Play all audios:
ABSTRACT A central regulator of metabolism, transcription factor carbohydrate response element binding protein (ChREBP) senses and responds to dietary glucose levels by stimulating the
transcription of glycolytic and lipogenic enzymes. Genetic depletion of ChREBP rescues β-cell dysfunction arising from high glucose levels, suggesting that inhibiting ChREBP might represent
an attractive therapeutic approach to manage diabetes and other metabolic diseases. However, the molecular mechanisms governing ChREBP activation are poorly understood and chemical tools to
probe the cellular activity of ChREBP are lacking. Here, we report a high-throughput pharmacological screen in INS-1E β-cells that identified novel inhibitors of ChREBP-driven transcription
at carbohydrate response element sites, including three putative covalent inhibitors and two likely non-covalent chemical scaffolds. This work affords a pharmacological toolkit to help
uncover the signaling logic controlling ChREBP activation and may ultimately reveal potential therapeutic approaches for treating metabolic disease. SIMILAR CONTENT BEING VIEWED BY OTHERS
MOLECULAR GLUES OF THE REGULATORY CHREBP/14-3-3 COMPLEX PROTECT BETA CELLS FROM GLUCOLIPOTOXICITY Article Open access 02 March 2025 PAN-AMPK ACTIVATOR O304 PREVENTS GENE EXPRESSION CHANGES
AND REMOBILISATION OF HISTONE MARKS IN ISLETS OF DIET-INDUCED OBESE MICE Article Open access 23 December 2021 IDENTIFICATION OF PROBUCOL AS A CANDIDATE FOR COMBINATION THERAPY WITH METFORMIN
FOR TYPE 2 DIABETES Article Open access 23 May 2023 BACKGROUND & SUMMARY Glucose serves as a ubiquitous source of energy for all organisms and its regulation is of central importance to
organismal function. Indeed, prolonged elevation of blood glucose levels dramatically increases risk of metabolic diseases such as type 2 diabetes, obesity, and nonalcoholic fatty liver
disease. Accordingly, pharmacological modulation of glucose responsive signaling pathways may provide novel therapeutic strategies for treating these disorders. One such metabolic regulator
that responds to changing glucose levels is the transcription factor carbohydrate response element binding protein (ChREBP). ChREBP is a member of the Mondo family of basic helix-loop-helix
leucine zipper (bHLH-zip) transcription factors1. It contains two nuclear export signals and a nuclear localization signal in the N-terminus region and the bHLH-zip domain as well as a
Zip-like domain in its C-terminus, which interacts with transcriptional co-activators like Max like protein x (Mlx)2 and p300/CBP3. ChREBP regulates multiple signaling and metabolic pathways
in several tissues including the liver, white and brown adipose tissue, small intestine, and pancreatic islets4. During fasting conditions, ChREBP is inactivated via phosphorylation by
protein kinase A5 and AMP activated protein kinase6, leading to its cytosolic retention. In response to carbohydrates however, certain glucose derived metabolites bind to the
glucose-response activation conserved element (GRACE) on the N-terminus of ChREBP7. This, in conjunction with dephosphorylation by protein phosphatase 2A8, activates ChREBP, promoting its
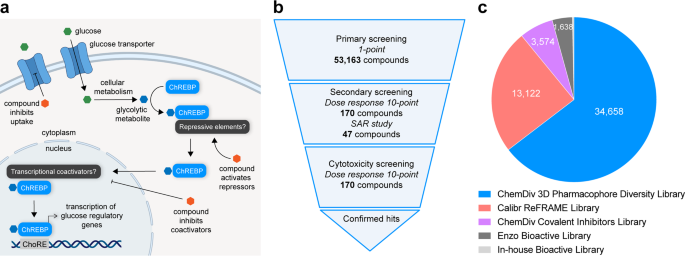
dimerization with Mlx9 and translocation to the nucleus, where it undergoes _O_-GlcNacylation10 and acetylation11 to promote binding to its cognate carbohydrate response element (ChoRE) in
the loci of target genes (Fig. 1a). Previous studies have proposed glucose-6-phosphate12 or xylulose-5-phosphate13 as a potential glucose derived metabolites with ChREBP activating
potential; however, the specific metabolite that ChREBP directly senses remains unclear. ChREBP is primarily expressed in organs with high levels of _de novo_ lipogenesis. In the liver,
ChREBP augments lipogenesis in hyperglycemic states by upregulating transcription of key enzymes, including pyruvate kinase, fatty acid synthase, acetyl-CoA carboxylase14, and indirectly
through ATP-citrate lyase (through its upregulation of the branched-chain ketoacid dehydrogenase complex)15. Secretion of very low density lipoprotein is also controlled in part by ChREBP16
as hepatic ChREBP knockdown decreases microsomal triglyceride transfer protein levels in the liver17. ChREBP overexpression in the liver of mice has been shown to increase hepatic
steatosis18 and genetic ChREBP ablation in the liver reverses hepatic steatosis19. In addition, ChREBP modulates fructose tolerance in the liver by increasing expression of fructokinase and
triose kinase20. In the small intestine, ChREBP drives expression of glucose transporters 2 and 5 as well as ketohexokinase and sucrose-isomaltase, promoting sugar absorption21. ChREBP is
also critically important in regulating pancreatic function. In pancreatic β-cells, ChREBP drives expression of thioredoxin-interacting protein (TXNIP), the accumulation of which causes
β-cell damage22 by increasing inflammation through activation of the NLRP3 inflammasome23, promoting apoptosis via inhibition of thioredoxin24, and decreasing insulin sensitivity by
promoting the internalization of glucose transporters25. Therefore, inhibiting expression of TXNIP may protect β-cells from the cytotoxic effect of high glucose levels, enabling β-cell
expansion and increasing insulin production. Notably, knockdown of ChREBP and/or TXNIP has been shown to improve long-term glucose tolerance and insulin sensitivity in mouse models of
obesity, suggesting that decreasing TXNIP levels pharmacologically could be a potential therapeutic route for treating diabetes and its complications. To date, W2476 is the only synthetic
small molecule that has been reported to inhibit TXNIP expression26. Mechanistically, W2476 was found to inhibit the phosphorylation of forkhead box O1 transcription factor (FOXO1), which
increases FOXO1 binding to the TXNIP promoter, therefore preventing ChREBP binding27. Given this compound inhibits FOXO1 signaling globally, W2476 does not necessarily act as a specific
inhibitor of ChREBP activity, and thus the discovery of additional pharmacological agents along with their associated ligandable targets will be essential in helping further define the logic
of ChREBP activation. Here, we report results from a cell-based high-throughput screen of 53,163 compounds aimed at identifying small molecule inhibitors of ChoRE driven transcription (Fig.
1b,c). We generated an INS-1E cell line stably expressing a ChoRE-dependent luciferase reporter (ChoRE-LUC) and developed a 384-well compatible screening assay to identify compounds that
inhibited transcriptional activity at ChoREs. The libraries that were screened were the comprehensive drug repurposing library ReFRAME, a structurally diverse pharmacophore library, a
covalent inhibitors library, and known bioactive small molecule libraries: the Enzo library and an In-house bioactive library (Fig. 2). Following primary screening, top hits from each
library were re-tested in dose response for modulating ChoRE-LUC activity and cytotoxicity. Further structure-activity relationship (SAR) analysis using commercially available analogs was
performed on hits from the covalent inhibitor library. METHODS COMPOUND LIBRARIES High-throughput screening was performed using five compound libraries, screened at the highest concentration
available from the source compound plates. The Calibr ReFRAME library contains 13,122 compounds and was screened at 2 µM final concentration. The Calibr ReFRAME library was supplied by
Calibr. Its construction and composition have been described in the literature28. The 3D-Pharmacophore Based Diversity Library contains 34,658 compounds screened at 20 µM final concentration
and was obtained from the commercial vendor ChemDiv. The Covalent Inhibitors Focused Library contains 3,574 compounds screened at 2 µM final concentration and was also obtained from
ChemDiv. The Enzo library contains 1,638 compounds and was obtained from Enzo Life Sciences. The In-house bioactive library contains 171 compounds and was obtained from several chemical
suppliers including Tocris and Sigma. The compounds were stored in DMSO at −20 °C. CELL CULTURE Rat INS-1E cells were a gift from Weijun Shen (Calibr, a division of Scripps Research). INS-1E
cells were maintained in RPMI medium (Corning, 10-040-CV) supplemented with 10% fetal bovine serum (FBS, Gibco, 10438-026), 10 mM HEPES (Corning, 25-060CI), 1 mM sodium pyruvate, (Gibco,
11360070), and 1% penicillin/streptomycin (Gibco, 15070063). HEK293T cells were obtained from the American Type Culture Collection (ATCC, CRL-11268) and were maintained in DMEM medium
(Corning, 10-013-CV) supplemented with 10% FBS and 1% penicillin/streptomycin. REPORTER PLASMIDS pGL4-ChoRE-LUC reporter plasmids were generated from codon-optimized ChoRE promoter
constructs (obtained from Integrated DNA Technologies as gBlock HiFi Gene Fragments) cloned into the pGL4 backbone from pGL4.40 (Promega, E4131) using the NEBuilder HiFi DNA Assembly Kit
(NEB, E5520S). pGF1-ChoRE WT-LUC plasmid was generated in the same way using the pGreenFire backbone (System Biosciences). For transient transfection of reporter plasmids in 384-well plate
format, INS-1E WT cells (5000 cells in 40 µL assay medium per well) were transfected with 100 ng plasmid DNA in 10 µL OptiMEM media (Gibco, 31985062) containing a 1:3 ratio (µg DNA:µL
reagent) of FuGENE HD (Promega, E2311) at the time of cell seeding. STABLE CELL LINES For production of lentivirus, 1 × 106 HEK293T cells were plated on poly-D-lysine coated 10 cm plates in
growth medium and transfected with 2 µg of pGF1-ChoRE WT-LUC and 2 µg of each packaging plasmid (pMD2.G and psPAX2, Addgene #12259, #12260) with 24 µL of FuGENE HD transfection reagent in
600 µL of OptiMEM medium at the time of cell seeding. 10 mL of cell culture supernatant was harvested after 48 h and clarified via centrifugation (3 min, 300 x_g_) and filtration with a 0.45
µm syringe filter, then concentrated to 1 mL using a 30 kDa MWCO centrifugal filter (Amicon). The concentrated supernatant was added to 4 × 105 INS-1E cells one day after seeding in a
six-well plate. After 72 h, INS-1E ChoRE-LUC stable cell lines were selecting using puromycin (1.6 µg/mL) for 48 h. Fluorescent activated cell sorting (FACS) was used isolate single clones
expressing green fluorescent protein. HIGH-THROUGHPUT SCREENING For ReFRAME library screening, 10 nL of 2 mM DMSO stock concentrations of compound were prespotted into 5 µL of assay medium
(RPMI with 2% FBS, 10 mM HEPES, 1 mM sodium pyruvate, 25 mM D-glucose (Sigma), and 1% penicillin/streptomcycin) in white 384-well plates (Grenier) using an Echo Acoustic Liquid Handler
instrument (Labcyte) before the addition of INS-1E ChoRE-LUC cells (5000 cells in 45 µL assay medium per well). Cells were incubated for 24 h before 30 µL of BrightGlo Luciferase Assay
solution (diluted 1:3 in water, Promega) was added to each well and luminescence signal values were recorded with an Envision plate reader (PerkinElmer). This protocol was used as the
ReFRAME library was supplied from off-site and necessitated pre-spotting for efficient transfer of materials. For the screening of all other libraries, which was performed in-house, INS-1E
ChoRE-LUC cells were plated in a white 384-well plate (5000 cells in 50 µL assay medium per well) and incubated for 24 h. 100 nL of DMSO stock concentrations of compound were transferred to
each well using a Bravo instrument with a pintool head (Agilent). Cells were incubated for 24 h before 30 µL of BrightGlo Luciferase Assay solution was added to each well and luminescence
signal values were recorded with an Envision plate reader. CYTOTOXICITY SCREENING INS-1E ChoRE-LUC cells were plated in a white 384-well plate (5000 cells in 50 µL assay medium per well) and
incubated for 24 h. 100 nL of DMSO stock concentrations of compound were transferred to each well using a Bravo instrument (Agilent) affixed with a pintool head (V&P Scientific). Cells
were incubated for 24 h before 30 µL of CellTiterGlo Cell Viability Assay solution (diluted 1:6 in water, Promega) was added to each well and luminescence signal values were recorded with an
Envision plate reader. DATA ANALYSIS Reporter activity and cell viability data was normalized to the DMSO control wells in each plate. Ten or twelve dose-response points and three
biological replicates were used to determine dose-response curves using the four-parameter inhibitor dose-response curve fitting function in Prism software (Graphpad). All graphed data are
plotted as the mean and the s.e.m denoted with error bars. CHEMICALS USED W2476 was obtained from ChemDiv. CYC065, RO-4584820, and PCA 4248 were obtained from Cayman Chemical. All chemicals
