Play all audios:
Calcification and biomass production by planktonic marine organisms influences the global carbon cycle and fuels marine ecosystems. The major calcifying plankton group coccolithophores are
highly diverse, comprising ca. 250–300 extant species. However, coccolithophore size (a key functional trait) and degree of calcification are poorly quantified, as most of our understanding
of this group comes from a small number of species. We generated a novel reference dataset of coccolithophore morphological traits, including cell-specific data for coccosphere and cell
size, coccolith size, number of coccoliths per cell, and cellular calcite content. This dataset includes observations from 1074 individual cells and represents 61 species from 25 genera
spanning equatorial to temperate coccolithophore populations that were sampled during the Atlantic Meridional Transect (AMT) 14 cruise in 2004. This unique dataset can be used to explore
relationships between morphological traits (cell size and cell calcite) and environmental conditions, investigate species-specific and community contributions to pelagic carbonate
production, export and plankton biomass, and inform and validate coccolithophore representation in marine ecosystem and biogeochemical models.
Coccolithophores (Prymnesiophyceae) are abundant calcifying marine phytoplankton that are major contributors to the marine carbon cycle1,2 through their dual production of organic carbon
(biomass) and biosynthesis of their inorganic carbon (calcium carbonate, calcite) exoskeletal plates. Coccolithophore calcification and photosynthesis are highly dynamic cellular processes
that respond to changes in cell size, cell stoichiometry (elemental content), and the morphology and production rates of the calcite plates (coccoliths) that form the exoskeleton
(coccosphere) of each cell3,4 that can occur as cell physiology responds to fluctuating environmental conditions. At the community level, changes in population growth rates and assemblage
composition, including changes in the relative and absolute abundance of species present with different morphological traits and therefore degrees of calcification, influences the
contribution of coccolithophores to pelagic carbonate production and carbonate flux to the deep ocean5,6,7. Variability in surface ocean carbonate production2, of which coccolithophores
represent ca. 50–90%1,8,9, and carbonate export affects ocean alkalinity on shorter timescales and therefore the air-sea flux of carbon dioxide9,10,11,12. On longer timescales (Myr), the
production and sedimentation of biogenic carbonate (the counter carbonate pump) acts as a long-term CO2 sink13 that helps to regulate global temperatures14. Given the major role of
coccolithophores in these global processes and the sensitivity of coccolithophore physiology (including calcification) to environmental stressors15,16,17,18,19,20,21,22, understanding the
biogeochemical and ecological consequences of the variability in coccolithophore calcification and productivity and the drivers of this variability have been pressing research questions for
more than 40 years.
Alternatively, morphometric traits can be used to estimate cellular-level calcite content for a wide diversity of extant coccolithophore species. A morphometric approach to calculating
cellular calcite content is based on estimating the volume of individual coccoliths and the number of coccoliths per cell30,31. Morphometric-based calcite estimates generally agree well with
calcite estimates derived through other approaches32,33,34,35 (see Technical Validation). With due consideration for the distinct morphological characteristics for each species,
morphometric-based cellular calcite estimates can be made across the full diversity of extant and extinct species. The approach can be applied to coccoliths of any size or thickness and uses
measurements taken on both entire, intact coccospheres and on individual coccoliths. Morphometric-based cellular calcite estimates are also truly cellular, as opposed to the assay-based
measurements that average population calcite (including the calcite of dead cells and shed coccoliths) across population abundance and overestimate mean cell calcite as a result.
Morphometric-based estimates of coccolith and cellular calcite content have been widely used to estimate pelagic carbonate production and carbonate export fluxes (sometimes using a species’
mean coccolith size and the number of coccoliths per cell only)36,37,38,39,40,41,42,43,44,45, and to quantify calcification responses to environmental and physiological change in both
micropaleontological studies46,47,48 and laboratory experiments on E. huxleyi, Coccolithus, Calcidiscus, and Helicosphaera4,49,50,51,52. For the remaining species of extant coccolithophores,
very little is known concerning their species- or genus-specific biomass and calcite.
