012). CAS Google Scholar * Croteau, R. Biosynthesis and catabolism of monoterpenoids. _Chem. Rev._ 87, 929–954 (1987). Article CAS Google Scholar * Luan,
F., Mosandl, A., Munch, A. & Wust, M. Metabolism of geraniol in grape berry mesocarp of _Vitis vinifera_ L. cv. Scheurebe: demonstration of stereoselective reduction, E/Z-isomerization,
oxidation and glycosylation. _Phytochemistry._ 66, 295–303 (2005). Article CAS Google Scholar * Fenoll, J., Manso, A., Hellin, P., Ruiz, L. & Flores, P. Changes in the aromatic
composition of the _Vitis vinifera_ grape Muscat Hamburg during ripening. _Food Chem._ 114, 420–428 (2009). Article CAS Google Scholar * Liu, B. _et al_. The free and enzyme-released
volatile compounds of distinctive _Vitis amurensis_ var. Zuoshanyi grapes in China. _Eur. Food Res. Technol._ 240, 985–997 (2015). Article CAS Google Scholar * Kalua, C. M. & Boss, P.
K. Evolution of volatile compounds during the development of Cabernet Sauvignon grapes (_Vitis vinifera L_.). _J. Agric. Food Chem._ 57, 3818–3830 (2009). Article CAS Google Scholar *
Kalua, C. M. & Boss, P. K. Comparison of major volatile compounds from Reisling and Cabernet Sauvignon grapes (_Vitis vinifera L_.) from fruitset to harvest. _Aus. J. Grape and Wine Res_
16, 337–348 (2010). Article CAS Google Scholar * Bureau, S. M., Razungles, A. J. & Baumes, R. L. The aroma of Muscat of Frontignac grapes: effect of the light environment of vine or
bunch on volatiles and glycoconjugates. _J. Sci. Food Agric_ 80, 2012–2020 (2000). Article CAS Google Scholar * Wang, Y. _et al_. Effects of cluster thinning on vine photosynthesis, berry
ripeness and flavonoid composition of Cabernet Sauvignon. _Food Chem._ 248, 101–110 (2018). Article CAS Google Scholar * Xu, X. Q. _et al_. Differences in volatile profiles of Cabernet
Sauvignon grapes grown in two distinct regions of China and their responses to weather conditions. _Plant Physiol. Biochem._ 89, 123–133 (2015). Article CAS Google Scholar * Koundouras,
S., Marinos, V., Gkoulioti, A., Kotseridis, Y. & Van, L. C. Influence of vineyard location and vine water status on fruit maturation of non-irrigated cv. Agiorgitiko (_Vitis vinifera
L_.). effects on wine phenolic and aroma components. _J. Agric. Food Chem._ 54, 5077–5086 (2006). Article CAS Google Scholar * Wilson, B., Strauss, C. R. & Williams, P. J. The
distribution of free and glycosidically-bound monoterpenes among skin, juice, and pulp fractions of some white grape varieties. _Amer. J. Enol.Viticult._ 37, 107–111 (1986). CAS Google
Scholar * Hjelmeland, A. K. & Ebeler, S. E. Glycosidically bound volatile aroma compounds in grapes and wine: a review. _Amer. J. Enol. Viticult._ 66, 1–11 (2015). Article CAS Google
Scholar * Voirin, S. G., Baumes, R. L., Bitteur, S. M., Gunata, Z. Y. & Bayonove, C. L. Novel monoterpene disaccharide glycosides of _vitis vinifera_ grapes. _J. Agric. Food Chem._ 38,
1373–1378 (1990). Article CAS Google Scholar * Dubey, V. S., Bhalla, R. & Luthra, R. An overview of the non-mevalonate pathway for terpenoid biosynthesis in plants. _J. Biosci._ 28,
637–646 (2003). Article CAS Google Scholar * Luan, F. & Wust, M. Differential incorporation of 1-deoxy-Dxylulose into (3S)-linalool and geraniol in grape berry exocarp and mesocarp.
_Phytochemistry_ 60, 451–459 (2002). Article CAS Google Scholar * Dudareva, N., Klempien, A., Muhlemann, J. K. & Kaplan, I. Biosynthesis, function and metabolic engineering of plant
volatile organic compounds. _New Phytol._ 198, 16–32 (2013). Article CAS Google Scholar * Schwab, W., Davidovich-Rikanati, R. & Lewinsohn, E. Biosynthesis of plant-derived flavor
compounds. _The Plant J_ 54, 712–732 (2008). Article CAS Google Scholar * Withers, S. T. & Keasling, J. D. Biosynthesis and engineering of isoprenoid small molecules. _Appl.
Microbiol. Biotechnol._ 73, 980–990 (2007). Article CAS Google Scholar * Degenhardt, J., Kollner, T. G. & Gershenzon, J. Monoterpene and sesquiterpene synthases and the origin of
terpene skeletal diversity in plants. _Phytochemistry_ 70, 1621–1637 (2009). Article CAS Google Scholar * Doligez, A., Audiot, E., Baumes, R. & This, P. QTLs for muscat flavour and
monoterpenic odorant content in grapevine (_Vitis vinifera L_.). _Mol. Breeding_ 18, 109–125 (2006). Article CAS Google Scholar * Battilana, J. _et al_. The 1-deoxy-D: -xylulose
5-phosphate synthase gene co-localizes with a major QTL affecting monoterpene content in grapevine. _Theor. Appl. Genet._ 118, 653–669 (2009). Article CAS Google Scholar * Duchene, E. _et
al_. A grapevine (_Vitis vinifera L_.) deoxy-d-xylulose synthase gene colocates with a major quantitative trait loci for terpenol content. _Theor. Appl. Genet._ 118, 541–552 (2009). Article
CAS Google Scholar * Battilana, J. _et al_. Functional effect of grapevine 1-deoxy-D-xylulose 5-phosphate synthase substitution K284N on Muscat flavour formation. _J. Exp. Bot._ 62,
5497–5508 (2011). Article CAS Google Scholar * Martin, D. M., Chiang, A., Lund, S. T. & Bohlmann, J. Biosynthesis of wine aroma: transcript profiles of hydroxymethylbutenyl
diphosphate reductase, geranyl diphosphate synthase, and linalool/nerolidol synthase parallel monoterpenol glycoside accumulation in Gewürztraminer grapes. _Planta._ 236, 919–929 (2012).
Article CAS Google Scholar * Wen, Y. Q. _et al_. Using the combined analysis of transcripts and metabolites to propose key genes for differential terpene accumulation across two regions.
_BMC Plant Biol._ 15, 240 (2015). Article Google Scholar * Costantini, L. _et al_. Drawing Links from Transcriptome to Metabolites: The Evolution of Aroma in the Ripening Berry of Moscato
Bianco. (_Vitis vinifera L_.). _Front. Plant. Sci._ 8, 780 (2017). Article Google Scholar * Wu, Y. _et al_. Aroma characterization based on aromatic series analysis in table grapes. _Sci.
Rep._ 6, 31116 (2016). Article ADS CAS Google Scholar * Vitulo, N. _et al_. A deep survey of alternative splicing in grape reveals changes in the splicing machinery related to tissue,
stress condition and genotype. _BMC Plant Biol._ 14, 99 (2014). Article Google Scholar * Coombe, B. G. Adoption of a system for identifying grapevine growth stages. _Aust. J. Grape Wine
Res._ 1, 100–110 (1995). Article Google Scholar * Wang, L., Si, Y., Dedow, L. K., Shao, Y., Liu, P. & Brutnell, T. P. A low-cost library construction protocol and data analysis
pipeline for Illumina-based strand specific multiplex RNA-seq. _PLoS One._ 6, e26426 (2011). Article ADS CAS Google Scholar * Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a
fast-spliced aligner with low memory requirements. _Nat. Methods_ 12, 357–360 (2015). Article CAS Google Scholar * NCBI Sequence Read Archive.
http://identifiers.org/ncbi/insdc.sra:SRP184152 (2019). * Sun, L. & Zhu, B. Transcriptome profiles of three Muscat table grape cultivars at three developmental stage. _Gene Expression
Omnibus_, http://identifiers.org/geo:GSE130386 (2019). * Sun, L. _et al_. Transcriptome profiles of three Muscat table grape cultivars to dissect the mechanism of terpene biosynthesis.
_figshare_, https://doi.org/10.6084/m9.figshare.c.4378256.v1 (2019). * Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. _Nat. Methods_ 9, 357–359 (2012). Article
CAS Google Scholar * Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. _BMC Bioinformatics_ 12, 323 (2011). Article
CAS Google Scholar * Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-Seq data with DESeq2. _Genome Biol._ 15, 550 (2014). Article
Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the Science and Technology Innovation Ability Construction Projects of Beijing Academy of Agricultural and
Forestry Sciences (KJCX20180411), Earmarked Fund for China Agriculture Research System (CARS-29) and Beijing Municipal Natural Science Foundation (6192017). AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Beijing Academy of Forestry and Pomology Sciences, Beijing, 100093, China Lei Sun, Xuanyin Zhang, Guojun Zhang, Ailing Yan, Huiling Wang & Xiaoyue Wang * College of
Biological Sciences and Technology, Beijing Forestry University, Beijing, 100083, China Baoqing Zhu * Key Laboratory of Biology and Genetic Improvement of Horticultural Crops (North China),
Ministry of Agriculture and Rural Affairs, Beijing, 100093, China Haiying Xu * Beijing Engineering Research Centre for Deciduous Fruit Trees, Beijing, 100093, China Haiying Xu Authors * Lei
Sun View author publications You can also search for this author inPubMed Google Scholar * Baoqing Zhu View author publications You can also search for this author inPubMed Google Scholar *
Xuanyin Zhang View author publications You can also search for this author inPubMed Google Scholar * Guojun Zhang View author publications You can also search for this author inPubMed Google
Scholar * Ailing Yan View author publications You can also search for this author inPubMed Google Scholar * Huiling Wang View author publications You can also search for this author
inPubMed Google Scholar * Xiaoyue Wang View author publications You can also search for this author inPubMed Google Scholar * Haiying Xu View author publications You can also search for this
author inPubMed Google Scholar CONTRIBUTIONS L.S. designed the experiments and wrote the manuscript. B.Q.Z. analysed the data. X.Y.Z. collected the samples and extracted RNA. G.J.Z.,
A.L.Y., H.L.W. and X.Y.W. reviewed the manuscript. H.Y.X. designed the experiments, reviewed the manuscript and supervised the study. CORRESPONDING AUTHOR Correspondence to Haiying Xu.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. ONLINE-ONLY TABLE ISA-TAB METADATA FILE DOWNLOAD METADATA FILE RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a
Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit
to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are
included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
license, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver http://creativecommons.org/publicdomain/zero/1.0/ applies to the metadata
files associated with this article. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Sun, L., Zhu, B., Zhang, X. _et al._ Transcriptome profiles of three Muscat table grape
cultivars to dissect the mechanism of terpene biosynthesis. _Sci Data_ 6, 89 (2019). https://doi.org/10.1038/s41597-019-0101-y Download citation * Received: 18 February 2019 * Accepted: 21
May 2019 * Published: 13 June 2019 * DOI: https://doi.org/10.1038/s41597-019-0101-y SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative