Play all audios:
Mammalian target of rapamycin (mTOR) is activated by numerous stimuli, including amino acids and growth factors. This kinase is part of the mTOR complex 1 (mTORC1) which regulates cell
proliferation, differentiation, and autophagy. Active mTORC1 is located on lysosomes and has been reported to disassociate from the lysosomal surface in the absence of amino acids.
Furthermore, mTORC1 activity has been linked to the vacuolar H+-ATPases (V-ATPases), the proton pumps responsible for lysosomal acidification; however, the exact role of the V-ATPases in
mTORC1 signaling is not known. To elucidate the mechanisms involved in mTORC1 regulation by the V-ATPases, we used primary osteoclasts derived from mice carrying a point (R740S) mutation in
the a3 subunit of the V-ATPase. In these cells, the mutant protein is expressed but the pump is not functional, resulting in higher lysosomal pH. By analyzing mTOR activation, mTOR/lysosome
co-localization, and lysosomal positioning using confocal microscopy, fractionation, and ultrapure lysosomal purification methods, we demonstrate that in primary osteoclasts, mTOR is
localized on the lysosomal surface even when mTOR activity is inhibited. Our findings reveal that mTOR targeting to the lysosome in osteoclasts is activity-independent, and that its
disassociation from the lysosome during starvation is not universal.
Mammalian target of rapamycin (mTOR) is a serine/threonine kinase responsible for cellular responses to growth factors and nutrient availability. In cells, it exists as part of two
complexes, complex 1 (mTORC1) and complex 2 (mTORC2)1, 2. mTORC1 activity is tightly linked to autophagy, a lysosomal degradation process necessary for maintenance of cellular homeostasis by
‘recycling’ of cellular proteins and organelles. In the absence of amino acids, inactive mTORC1 allows initiation of autophagic protein degradation to upregulate intracellular free amino
acid levels. In the presence of amino acids, active mTORC1 suppresses autophagy and promotes cell proliferation, differentiation, and growth3.
mTORC1 regulation is tightly controlled and closely connected to the lysosomes and to the vacuolar H+-ATPases (V-ATPases)4. It has been shown that in the presence of amino acids, active mTOR
translocates to the lysosome, while in the absence of amino acids, inactive mTOR disassociates from the lysosome (becomes cytosolic)4. Lysosomal positioning (peripheral vs. perinuclear) has
also been reported to play a role in mTORC1 regulation: peripheral positioning correlates with active mTORC1, while amino acid deprivation and mTORC1 inactivation leads to perinuclear
positioning5. In addition, the V-ATPases, the proton pumps responsible for maintenance of lysosomal pH, are also involved in mTORC1 regulation: inhibition of the V-ATPases by siRNA or by
specific inhibitors decreases mTORC1 activity, confirming the importance of the V-ATPases in mTORC1 signaling. Furthermore, it has been shown in HEK-293T cells that active mTORC1 attaches to
the lysosome via the Ragulator complex, and these Ragulator complex proteins, in turn, directly interact with several subunits of the V-ATPase4, 6. It has been proposed that the V-ATPases
serve as amino acid sensors, controlling mTORC1 activation via the ‘unknown inside-out mechanism’3; however, the precise role of the V-ATPases in mTORC1 signaling has not been elucidated.
The V-ATPases are ubiquitously expressed multi-subunit proton pumps involved in maintenance of intracellular/organellar and extracellular pH7. These pumps consist of at least 14 different
subunits which are divided into two distinct domains: the ATP-hydrolyzing V1 domain (subunits A, B, C, D, E, F, G, H), and the proton-translocating V0 domain (subunits a, c, c′, c″, d and
e). Some of the V-ATPase subunits have several isoforms and these isoforms are cell-type and tissue specific. For example, the V0 domain ‘a’ subunit, the subunit responsible for proton
translocation, has four isoforms, a1, a2, a3 and a4. The a1 isoform is highly expressed in neurons, while the a2 subunit is endosomal and is considered to be ubiquitous; the a4 isoform is
found primarily in renal intercalated cells and epididymal clear cells. The V-ATPases containing the a3 subunit, although also ubiquitously expressed, are enriched over 100 fold in
osteoclasts7, 8, the bone resorbing cells.
Osteoclasts are terminally differentiated multinucleated cells formed by fusion of hematopoietic precursors9. To resorb bone, osteoclasts change their morphology and form a unique ‘ruffled
border’ membrane, a convoluted membrane structure adjacent to the bone surface. This ‘ruffled border’ membrane is enriched with the a3-containing V-ATPases, where these proton pumps acidify
the area of resorption to dissolve the mineral component of bone and to create optimal environment for the enzymes to digest bone matrix proteins9. In non-resorbing osteoclasts, the
a3-containing V-ATPases are located in the lysosomes and are responsible for maintaining lysosomal pH in these cells10. Mutations in the a3 subunit cause autosomal recessive osteopetrosis, a
rare genetic disease characterized by dense but brittle bones due to inability of the osteoclasts to resorb bone11. There are several mouse models of autosomal recessive osteopetrosis that
involve mutations or deletions of a312,13,14; however, only the osteoclasts derived from mice with a point mutation in the a3 subunit of the V-ATPase have been reported to have an altered
lysosomal pH15. In this mouse model, an evolutionary conserved arginine responsible for proton translocation across the membrane in a3 is mutated to serine (R740S), making the pump inactive:
the mutant a3 protein is expressed and is targeted to the lysosomal membrane; however, its activity is significantly impaired14, 15. Lysosomal pH in heterozygous (+/R740S) and homozygous
(R740S/R740S) osteoclasts is higher compared to the wild type (+/+) cells15, 16. This gene-dosage dependent ‘pre-set’ lysosomal pH in osteoclasts (4.5 vs. 5.7 vs. 6.2, in +/+, +/R740S, and
R740S/R740S cells, respectively16) creates a unique in vitro model to study the role of the V-ATPases and lysosomal pH in signaling regulation in primary cells.
We have previously demonstrated that mTORC1 activity was significantly upregulated in +/R740S osteoclasts compared to the +/+ cells17, suggesting that lysosomal pH also plays a role in
mTORC1 regulation. We decided to utilize the unique property of this ‘naturally’ pre-set high lysosomal pH of the R740S osteoclast model to decipher the role of lysosomal pH and the
V-ATPases in mTORC1 regulation and lysosomal positioning. Here, we show that in primary osteoclasts, lysosomal pH does play a role in mTORC1 activation; however, the amino acid status does
not affect mTOR co-localization with the lysosome, suggesting that mTORC1 regulation is not universal and is different in highly specialized cells, such as osteoclasts.
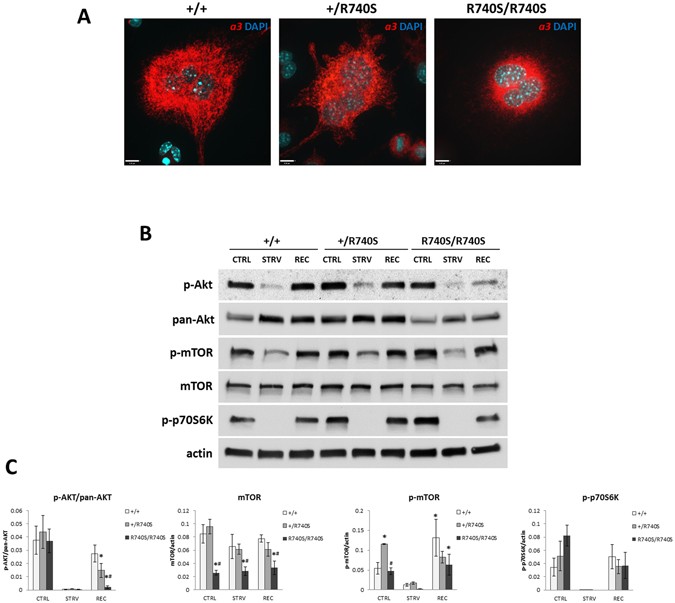
We have previously shown that the V-ATPases containing the R740S mutation in the a3 subunit are not functional14,15,16. The mutant a3 protein R740S is expressed (Fig. 1A) and is localized to
the lysosomes15. Using bone-marrow derived +/R740S osteoclasts, we have demonstrated that mTORC1 activity is increased17. To elucidate the connection between lysosomal pH, V-ATPases, and
mTORC1 activity, we examined mTORC1 pathway in homozygous R740S/R740S osteoclasts. Due to severe osteopetrosis and an early lethality in these animals, osteoclasts were derived from the
spleens of the 5–6 day old mice. We assessed mTORC1 activation and function by measuring the levels of total mTOR, p-AKT, p-mTOR, and phosphorylation of p70S6 kinase (p-p70S6K) under basal,
starvation (HBSS, 1 hr), and recovery conditions. Assessing p-AKT levels represents signaling upstream of mTORC1, while p-mTOR represents signaling downstream of AKT, and p-p70S6K, in turn,
is a well-characterized mTORC1 substrate.
mTORC1 activity is increased in R740S/R740S cells. Spleen-derived osteoclasts were differentiated as described in “Materials and Methods”. On day 4 of culture, the cells were incubated with
HBSS (starvation) for 60 min, and then in fully supplemented media for additional 30 min. (A) Immunofluorescence. Cells were fixed and stained with an anti-a3 antibody as described in
“Materials and Methods”; nuclei were stained with DAPI. Representative images of three independent experiments. Bars, 15 μm. (B) Immunoblotting, representative cropped blots of 4 independent
experiments. Whole cell lysates were separated on 4–20% gradient gels, transferred to a nitrocellulose membrane and probed for mTOR, p-mTOR, p-AKT, pan-AKT, p-p70S6K, and actin. (C)
Quantification of immunoblots; combined normalized data of at least 4 independent experiments, mean ± std. dev; * indicates statistical significance compared to +/+ control, # indicates
statistical significance compared to +/R740S control, p