Samples were collected during the Atlantic Meridional Transect 14 cruise (28 April to 1 June 2004). The cruise track ran from the Falkland Islands to the UK covering a sea surface
temperature range of 5 to 27 °C and a dissolved nitrate range of 1 to >10 µmol N L−1 54,58. The coccolithophore community during AMT-14 has previously been investigated over the euphotic
zone at 19 stations55. For coccolithophore analysis, 1-2 L of a 20 L seawater sample collected using a rosette sampler from between 5 and 13 water depths (55, 33, 14, 1, and 0.1% of surface
irradiance plus additional water depths at some stations) was gently filtered onto 25 mm polycarbonate filters with a 0.45 µm pore size. Filters were dried at room temperature and stored
until imaging. For the purposes of this morphological trait dataset, a selection of samples were examined, covering a range of latitudes and water depths (Table 1) to ensure that
morphometric data from a wide range of both common and less common extant coccolithophore species could be measured.
Coccolithophore morphometric data were collected from six stations at 55% surface irradiance (7 to 17 m water depth) and at a further seven water depths (30 m, 60 m, 90 m, 120 m, 140 m, 160
m and 200 m) at station CTD 34 in the southern subtropical gyre (Table 1). A further three samples identified as having higher abundances of generally less abundant taxa (Oolithotus,
Calciosolenia, Gephyrocapsa) were also examined (Table 1).
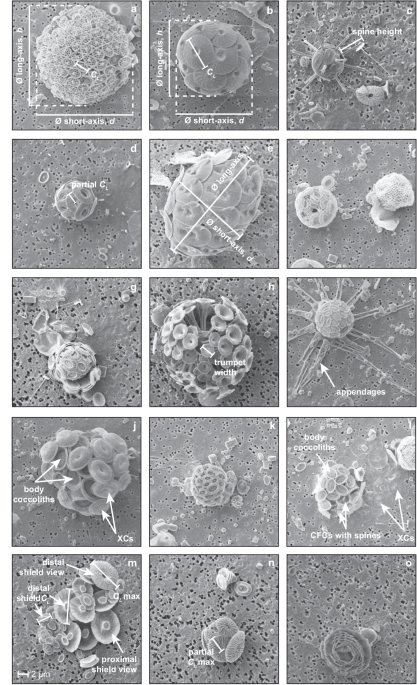
Illustrative examples of morphometric measurements and taxonomic specific morphological features. (a) Coccosphere of Calyptrolithina multipora (holococcolith-bearing; CTD34 60 m)
illustrating the measurement of coccolith length (CL) and coccosphere diameter (∅) short-axis (d) and long-axis (h). (b) Same measurements illustrated as in (a) for a coccosphere of
Calcidiscus leptoporus (CTD34 30 m). (c) Coccosphere of Rhabdosphaera clavigera var. stylifera (CTD15 13 m) illustrating the measurement of spine height. (d) Coccosphere of Umbilicosphaera
hulburtiana (CTD36 17 m) showing the partial measurement of CL between the centre point of the coccolith to distal shield rim edge, which is then multiplied by two in order to approximate CL
for specimens where the most flat-lying coccolith is partially overlapped by adjacent coccoliths. (e) Coccosphere of Helicosphaera carteri (CTD6 11 m) showing h and d measurements on a
prolate spheroid coccosphere shape. (f) Coccospheres of Emiliania huxleyi (left) and the polycrater phase of Alisphaera spp. (right) illustrating very high numbers of very small coccoliths.
(g) Coccosphere of E. huxleyi (CTD28 7 m) illustrating visible evidence of multi-layered coccospheres. (h) Coccosphere of Discosphaera tubifera (CTD28 7 m) illustrating the measurement of
trumpet width used in the calculation of coccolith calcite for this taxon. (i) Coccosphere of Michaelsarsia elegans (CTD34 90 m) showing appendages. (j) Coccosphere of Syracosphaera pulchra
(CTD34 30 m) showing the combination of body coccoliths and overlying exothecal coccoliths (XCs) on the same coccosphere. (k) Coccosphere of Syracosphaera molischii (CTD34 120 m). (l)
Coccosphere of Syracosphaera nodosa (CTD34 120 m) showing circum-flagellar coccoliths (CFCs) with small spines and XCs. (m) A collapsed coccosphere of Umbellosphaera tenuis (CTD28 7 m)
illustrating the wide range in coccolith sizes present on an individual coccosphere and distal shield measurements. (n) Intact Umbellosphaera coccosphere (CTD28 7 m) for comparison. (o)
Coccosphere of Florisphaera profunda (CTD34 160 m) showing strongly overlapping layers of coccoliths. The 2 µm scale bar shown in (m) applies to all image panels.
where d is the short-axis measurement (µm) and h is the long-axis measurement (µm) of the coccosphere (see also Fig. 1). Many spherical species (particularly those with placolith-type
coccoliths) do not have precisely spherical coccosphere dimensions, as slight variability in coccosphere thickness can arise from the arrangement of overlapping coccoliths. As such, both
long axis and short axis measurements are always made (Fig. 1a,b,e) and the volume of spherical, sub-spherical and prolate spheroid coccospheres (Supplementary Table 1) were all calculated
using the equation for volume of a prolate sphere (Eq. 1). Although some coccosphere shapes are best described as ellipsoid, it was not possible to measure all axes necessary to calculate
ellipsoid volume from two-dimensional SEM images, so volume was calculated as prolate spheroid (PS) for species with more elongated shapes (Supplementary Table 1).
In SEM images, only one surface of the coccosphere is visible (Fig. 1). To count the number of coccoliths per cell, therefore, the number of coccoliths on the visible surface of the
coccosphere was counted and this number was doubled to approximate the total number of coccoliths in the coccosphere (CN). Some species have coccospheres comprised of a very large number
(>100) of very small (ca. 0.5 to 2 µm) coccoliths (e.g., the polycrater phase of Alisphaera; Fig. 1f). For these coccospheres, the number of coccoliths per cell was counted as accurately as
possible but there is a degree of uncertainty in the counts (see Technical Validation).
Morphometric-based estimates of cellular calcite (particulate inorganic carbon, PIC) depend on the number of coccoliths per cell and the PIC of each of those coccoliths. For each individual
coccosphere, we first estimated coccolith PIC using the method of Young and Ziveri30 based on coccolith size (usually using coccolith length) and a shape factor related to coccolith
cross-sectional shape, as follows:
Where CL is coccolith size (µm), Ks is a species- or genus-specific shape factor, and 2.7 is the mass of calcite (pg µm−3).
Between species, Ks values vary by around an order of magnitude (minimum of ca. 0.01 to maximum of ca. 0.2 in extant species30). We used recommended species-specific Ks values from the
literature30,32,44,65,66 or adapted the Ks values of a species with similar morphologies for species lacking a published Ks value. The specific coccolith length parameter used in the
calculation (e.g., distal shield length or process height) and Ks value used for each genus or morpho-species group in the calculation of coccolith PIC are detailed in Supplementary Table 1.
Cellular PIC was then calculated by multiplying coccolith PIC by the number of coccoliths per cell, as follows:
Where CN is the number of coccoliths per coccosphere/cell and coccolith PIC is calculated as in Eq. 4.
The relationship between coccolith morphology and coccolith calcite mass parameterised in Eq. 4, where cell-specific variability in coccolith calcite is driven principally by variability in
coccolith size, represents a simplification of true cellular variation in coccolith calcite mass. Caveats and uncertainties related to this methodological approach, including variability in
coccolith thickness, the use of species-specific shape factors, and measurement uncertainties, are discussed in detail in Young and Ziveri30 and in the Technical Validation.
The organic biomass of phytoplankton cells is strongly related to cell volume67. In coccolithophores, the organic cell cytoplasm is covered by the calcite coccosphere so it is useful to
report both coccosphere size and cell size dimensions. The inner coccosphere size (approximating the size of the organic cell) cannot be measured directly from SEM images, as the cell is
obscured by the coccosphere (Fig. 1). We therefore estimated cell diameter as a function of coccosphere diameter, which can be precisely measured from SEM images (described above), using a
species- or genus-specific function that estimates the percentage of coccosphere volume (from coccosphere diameter) that is occupied by cell volume, as follows:
The majority of extant coccolithophore species have coccospheres formed of a single layer of abutting, overlapping and/or interlocking coccoliths. A notable exception to this is the species
Emiliania huxleyi, which is well-known for producing coccospheres with multiple layers70. Other species that produce multilayer or pseudo-multilayer coccospheres include Florisphaera
profunda and Umbellosphaera tenuis. We have accounted for the presence of (pseudo-)multi-layered coccospheres when converting from coccosphere size to cell size (see also details under
Emiliania, Florisphaera and Umbellosphaera in the taxonomic-specific consideration below).
There are two additional factors influencing the calculation of cellular calcite in Emiliania huxleyi: variable degrees of coccolith calcite across the different morphotypes and the presence
of multi-layered coccospheres.
E. huxleyi often makes coccospheres consisting of more than one layer of coccoliths, with as many as 3–5 layers observed in culture70,75,76 and field samples77,78,79. This occurs especially
under nutrient depleted conditions68, when cell division has slowed or ceased. For intact E. huxleyi coccospheres imaged using SEM, the presence of any additional coccolith layers is not
always apparent, as the outermost coccolith layer may conceal underlying coccolith layers. Presence of multiple coccolith layers is often revealed when one or more of the coccoliths forming
the outer coccolith layer becomes dislodged or the outermost coccolith layer has sheared off (Fig. 1g). Estimating cell diameter from the coccosphere diameter of multi-layered coccospheres
by directly applying a fixed percentage conversion factor between coccosphere volume and cell volume (y = 86% for E. huxleyi; Supplementary Table 1) would introduce error, cell volume would
be overestimated. For the purpose of estimating cell size using y for multilayer coccospheres of E. huxleyi, we have therefore artificially reduced the coccosphere diameter measurement to
the diameter of an equivalent single-layer coccosphere using an estimate of mean E. huxleyi coccolith thickness. For two-layered coccospheres, we subtracted 2x coccolith thickness (0.13 µm
x2 = 0.26 µm) from measured coccosphere diameter to simulate reducing the coccosphere diameter by the one extra layer of coccoliths present, for three-layered coccospheres we reduced
coccosphere diameter by 4x coccolith thickness (0.13 µm x4 = 0.52 µm) to simulate reducing the coccosphere diameter by the two extra layers of coccoliths present, and so on. In SEM images,
intact coccospheres of E. huxleyi sometimes have loose coccoliths lying directly next to the coccosphere (especially larger cells with multiple layers) and these were included in the CN of
the coccosphere to account for their calcite as they are presumably shed from the adjacent coccosphere.
No specific considerations were necessary for Calcidiscus, Oolithotus, or Umbilicosphaera.
This genus produces rhombic coccoliths. We are not aware of any previously published Ks value for Calciosolenia and so have back calculated a Ks value of 0.007 based on values for coccolith
calcite (2.5 pg) and coccolith length (5 µm) published in Bollmann et al.83 based on circular polarizer birefringence-based measurements (n = 10) of coccolith thickness on specimens of
Holocene age. Note that both the reported mass estimate and the coccolith length are likely to be underestimated, as the rim of Calciosolenia coccoliths is largely formed of vertically
orientated crystal units (V-units) that are not birefringent under either cross polarised or circular polarised light. Coccospheres of Calciosolenia are strongly elongated and taper to a
point at either end, giving them a shape that is best described mathematically as a ‘double cone’64. Measuring the dimensions of Calciosolenia coccospheres was sometimes challenging as the
large coccospheres were often split across more than one SEM image. In these instances, if appropriate, we estimated the mid-point of the coccosphere within the image that contained the
largest portion of the coccosphere and used 2x the measurement from this mid-point to the tip as the measure of length. For instances where the coccosphere was split over more than one SEM
image, CN was counted from all SEM images that contained parts of the coccosphere.
Some coccoliths have well-developed spines, for example the polar coccoliths on Acanthoica quattrospina. The additional calcite content of these spinose coccoliths has not been explicitly
accounted for in our calculations of cell PIC. Spine lengths are variable, ca. 6–17 µm in some specimens, and if we assume that the Ks of these spinose coccoliths is broadly similar to that
of Rhabdosphaera stylifera (spine length for the calculation of calcite), the calcite of these spines would be ca. 9–136 pg, compared to a coccolith calcite of ca. 1 pg for regular body
coccoliths (although this is likely an overestimate as Acanthoica spines have a less complex structure and are more tapered than Rhabdosphaera). The coccosphere calcite of Acanthoica
specimens with spinose polar coccoliths is therefore underestimated. The average coccosphere thickness of Acanothoica is 0.7 µm and cell volume is on average 56% of coccosphere volume (n =
6) but can be smaller than this in some coccospheres (20–40% coccosphere volume).
Coccospheres of Palusphaera and R. xiphos were difficult to distinguish at the resolution of the SEM images available. The primary distinguishing characteristic is that Palusphaera is
monomorphic (all coccoliths have spines) but Rhabdosphaera is dimorphic (some but not all coccoliths have spines) (see taxonomic discussion in Archontikis and Young61). Coccospheres were
distinguished where possible in the SEM images but were largely collapsed, which further inhibited confirmation of mono- or dimorphism and made accurate CL and CN measurements extremely
challenging. As direct cellular morphometrics could not be taken accurately, Palusphaera and Rhabdosphaera xiphos were not included in the database.
Calciopappus coccospheres were extremely rare in the samples investigated and were not observed as intact coccospheres. We therefore do not report any data for Calciopappus.
Michaelsarsia coccospheres were rare in our samples (n = 5). Each coccosphere typically has ca. 8–12 appendages each formed of 3–4 coccoliths86 (Fig. 1i). To avoid underestimating
Michaelsarsia coccosphere calcite by omitting the calcite in these appendages, we assigned a value of 12.5 pg calcite per appendage (informed by a typical length of 5.5 µm for each appendage
segment, a Ks value of 0.007 and assuming 4 segments per appendage). If the number of appendages is unknown, we assumed that 8 appendages were present (i.e., an addition of 100 pg calcite).
This additional calcite was then added to the calcite of the main body coccoliths, which were calculated using a Ks value adapted from Syracosphaera.
The appendage coccoliths of Ophiaster are also elongated but with a more solid structure than those of Michaelsarsia and each coccosphere usually has 4–7 long ‘arms’ of these osteoliths86.
As the appendages of Ophiaster are longer and with a much greater number of component osteoliths, an approximation of the additional calcite represented by the appendages was estimated as
follows: assuming that each of the osteoliths has the same Ks value as a Florisphaera profunda nannolith (Ks = 0.0332 based on the broadly similar shape) and each ‘arm’ contains on average
14 nannoliths of ca. 1.8 µm length, this would equate to 0.47 pg calcite per nannolith, 6.6 pg calcite per ‘arm’ and 33 pg additional calcite per coccosphere in total (assuming on average 5
arms). This fixed estimate of an additional 33 pg calcite for appendages was applied where appendages were clearly present (as many specimens are without appendages86). This estimate of
appendage calcite is broadly comparable to the calcite contained in the body coccoliths of many specimens (i.e., including a calcite estimate for the appendages approximately doubles cell
calcite content calculated on body coccoliths alone).
For Syracosphaera species that have a well-developed inner wall cycle and robust central area structures such as a boss or spine, we use the published Ks value of Young and Ziveri30 for
Syracosphaera pulchra (Ks = 0.03). This includes species in the ‘pulchra group’ and Syracosphaera mediterranea, which has a thick rim cycle and low central mound. For this morpho-group
(e.g., Fig. 1j), cell volume was assumed to be 65% of coccosphere volume (ranging between ca. 60 and 80% for species in the group).
For species without either of these features we use the Ks values of Young and Ziveri30 for a ‘small’ Syracosphaera species (Ks = 0.015), even though the coccoliths in question may not be
‘small’. This includes ‘borealis type’ species, ‘nodosa group’ species (e.g., Fig. 1l), and Syracosphaera maxima. For this morpho-group, cell volume was assumed to be 75% of coccosphere
volume.
For species that have well-developed rims but no central area bosses or spines, we chose a relatively arbitrary ‘intermediate’ value of Ks = 0.022. This includes species in the ‘molischii
group’ (other than ‘borealis type’ species; e.g., Fig. 1k). For this morpho-group, cell volume was assumed to be 75% of coccosphere volume.
For coccospheres that could not be confidently identified to species level, a Ks value of 0.02 was used to calculate coccosphere calcite.
The morphology of CFCs is often similar to that of the body coccoliths but with relatively small spines (Fig. 1l). We therefore used the same Ks value for CFCs as for body coccoliths.
There is a much broader morphological diversity across XCs, which makes it challenging to apply any ‘one size fits all’ approach to account for differences in the calcite content of XCs in
the calculation of cellular calcite across all Syracosphaera species. The morphologies of XCs have previously been tentatively classified into 12 groups88 spanning disk-like coccoliths
(e.g., Syracosphaera anthos, Syracosphaera nodosa, Syracosphaera lamina, Syracosphaera nana, Syracosphaera bannockii), undulating coccoliths that have a distinct rim and central area (e.g.,
Syracosphaera borealis, Syracosphaera molischii, Syracosphaera ossa), dome-like/vaulted coccoliths (e.g., Syracosphaera pulchra), and caneoliths with a murolith morphology (e.g.,
Syracosphaera dilatata, Syracosphaera prolongata, Syracosphaera noroitica). As XCs can be numerous (e.g., Fig. 1j,l), we attempted to account broadly for the calcite of these XCs when
present by counting the number of XCs present and using a Ks value of 0.02 for all morphologies to calculate the additional calcite contained in these coccoliths, recognising that this Ks
value is an underestimate in some instances and an overestimate in others.
An additional challenge presented by the presence of XCs is that they partially or sometimes entirely obscure the visibility of body coccoliths (Fig. 1j). In these instances, a best estimate
of CN was determined by either scaling up the number of body coccoliths visible over an unobscured portion of the cell, doubling the CN of visible XCs when the size of XCs and body
coccoliths are broadly comparable and the entire coccosphere is covered in XCs, or estimating CN based on the range of CN typical for the species (this is noted in the raw data file when
applied).
Many species of Syracosphaera are known to have holococcolith stages. See the remarks on ‘Holococcolithophores’ for further details.
No specific considerations were necessary for Helicosphaera.
Umbellosphaera coccoliths have umbelliform morphology, reminiscent of a flaring trumpet shape. Coccoliths forming the coccosphere have a range of distal shield sizes (as seen easily from
collapsed coccospheres; Fig. 1m) that leads Umbellosphaera coccospheres to have a pseudo-multilayered structure that is reminiscent of a rainforest canopy structure, where the distal shields
of the largest coccoliths overlay the distal shields of smaller coccoliths (Fig. 1n) whilst the proximal shields sit adjacent on the cell surface. This presents challenges for counting CN
accurately and accurately measuring a ‘representative’ coccolith size in Umbellosphaera. For CN, we therefore multiplied counted coccoliths by three (rather than by two for regular,
single-layer coccospheres) to better account for the ‘hidden’ layer of coccoliths underneath the visible outer layer. For intact coccospheres, CL and CW of the largest coccolith distal
shield were measured.
Coccolithophores have a haplo-diplontic life cycle, and typically produce heterococcoliths in the diploid life cycle stage and holococcoliths (HOL) during the haploid life cycle stage (e.g.,
Fig. 1a). Holococcoliths are formed from small, simple rhombohedral crystals and are morphologically distinct from heterococcoliths89. Holococcoliths do, however, occur in a range of
different shapes and forms60. The calcite content of individual holococcoliths is currently unknown and we are not aware of any published values for inorganic carbon per cell measured from
cultured holococcolith-bearing strains from which coccolith calcite and therefore Ks could be estimated. Here, we therefore used an estimated Ks value of 0.036 for all holococcolith-bearing
coccospheres45.
If it was not possible to directly count CN (as holococcoliths are often very small and coccospheres have high CN), CN was estimated by back-calculating CN using the surface area of the
coccospheres and the surface area of the measured coccolith or an average CN of that species was used if available from another published source. We assumed that cell volume is 80% of
coccosphere volume for all holococcolithophore coccospheres.
Ceratolithus produces three different lith morphologies depending on the life cycle stage, including distinctive horseshoe-shaped nannoliths (ceratoliths, CER). No intact Ceratolithus
coccospheres were observed in any of our samples so they are not present in the database.
The Cellular morphometric trait dataset for extant coccolithophores from the Atlantic Ocean is formatted as a column-orientated table in Microsoft Excel format. Each row of the dataset
represents the raw morphometric data (number of coccoliths per cell, coccolith length, coccolith width, coccosphere diameter long axis, coccosphere diameter short axis) and calculated
morphological trait data (coccosphere volume, equivalent spherical coccosphere diameter, cellular calcite) obtained from a single intact coccosphere. Each entry includes ancillary
information, including sample identifier (station and water depth), SEM image number and taxonomic classification (family, genus, species). A column entitled ‘Additional information’ records
any additional details relevant to the accuracy of the measurements and calculated parameters, for instance stating the presence and amount of XCs additionally included in the cellular
calcite calculation or the presence of a multi-layered coccosphere where this has been accounted for in the estimation of cell size from coccosphere size.
File 2. Descriptor describes the column headings contained in the Cellular morphometric trait dataset.
File 3. Coccolith size, coccolith calcite and cellular calcite in collapsed Umbellosphaera coccospheres contains the coccolith size and coccolith calcite data measured from collapsed
coccospheres of Umbellosphaera used to derive the adjustment of coccolith size used in the calculation of cellular calcite in Umbellosphaera.
File 4. Sensitivity of cellular calcite content to uncertainties in coccolith size, number of coccoliths per cell and shape factors contains the data used to perform the sensitivity analysis
of cellular calcite to variability in morphometric parameters (see Technical Validation).
File 5. Partial coccolith length and coccolith length measurements contains coccolith measurements used to calculate the measurement uncertainty associated with measuring partial CL on
coccoliths where the rim of the distal shield is partly obscured by an overlapping coccolith.
In total, 4712 morphometric measurements were measured on 1074 individual coccospheres representing 61 species from 25 genera (of the 31 most common genera that collectively constituted >95%
total cell numbers during AMT 1455) (Fig. 2). The number of measurements for each genus represented in the dataset largely reflects the relative abundance of different species in the
samples examined, as well as the structural stability of different coccosphere architectures (as some species coccospheres have a greater tendency to collapse, even under gentle filter
pressure, and were therefore not measured).
Taxonomic coverage of the morphometric dataset. (a) Coverage by coccolithophore Family. (b) Coverage by coccolithophore genus. Number of coccospheres in the morphometric dataset are labelled
above each bar.
Five genera (20%) have more than 50 coccospheres in the dataset and 14 genera (70%) have 10 or more coccospheres in the dataset (Fig. 2b). Some extant taxa are not present in our dataset for
the following reasons: (1) the species was not observed because it is generally not present in the temperate to equatorial latitudes of the oceanic Atlantic ocean sampled during AMT-14
(e.g., Coccolithus pelagicus that has a biogeographic distribution in the northern hemisphere high latitudes, the genus Braarudosphaera that is known to thrive in hyposaline conditions, the
predominantly polar Family Papposphaeraceae, and the neritic genera in the Families Hymenomonadaceae and Pleurochrysidaceae); (2) the species is generally rare (e.g., Calyptrosphaera,
Navilithus, Placorhombus, and Turrilithus) or rare in AMT samples (140 of 171 species, or 82%, recorded during AMT-14 constituted