were dissolved in DMSO and used without further purification. DATA RECORDS PRIMARY AND SECONDARY SCREENING COMPOUNDS The compounds used in the primary and secondary luciferase reporter and
viability assay screens are detailed in “Primary and Secondary Screening Compounds.xlsx” within figshare29. We provide compound ID, common identifiers, and chemical structures for the
compounds used in the ReFRAME, ChemDiv 3D Pharmacophore, ChemDiv Covalent Inhibitors, Enzo, and In-house Bioactive libraries used in the primary and secondary screens. The table headings
present in the data file are defined in Table 1. PRIMARY SCREENING CHORE-LUC ACTIVITY DATA The primary luciferase reporter screen data set can be found in “Primary Screening ChoRE-LUC
Activity Data.xlsx” within figshare29. We provide screen-wide normalized data, raw data, and median and mean z-scores for the ReFRAME, ChemDiv 3D Pharmacophore, ChemDiv Covalent Inhibitors,
Enzo, and In-house bioactive libraries used in the primary screen. The table headings present in the data file are defined in Table 2. SECONDARY DOSE RESPONSE SCREENING CHORE-LUC ACTIVITY
DATA The secondary dose response luciferase reporter screen data set can be found in “Secondary Dose Response Screening ChoRE-LUC Activity Data.xlsx” within figshare29. We provide
dose-response normalized data and raw data for hits from the ReFRAME, ChemDiv 3D Pharmacophore, ChemDiv Covalent Inhibitors, ChemDiv Covalent Inhibitors SAR, and In-house Bioactive
libraries. The table headings present in the data file are defined in Table 3. SECONDARY DOSE RESPONSE SCREENING VIABILITY DATA The secondary dose response cytotoxicity screen data set can
be found in “Secondary Dose Response Screening Viability Data.xlsx” within figshare29. We provide dose-response normalized data and raw data for hits from the ReFRAME, ChemDiv 3D
Pharmacophore, ChemDiv Covalent Inhibitors, ChemDiv Covalent Inhibitors SAR, and In-house Bioactive libraries. The table headings present in the data file are defined in Table 3. TECHNICAL
VALIDATION REPORTER OPTIMIZATION To establish a reliable assay with a suitable dynamic range for high-throughput screening, we first optimized the composition of the DNA regulatory sequences
within the ChoRE-LUC reporter. Previous studies showed that the minimal promoter required for ChoRE-driven TXNIP expression induction by glucose consists of a ChoRE sequence (two E-boxes,
CACGTG, separated by 5 nucleotides), a CCAAT box, a FOXO1 binding site, an inverted CCAAT box, and a second degenerate ChoRE sequence30. To determine whether this combination of regulatory
elements was robust enough to test general ChoRE activity, we constructed six pGL4-based luciferase reporter plasmids with different numbers and/or combinations of ChoRE and FOXO sequences
from the promoter of the human TXNIP gene (Fig. 3a). The wild-type (WT) sequence represents the intact minimal promoter of the TXNIP gene (GRCh38.p14, chr1:145,996,639–145,996,759). The six
plasmids were transiently transfected into INS-1E cells using cationic lipid-based transfection reagents and the induction of luciferase after 24 h in 25 mM glucose containing media was
compared (Fig. 3b). In addition, we tested various glucose concentrations in the assay media to determine the concentration that results in the highest stimulation. We determined that the WT
ChoRE-LUC construct in 25 mM glucose media resulted in the most maximal and consistent increase in luminance signal (Fig. 3c). The WT ChoRE-LUC construct was then used to generate an INS-1E
stable cell line (INS-1E ChoRE-LUC) for screening in 384-well plate format. For high-throughput screening, the treatment time, serum percentage in media, and cell count per well were
optimized further. The final assay design consisted of 5,000 INS-1E ChoRE-LUC cells per well of a 384-well plate in 25 mM glucose media supplemented with 2% FBS and a compound treatment time
of 24 h. SCREENING ASSAY Pharmacological validation of the assay was performed using the known TXNIP expression inhibitor W2476. The ChoRE-LUC IC50 value (1.33 µM) obtained for W2476 in our
assay was similar to the previously reported measurement (1.70 µM)26, indicating the assay was likely suitable for interrogating inhibitory effects on TXNIP expression via ChoREs (Fig. 4a).
The primary screen was validated by calculating the Z’-factor for each plate (Fig. 4b). All 162 plates had a Z’-factor greater than 0.40, indicating good assay quality (Fig. 4c). The
average Z’-factor for all plates was 0.55. The Z’-factor per library is shown in Table 4. Hit compounds from each library were identified by z-score cutoff values (Table 4). The primary
screen returned 170 hits (0.31% hit rate) which were re-purchased from chemical suppliers and subjected to secondary dose-response reporter activity screening. 13 hits were identified from
the ReFRAME library (Fig. 5a,b). The secondary dose-response screening of the top 3 hits is shown in Fig. 5c. 3 hits were identified from the ChemDiv Covalent Inhibitors library (Fig. 6a)
and their dose-response activities are shown in Fig. 6b. We performed a structure activity relationship study by supplier inventory of 47 additional covalent inhibitor compounds based on the
scaffolds of the top 3 hits which revealed more potent inhibitors including V011-8130 (ChoRE-LUC IC50 = 1.1 µM). 142 hits were identified from the 3D Pharmacophore Diversity library. Of
those, 9 shared the common SD60 scaffold and 8 shared the common SC41 scaffold (Fig. 7a). Secondary dose-response screening of these hits yielded 9 confirmed hits as shown in Fig. 7b.
CYTOTOXICITY Hit compounds were further evaluated in dose-response for potential cytotoxicity to determine if decreased luciferase levels were not derived from the induction of cell death.
Evaluation of cellular viability was measured using a CellTiterGlo assay. The top 3 most potent hits from the ReFRAME library had significant cytotoxicity as shown in Fig. 5d. For the
covalent inhibitor compounds, only V029-2992 (ChoRE-LUC IC50 = 2.9 µM) had good potency without significant cytotoxicity (Fig. 6c). Out of the 9 hits in the 3D Pharmacophore Diversity
library, 7 were not cytotoxic, with SD60-0149 and SC41-0178 being the most potent hits from their respective scaffolds (Fig. 7d). CODE AVAILABILITY A collection of Java scripts for the
analysis of our datasets have been made available on figshare with no usage restrictions29. REFERENCES * Yamashita, H. _et al_. A glucose-responsive transcription factor that regulates
carbohydrate metabolism in the liver. _Proc. Natl. Acad. Sci. USA_ 98, 9116–9121 (2001). Article ADS PubMed PubMed Central CAS Google Scholar * Stoeckman, A. K., Ma, L. & Towle, H.
C. Mlx is the functional heteromeric partner of the carbohydrate response element-binding protein in glucose regulation of lipogenic enzyme genes. _J. Biol. Chem._ 279, 15662–15669 (2004).
Article PubMed CAS Google Scholar * Feng, Y., Wang, H., Chen, Z. & Chen, B. High glucose mediates the ChREBP/p300 transcriptional complex to activate proapoptotic genes Puma and BAX
and contributes to intervertebral disc degeneration. _Bone._ 153, 116164 (2021). Article PubMed CAS Google Scholar * Ortega-Prieto, P. & Postic, C. Carbohydrate Sensing Through the
Transcription Factor ChREBP. _Front. Genet._ 10, 472 (2019). Article PubMed PubMed Central CAS Google Scholar * Kawaguchi, T., Osatomi, K., Yamashita, H., Kabashima, T. & Uyeda, K.
Mechanism for fatty acid “sparing” effect on glucose-induced transcription: regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase. _J. Biol. Chem._
277, 3829–3835 (2002). Article PubMed CAS Google Scholar * Sato, S. _et al_. Metabolite regulation of nuclear localization of carbohydrate-response element-binding protein (ChREBP): role
of AMP as an allosteric inhibitor. _J. Biol. Chem._ 291, 10515–10527 (2016). Article PubMed PubMed Central CAS Google Scholar * Li, M. V., Chang, B., Imamura, M., Poungvarin, N. &
Chan, L. Glucose-dependent transcriptional regulation by an evolutionarily conserved glucose-sensing module. _Diabetes._ 55, 1179–1189 (2006). Article PubMed CAS Google Scholar *
Kawaguchi, T., Takenoshita, M., Kabashima, T. & Uyeda, K. Glucose and cAMP regulate the L-type pyruvate kinase gene by phosphorylation/dephosphorylation of the carbohydrate response
element binding protein. _Proc. Natl. Acad. Sci. USA_ 98, 13710–13715 (2001). Article ADS PubMed PubMed Central CAS Google Scholar * Ma, L., Tsatsos, N. G. & Towle, H. C. Direct
role of ChREBP.Mlx in regulating hepatic glucose-responsive genes. _J. Biol. Chem._ 280, 12019–12027 (2005). Article PubMed CAS Google Scholar * Yang, A. Q., Li, D., Chi, L. & Ye, X.
S. Validation, Identification, and Biological Consequences of the Site-specific O-GlcNAcylation Dynamics of Carbohydrate-responsive Element-binding Protein (ChREBP). _Mol. Cell.
Proteomics._ 16, 1233–1243 (2017). Article PubMed PubMed Central CAS Google Scholar * Bricambert, J. _et al_. Salt-inducible kinase 2 links transcriptional coactivator p300
phosphorylation to the prevention of ChREBP-dependent hepatic steatosis in mice. _J. Clin. Invest._ 120, 4316–4331 (2010). Article PubMed PubMed Central CAS Google Scholar * McFerrin,
L. G. & Atchley, W. R. A novel N-terminal domain may dictate the glucose response of Mondo proteins. _PLoS One._ 7, e34803 (2012). Article ADS PubMed PubMed Central CAS Google
Scholar * Kabashima, T., Kawaguchi, T., Wadzinski, B. E. & Uyeda, K. Xylulose 5-phosphate mediates glucose-induced lipogenesis by xylulose 5-phosphate-activated protein phosphatase in
rat liver. _Proc. Natl. Acad. Sci. USA_ 100, 5107–5112 (2003). Article ADS PubMed PubMed Central CAS Google Scholar * Ishii, S., Iizuka, K., Miller, B. C. & Uyeda, K. Carbohydrate
response element binding protein directly promotes lipogenic enzyme gene transcription. _Proc. Natl. Acad. Sci. USA_ 101, 15597–15602 (2004). Article ADS PubMed PubMed Central CAS
Google Scholar * White, P. J. _et al_. The BCKDH Kinase and Phosphatase Integrate BCAA and Lipid Metabolism via Regulation of ATP-Citrate Lyase. _Cell. Metab._ 27, 1281–1293 e1287 (2018).
Article PubMed PubMed Central CAS Google Scholar * Hussain, M. M., Nijstad, N. & Franceschini, L. Regulation of microsomal triglyceride transfer protein. _Clin. Lipidol._ 6, 293–303
(2011). Article PubMed PubMed Central CAS Google Scholar * Lei, Y. _et al_. Hepatic Carbohydrate Response Element Binding Protein Activation Limits Nonalcoholic Fatty Liver Disease
Development in a Mouse Model for Glycogen Storage Disease Type 1a. _Hepatology._ 72, 1638–1653 (2020). Article PubMed CAS Google Scholar * Benhamed, F. _et al_. The lipogenic
transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. _J. Clin. Invest._ 122, 2176–2194 (2012). Article PubMed PubMed Central CAS Google
Scholar * Dentin, R. _et al_. Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob mice. _Diabetes._ 55, 2159–2170 (2006). Article PubMed CAS
Google Scholar * Iizuka, K., Bruick, R. K., Liang, G., Horton, J. D. & Uyeda, K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as
glycolysis. _Proc. Natl. Acad. Sci. USA_ 101, 7281–7286 (2004). Article ADS PubMed PubMed Central CAS Google Scholar * Kato, T. _et al_. ChREBP-Knockout Mice Show Sucrose Intolerance
and Fructose Malabsorption. _Nutrients_. 10 (2018). * Cha-Molstad, H., Saxena, G., Chen, J. & Shalev, A. Glucose-stimulated expression of Txnip is mediated by carbohydrate response
element-binding protein, p300, and histone H4 acetylation in pancreatic beta cells. _J. Biol. Chem._ 284, 16898–16905 (2009). Article PubMed PubMed Central CAS Google Scholar * Zhou,
R., Tardivel, A., Thorens, B., Choi, I. & Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. _Nat. Immunol._ 11, 136–140 (2010). Article
PubMed CAS Google Scholar * Ray, P. D., Huang, B. W. & Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. _Cell. Signal._ 24, 981–990
(2012). Article PubMed PubMed Central CAS Google Scholar * Waldhart, A. N. _et al_. Phosphorylation of TXNIP by AKT Mediates Acute Influx of Glucose in Response to Insulin. _Cell. Rep._
19, 2005–2013 (2017). Article PubMed PubMed Central CAS Google Scholar * Li, T. _et al_. W2476 ameliorates beta-cell dysfunction and exerts therapeutic effects in mouse models of
diabetes via modulation of the thioredoxin-interacting protein signaling pathway. _Acta. Pharmacol. Sin._ 38, 1024–1037 (2017). Article PubMed PubMed Central CAS Google Scholar * Zhong,
L. _et al_. W2476 represses TXNIP transcription via dephosphorylation of FOXO1 at Ser319. _Chem. Biol. Drug. Des._ 97, 1089–1099 (2021). Article PubMed CAS Google Scholar * Janes, J.
_et al_. The ReFRAME library as a comprehensive drug repurposing library and its application to the treatment of cryptosporidiosis. _Proc. Natl. Acad. Sci. USA_ 115, 10750–10755 (2018).
Article ADS PubMed PubMed Central CAS Google Scholar * You, S. & Bollong, MJ. A high throughput screen for pharmacological inhibitors of the carbohydrate response element,
_figshare_, https://doi.org/10.6084/m9.figshare.c.6533509.v1 (2023). * Yu, F. X. & Luo, Y. Tandem ChoRE and CCAAT motifs and associated factors regulate Txnip expression in response to
glucose or adenosine-containing molecules. _PLoS One._ 4, e8397 (2009). Article ADS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We would like to thank
Kristen Johnson at Calibr for screening assistance and Luke Lairson and R. Luke Wiseman for providing the Enzo and ChemDiv 3D Pharmacophore Diversity compound libraries, respectively. We
would also like to thank Fereshte Ghorbani for helpful discussions. This work was supported by the NIH (GM146865 to MJB). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of
Chemistry, The Scripps Research Institute, La Jolla, California, 92037, USA Shaochen You & Michael J. Bollong Authors * Shaochen You View author publications You can also search for this
author inPubMed Google Scholar * Michael J. Bollong View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS S.Y. conducted all experimental work
and performed data analysis. M.J.B. supervised the work. S.Y. and M.J.B. wrote the manuscript. CORRESPONDING AUTHOR Correspondence to Michael J. Bollong. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,
adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE You, S., Bollong, M.J. A high throughput screen for pharmacological inhibitors of the carbohydrate response element. _Sci Data_ 10, 676 (2023).
https://doi.org/10.1038/s41597-023-02596-z Download citation * Received: 04 April 2023 * Accepted: 26 September 2023 * Published: 04 October 2023 * DOI:
https://doi.org/10.1038/s41597-023-02596-z SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative