Play all audios:
ABSTRACT Alphonso is known as the “King of mangos” due to its unique flavor, attractive color, low fiber pulp and long shelf life. We analyzed the transcriptome of Alphonso mango through
Illumina sequencing from seven stages of fruit development and ripening as well as flower. Total transcriptome data from these stages ranged between 65 and 143 Mb. Importantly, 20,755 unique
transcripts were annotated and 4,611 were assigned enzyme commission numbers, which encoded 142 biological pathways. These included ethylene and flavor related secondary metabolite
biosynthesis pathways, as well as those involved in metabolism of starch, sucrose, amino acids and fatty acids. Differential regulation (p-value ≤ 0.05) of thousands of transcripts was
evident in various stages of fruit development and ripening. Novel transcripts for biosynthesis of mono-terpenes, sesqui-terpenes, di-terpenes, lactones and furanones involved in flavor
formation were identified. Large number of transcripts encoding cell wall modifying enzymes was found to be steady in their expression, while few were differentially regulated through these
stages. Novel 79 transcripts of inhibitors of cell wall modifying enzymes were simultaneously detected throughout Alphonso fruit development and ripening, suggesting controlled activity of
these enzymes involved in fruit softening. SIMILAR CONTENT BEING VIEWED BY OTHERS TRANSCRIPTOME ANALYSIS INSIGHT INTO ETHYLENE METABOLISM AND PECTINASE ACTIVITY OF APRICOT (_PRUNUS
ARMENIACA_ L.) DEVELOPMENT AND RIPENING Article Open access 30 June 2021 RNASEQ REVEALS DIFFERENT TRANSCRIPTOMIC RESPONSES TO GA3 IN EARLY AND MIDSEASON VARIETIES BEFORE RIPENING INITIATION
IN SWEET CHERRY FRUITS Article Open access 22 June 2021 INTEGRATED ENDOGENOUS HORMONES AND TRANSCRIPTOME ANALYSIS CONTRIBUTE TO FRUIT DEVELOPMENT RELATED GENE MINING IN _ERIOBOTRYA JAPONICA_
Article Open access 28 April 2025 INTRODUCTION Mango (_Mangifera indica_ L.) is one of the most popular and highly favored fruit. Global mango production was reported to be 43.3 million
metric tons in 2013 preceding banana, apple, grape and orange. (https://www.statista.com/statistics/237064/top-world-producers-of-selected-fresh-fruit-by-value-2009/). There are thousands of
mango cultivars worldwide, among which Alphonso, Keitt, Kent, Lilli, Zill, Osteen, Haden, Kesar, Pairi, Dashehari, Langra and Banganapalli are well known. These varieties vary in their
fruit color, size, shape, flavor, taste and ripening period and pattern. To understand the composition and biosynthesis of their unique flavor and complex ripening process various studies
have been carried out at metabolic1,2,3, proteomic4,5,6, genetic7,8,9,10,11,12,13,14,15 and post-harvest processing16,17,18,19,20 levels. Whole genome sequencing and RNA sequencing (RNAseq)
are the two important high throughput technologies recently adopted to understand the complex cellular and physiological processes in fruits such as citrus21, tomato22 and strawberry23 as
well as domestication and diseases tolerance in citrus21, 24. Although mango genome sequence is yet not available, few recent studies have described the transcriptomic analysis from various
tissues of few mango cultivars. The first report from Zill mango25 provided extensive transcriptomic and proteomic profiling from pulp and skin tissues of four fruit developing stages using
pooled RNA but not stage specific and differentially expressed transcripts. Another study of leaf transcriptome and chloroplast genome sequencing from cultivar Langra provided information
about the production of several bioactive compounds26. Transcriptome analysis from two (raw and ripe) and three (raw, mid ripe and ripe) stages of Kent27 and Dashehari fruit pulp28,
respectively provided important insights into the ripening process and flavor biogenesis in these mango cultivars. India is the largest producer and exporter of mango with 40.6% share in
international mango market (http://www.fao.org). Among the Indian mango cultivars Alphonso is globally favored and highly exported mango due to its unique and attractive flavor, low fiber
containing pulp and high carotene content29, 30. The ripening duration of Alphonso mango is 15 days from harvest, which is the highest among all mango cultivars; for example, the ripening
duration for Kent and Dashehari mango fruit is 10 and 6 days, respectively27, 28. Fruit ripening in Alphonso mango progresses from skin towards the stone leading to attractive skin color and
easy monitoring of ripening progress. On the other hand various mango varieties, _viz_. Haden, Keitt, Kent, Tommy Atkins (National Mango Board, USA; http://www.mango.org) and Dashehari28
show polarity of their ripening from fruit stone to skin, making it difficult to identify ripened fruits. Longer ripening duration and shelf life of Alphonso mango provides sufficient time
for its transportation globally. The mechanisms underlying these unique properties of Alphonso mango need to be explored in depth at spatial and temporal level of fruit development and
ripening using transcriptomic, proteomic and metabolomic approaches as they can be correlated to the specific phenotype. In the present study, we performed transcriptome analysis of Alphonso
mango through eight different tissues such as flower, whole fruit at 30 and 60 DAP (Days After Pollination), pulp and skin of 90 DAP fruit (mature raw fruit) and pulp from three fruit
ripening stages i.e. 5 DAH (Days After Harvest): table green stage; 10 DAH: mid ripe stage and 15 DAH: ripe stage to analyze various fruit developing and ripening processes in Alphonso
mango. RESULTS ALPHONSO MANGO TRANSCRIPTOME Alphonso mango transcriptome was screened through eight tissue samples. To map differentially expressed transcripts a merged assembly was
generated from the reads of all the tissues, which reflected upon overall Alphonso mango flower and fruit transcriptome. For each tissue read numbers were more than 100 million, which were
assembled using k-mers 67, 75 and 83 separately and then merged for individual tissue. Average number of unique transcripts post assembly was 76,043 and with N50 and N80 values as 1,835 and
1,008 bp, respectively (Table 1). The minimum and maximum lengths of transcript from these assemblies were 100 and 17,342 bp, respectively (Table 1). Average number of transcripts was 11,925
upon filtering for redundancy and identifying candidate coding regions with maximum 90% identity and minimum 70% coverage (Table 2). Unique transcripts from each assembled and filtered set
were subjected to BLASTx against the non-redundant dataset from NCBI (http://www.ncbi.nlm.nih.gov). From a total of 20,755 unique transcripts from the merged assembly, 92.22% transcripts
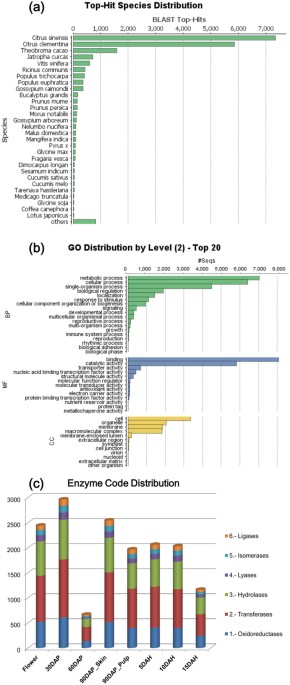
were annotated while 954 transcripts (4.59%) encoded hypothetical proteins and 661 (3.17%) remained unidentified. BLASTx statistics revealed maximum hits from _Citrus sinensis_ and _C.
clementina_ followed by _Theobroma cacao, Jatropha curcas, Vitis venifera_ and _Ricinus communis_ (Fig. 1a). Total 74,330 GO terms related to various biological processes (BP), molecular
functions (MF) and cellular components (CC) were assigned to these 20,755 unique transcripts. Metabolic, cellular and single organism processes followed by biological regulation and
localization were the most abundant terms under the BP category; binding and catalytic activity related terms under MF category; while, organelle, membrane and macromolecular complex terms
under the CC category, respectively (Fig. 1b, Supplementary Figure SF1). Unique transcripts were assigned enzyme commission (EC) number to determine the involvement of these transcripts in
various BPs. Total 4,611 ECs were assigned from oxidoreductase, transferase, hydrolase, ligase, lyase and isomerase classes; wherein, transferases were the most abundant followed by
hydrolases in all the eight tissues (Fig. 1c). These assigned ECs represented 142 known pathways from the KEGG database (http://www.genome.jp/kegg/pathway.html)31,32,33, which are
potentially functional in Alphonso mango fruit development and ripening. Most of these pathways were saturated with higher number of annotations from transcriptome data e.g. metabolism of
starch, sucrose and various amino acids including methionine and biosynthesis pathway of ethylene, phenylpropanoids and flavonoids (Supplementary Figure SF2). TRANSCRIPTOME CHANGES THROUGH
FLOWER TO FRUIT AND FRUIT DEVELOPMENT TO RIPENING Variations in the transcriptome were studied using several parameters, such as differentially expressed transcripts, transcripts distinctive
to a stage and gene ontology enrichment during flower to fruit transition and through process of fruit development and ripening. DIFFERENTIALLY EXPRESSED TRANSCRIPTS Comparison between
adjacent tissue stages was carried out to identify differentially expressed transcripts at each stage of fruit development and ripening (Table 3, Supplementary Table ST1). Flower to fruit of
30DAP, 524 transcripts were down regulated while 181 were up-regulated. Among the down-regulated transcripts alpha-amylase and subtilisin inhibitor-like (contig_6593), carbonic anhydrase
(contig_5377), chitinase (contig_4907) and maternal effect embryo arrest 59 (contig_4949) exhibited the highest fold change (>2-fold); while homeodomain-like protein (contig_12912 and
12913), cytochrome p450 - cyp72a219-like (contig_4083), heat shock cognate 70 kDa protein (contig_5948) and inositol-3-phosphate synthase (contig_9620) were highly up-regulated (>2-fold).
Transition from 30 DAP to 60 DAP resulted in up-regulation of 73 and down-regulation of seven transcripts. Important down-regulated transcripts were, n-acetyltransferase (contig_1261),
9-_cis_-epoxycarotenoid dioxygenase (contig_1680), protein reversion-to-ethylene sensitivity (contig_9164) and ethylene receptor 2 (contig_6147). While important up-regulated transcripts
were nucleotide sugar transporter family protein (contig_6337), beta-xylosyltransferase (contig_4150), various cellulose synthase catalytic subunits and laccases. Comparison of 60 DAP fruit
tissue with 90 DAP pulp and skin tissue, respectively revealed down-regulation of beta-xylosyltransferase, beta-d-xylosidase, cellulose synthase and galacturonosyl transferase; whereas spx
and exs domain-containing protein was up-regulated in both 90 DAP pulp and skin. Up-regulation of homeobox protein sbh1, gdsl esterase lipase, caffeoyl shikimate esterase, hydroperoxide
lyase, udp-rhamnose:rhamnosyl transferase and pectinesterase inhibitor was evinced from 90 DAP pulp tissue compared to that in 60 DAP fruit tissue. Evaluation of differentially expressed
transcripts between pulp and skin of 90 DAP revealed very few transcripts down-regulated in skin with none of them showing change more than 2-fold, while 54 transcripts were up-regulated in
90 DAP skin tissue including the important ones as DNA mismatch repair protein msh5, acyl carrier protein, amino-acid permease, calcium-transporting ATPase, isoflavone reductase and various
disease resistance proteins. During ripening of Alphonso pulp from 90 DAP to 5 DAH, 42 transcripts were up-regulated (>2-fold) including methyltransferase, amino-acid permease,
chloroplastic 9-_cis_-epoxycarotenoid dioxygenase, beta-galactosidase and protein phosphatase mainly, whereas 52 transcripts were down-regulated, important ones being few disease resistance
proteins, peroxidase, sucrose synthase and glycerol-3-phosphate dehydrogenase. The highest level of differential expression was evinced through transition from 5 DAH to 10 DAH among all the
ripening tissues wherein 418 transcripts were down and 191 were up-regulated. These stages are known for non-climacteric to climacteric transition during Alphonso fruit ripening based on
metabolite analysis17, 34. Prominently down regulated transcripts were phospholipase-A LCAT3, sugar phosphate exchanger, auxin-responsive protein IAA9, abscisate β-glucosyltransferase and
membrane-associated kinase regulator with more than 3-fold change, while up-regulated transcripts were aspartic proteinase nepenthesin, UDP-glucose 6-dehydrogenase,
1-aminocyclopropane-1-carboxylate oxidase, peroxidase, bidirectional sugar transporter sweet1-like, omega-6 fatty acid desaturase and squalene monooxygenase. During the transition from 10
DAH to 15 DAH stage, increased phosphate metabolism was evident. Total 30 transcripts were up-regulated mainly including phospholipase-D, inorganic pyrophosphatase, phosphate transporter,
transcription factor glk2 and DNA translocase, whereas 12 transcripts were down-regulated and important ones were aspartic protease, plastocyanin-like domain protein, annexin d2-like and
glucuronosyltransferase (>2-fold). IDIOSYNCRATIC TRANSCRIPTS FOR THE STAGE Various transcripts unique to each of these stages (Fig. 2, Table 4, Supplementary Table ST2) representing
multiple stage specific processes were identified during this comparative analysis. A total of 388 transcripts were identified as unique to flower tissue, which mostly included various
transcription and translation factors, late embryogenesis abundant proteins, stress-sensitive and dehydration-responsive element-binding protein-1b and various ribosomal proteins. Similarly,
distinct transcripts specific to fruit developing and ripening stages were identified, wherein 1,090 and 27 transcripts were idiosyncratic to the fruit developing and ripening stages,
respectively (Supplementary Table ST2). Various auxin and gibberellin induced and regulated proteins as well as several proteins responsible for various vacuole activities, multiple disease
resistance proteins and various terpene synthases were distinct to the developing stages. Transcripts encoding for multiple transcription and translation factors during Alphonso fruit
development were also detected. Interestingly multiple ethylene responsive transcription factors along with the protein reversion-to-ethylene sensitivity were uniquely revealed in the
developing stages. Whereas, NIN-like protein, respiratory burst oxidase homolog protein d-like, rhamnogalacturonate lyase b-like, lectin receptor kinase, gag protein and methionine Υ-lyase
were exclusive to the Alphonso ripening stages. Similarly, WRKY transcription factor 43, b3 domain-containing val3 and ap2 ERF domain-containing transcription factors were uniquely
identified from the ripening stages. Several hypothetical and uncharacterized proteins were also found to be Alphonso ripening specific and their characterization might help to reveal the
ripening process in Alphonso. GENE ONTOLOGY (GO) ENRICHMENT Fisher’s exact test was performed to understand over- and down-represented GOs (p-value ≤ 0.001) during the transition, which gave
an overall picture of the Alphonso mango development and ripening (Fig. 3). During the flower to 30 DAP fruit transition certain GOs were over-expressed such as post-embryonic developmental
and anatomical structure morphogenesis process, response to abiotic stimulus and various plastid and thylakoid processes. At the same time, hydrolysis of _O-_glycosyl compounds and
UDP-glycosyltransferase activity along with the methyltransferase, sucrose metabolic and lipid biosynthetic activities coding GOs were down-represented. 30 DAP to 60 DAP transition described
enriched GOs for starch and sucrose metabolic process, fatty acid, cellulose and vitamin biosynthetic processes, protein glycosylation, response to oxidative stress and heat along with HSP
binding. During the same event cell differentiation, growth and cell death in addition to the anatomical structure morphogenesis, secondary metabolic process and response to biotic stimulus
coding GOs were observed to be decreased. Comparison between 60 DAP fruit and 90 DAP pulp revealed over-represented GOs for cell differentiation and cell growth, response to biotic stimulus,
secondary metabolic process, tropism and regulation of gene expression. While, starch and sucrose metabolic processes, _O-_glycosyl hydrolase UDP-glucosyltransferase activities, fatty acid
biosynthesis and catabolic processes along with the lipid transport, various amino acid and terpenoid biosynthesis and cell wall biogenesis were down-represented. 90 DAP pulp and skin
tissues showed over-expression of various ion, ADP and sequence-specific DNA binding activities along with the response to hormone, chemical and organic substances in the skin tissue;
whereas only cytoplasm and cytoplasmic part related GOs were found to be down-expressed in the skin compared to that in the pulp at 90 DAP. Transition of 90 DAP pulp to 5 DAH pulp revealed
only over-representation of GOs such as biosynthesis of RNA, aromatic compounds, and nucleobase containing compounds; binding of various ions; regulation of primary, cellular, nitrogenous
compounds, RNA biosynthesis processes and gene expression and response to hormone, chemical and organic substance. Surprisingly, none of the GOs showed significant (P-value ≤ 0.001)
enrichment during the transition from 5 DAH to 10 DAH and reflected as the stationary phase of Alphonso mango ripening. The 10 DAH (mid ripe stage) to 15 DAH (ripe stage) transition showed
over-expression of many GOs such as binding of various ions; phospholipid, glycolipid, amino acid, co-enzyme and water soluble vitamin biosynthetic processes; methyltransferase,
UDP-glycosyltransferase and _O-_glycosyl hydrolase activities; starch, sucrose metabolic processes, gluconeogenesis and glycolytic process; protein phosphorylation, dephosphorylation,
folding, protein modification and small protein conjugation along with the regulation and response to oxidative stress, oxidoreductase activity and redox homeostasis. While the GOs related
to plastid and cytosol; post embryonic development; response to abiotic and endogenous stimulus; anatomical structure morphogenesis, plasma membrane, cell differentiation and communication
were down-represented. SPATIAL CHANGES IN TRANSCRIPTOME AT 90 DAP In case of Alphonso mango, 90 DAP stage is a mature raw stage of the fruit and is considered as the right stage of fruit
harvest (0 DAH) for further artificial ripening of the fruit35. Hence transcriptome analysis of skin and pulp were separately carried out at this stage. Overall 90 DAP skin was found to be
metabolically more active compared to the 90 DAP pulp with respect to differentially expressed genes (Supplementary Table ST1), unique genes (Supplementary Table ST2) and enriched GOs (Fig.
3). In the skin, 54 transcripts were up-regulated whereas only four were down-regulated compared to the pulp. Among the up-regulated transcripts (>2-fold), isoflavone reductase,
transcription and translation regulatory proteins, hydrolases, methyltransferase etc. were more prominent; whereas four down-regulated transcripts were membrane and cytoplasm related GOs.
Unique transcripts upon comparison between 90 DAP pulp and skin showed carotene and xanthophylls biosynthesis related contigs, beta-carotene hydroxylase (contig_5406) and anthocyanidin
3-o-glucosyltransferase, flavor related various terpene synthases and ripening related contigs such as ethylene-responsive transcription factors, pectate lyase, pectin esterase and cellulase
as unique to skin compared to the pulp. Interestingly, in comparison of 5DAH stage with 90 DAP pulp few of these transcripts such as isoflavone reductase (contig_5337) and beta-carotene
hydroxylase (contig_5406) were observed to be idiosyncratic to 5 DAH stage as seen in 90 DAP skin stage. Similarly, transcripts encoding enzymes involved in pectin degradation (contigs_9578,
9579, 18096 and 3603) were found to be upregulated (>2-fold) in the comparison between 10DAH vs 90 DAP pulp and 15 DAH vs 90 DAP pulp. These finding suggest progression of ripening
related molecular processes in the pulp of 5 DAH and onward stages which were initiated at 90 DAP skin and highlight initiation of Alphonso ripening process from skin and its probable
progress towards fruit stone. GENES INVOLVED IN THE FLAVOR BIOGENESIS IN ALPHONSO MANGO Quantitatively mono-terpenes are abundant in Alphonso followed by sesqui-terpenes2, 35. The present
data revealed six contigs encoding mono-terpene synthases (_limonene synthase1, limonene synthase2, beta ocimine synthase1, beta ocimine synthase2_, _isoprene_ synthase1 and _isoprene
synthase2_), five contigs encoding sesquiterpene synthases (_germacreneD synthase1, germacreneD synthase2, nerolidol synthase1, nerolidol synthase2_ and _alpha farnesene synthase_) and three
contigs coding for di-terpene synthases (_ent-kaurene synthase_ and _casbene synthase (E,E)-geranillinalool synthase_). Phylogenetic analysis (Fig. 4) of these genes along with other plant
terpene synthases (TPS) showed distribution of these genes in to the TPS-a, TPS-b, TPS-e and TPS-f clades, respectively36. Furaneol and mesifuran are the two furanones from Alphonso mango
and their synthesis by _enone oxidoreductase_ (_EO_) and _O-methyltransferase_ (_OMTS_), respectively have been described earlier10, 11. Multiple contigs coding for _quinone oxidoreductase_
and _O-methyltransferases_ were detected in the present analysis. Phylogenetic analysis of these contigs with the characterized genes revealed another similar transcript variant for the
_MiEO_ (Supplementary Figure SF3), whereas none of the contigs showed similarity to the _MiOMTS_ (Supplementary Figure SF4). Green grassy aroma of unripe fruits is due to the C6 volatiles
formed during the lipoxygenase and hydroperoxide lyase (HPL) pathways. In the present study a single contig encoding the _hydroperoxide lyase_ and six contigs encoding _13-lipoxygenase_ were
detected. Involvement of _9-lipoxygenase_ (_Mi9LOX_) and epoxide _hydrolase 2_ (_MiEH2_) in the biogenesis of lactones from Alphonso mango was confirmed in our recent study15. One more
transcript encoding _9-lipoxygenase_ similar to that of the characterized _Mi9LOX_ (Supplementary Figure SF5) and three contigs coding for _epoxide hydrolase 2_ grouping with the _MiEH2_
(Supplementary Figure SF6) were additionally detected in the present study. Three more novel contigs coding for _epoxide hydrolase_ were also detected but neither grouped with the other
_EH1_or _EH2_ from different plant species (Supplementary Figure SF6). We further analyzed the differential expression of all these flavor related genes in terms of transcript abundance in
flower and fruit developing and ripening stages (Supplementary Figure SF7). Among these the contigs encoding various TPS were abundant in flower and during early developing stages.
Interestingly, many contigs encoding EH (contigs 8280, 3904, 3901, 14123 and 8281), LOX (contigs 18105, 12748, 12747 and 12385) and EO (contigs 8026, 5618, 15594, 14137 and 13600) were found
to be ripening specific and might be playing crucial role in generating unique aroma volatiles during Alphonso fruit ripening. GLYCOSIDASES AND CELL WALL DEGRADING ENZYMES FROM ALPHONSO
MANGO Glycosidases are involved in a variety of functions such as hydrolysis of complex carbohydrates (storage and structural) to mono-saccharides, removal of sugars from various glycans
including glycosidically bound aroma volatiles, which serve as storage pool for the aroma compounds. In the present study, many glycosidases acting on various sugars i.e. glucose, galactose,
mannose, fructose, xylose, fucose and rhamnose, were detected. Among these, class glucosidase with the highest number of contigs (51 contigs) was observed to contain 28 and 21contigs coding
for glucan β-glucosidase and general β-glucosidase, respectively. Two contigs coding for α-glucosidases were identified of which one coded for glucan α-glucosidase and the other for general
α-glucosidase. Among these, at 30 DAP stage contig_7442 and contig_7857 were found to be down-regulated (>3-fold) compared to those in flower stage. Contig_16888 was down-regulated
whereas contig_17138 was up-regulated at 10 DAH than those at 5 DAH. At 15 DAH contig_9072 was down-regulated (1.5-fold) compared to that at 10 DAH. Among the galactosidase class, nine
contigs encoding α-galactosidase and 21 contigs encoding β-galactosidase were detected. Two of these were down-regulated (contig_1095 and contig_1096) at 30 DAP compared to those in flower.
Contig_3844 was down regulated in 90 DAP pulp compared to 60 DAP. Transition from 90 DAP to 5 DAH reflected in to up-regulation of contig_3844 (2.72-fold) whereas, contig_1525 coding for
α-galactosidase was down-regulated in 10 DAH compared to that at 5 DAH. In the mannosidase class, five α-mannosidase and eight endo-β-mannosidase coding contigs were identified out of which
two encoding for endo-beta-mannosidase (contig_15554 and contig_15558) were down-regulated at 10 DAH compared to 5 DAH, rest didn’t show differential regulation. Among two α-xylosidase and
five β-xylosidase from Alphonso mango only contig_1633 showed differential regulation which was up-regulated at 60 DAP compared to 30 DAP and was further down-regulated in both the 90 DAP
tissues. Two transcripts coding for the acid beta-fructofuranosidase (contig_12501 and contig_12502) were detected but did not show any differential regulation in various tissues analyzed.
Also, seven contigs coding for the alpha-l-fucosidase were identified from Alphonso mango but did not show differential regulation. Degradation of plant cell wall components, namely
cellulose, hemi-cellulose and pectin by cellulases and glucanases in ripening fruits is responsible for the fruit softness. Cellulases encoding five contigs were identified among which one
coded for acidic cellulase and found to be abundant during flower and early fruit developing stages. Total 18 contigs encoding glucanase were detected of which contig_4148 was up-regulated
in flower compared to 30 DAP, whereas, four transcripts (contig_9268, contig_19283, contig_9267 and contig_17145) were down-regulated (>2-fold) at 10 DAH compared to 5 DAH stage. Pectin
is another component of fruit cell wall and is degraded by a set of enzymes _viz_. pectate lyase (PL), pectin esterase (PE), polygalacturonase (PG) and rhamnogalacturonate lyase. In the
present study 18 PL, 24 PG and 10 PE coding transcripts were detected. However, only few were differentially expressed, for example, only PL contig_7696 was down-regulated in 30 DAP fruit
compared to flower and PL contig_9578 was up-regulated in 10 DAH fruit than 5 DAH fruit. PG non-catalytic subunit jp650 coding contig_9895 was down-regulated in 5 DAH pulp than in 90 DAP
pulp, whereas PG contig_3471 was down regulated and PG contig_1614 was up-regulated in 10 DAH compared to 5 DAH stage. Likewise, PE contig_7997 was down-regulated and contig_9162 was
up-regulated at 30 DAP stage compared to flower. Similarly, eight transcripts encoding rhamnogalacturonate lyase were detected of which two (contig_7745 and contig_7746) were distinct to the
ripening stages (Supplementary Table ST2). TRANSCRIPTOME ANALYSIS IDENTIFIED NOVEL ENZYME INHIBITORS FROM ALPHONSO MANGO We identified various classes of enzyme inhibitors (Supplementary
Table ST3) such as α- amylase inhibitor (2 contigs), inhibitor of proliferation pds5, apoptosis inhibitor (2 contigs), bax inhibitor (4 contigs), lipid transfer protein inhibitor (6
contigs), various kinase inhibitors (16 contigs), cysteine proteinases inhibitor (4 contigs), inter-alpha-trypsin inhibitor (3 contigs), serine protease inhibitor, kunitz family trypsin and
protease inhibitor, guanosine nucleotide diphosphate dissociation inhibitor, nf-kappa-b inhibitor (2 contigs), pectinesterase inhibitor (12 contigs), polygalacturonase inhibitor, proteasome
inhibitor, protein transport inhibitor (4 contigs), protein phosphatase inhibitor and rho gdp-dissociation protein inhibitor (4 contigs). Along with these, contigs coding for protein
reversion-to-ethylene sensitivity (3 contigs), cell wall and vascular inhibitor of beta-fructosidase (2 contigs) and macrophage migration inhibitory factor were also identified from Alphonso
transcriptome. These inhibitors showed differential regulation during fruit development and ripening (Supplementary Figure SF8). Most of the inhibitors were found to be expressing
throughout all the stages except for the group 4 (coding for bax inhibitor), which were abundant only during fruit ripening stages of Alphonso mango and probably played important role in
ripening physiology of Alphonso mango. TRANSCRIPTOME VALIDATION THROUGH QRT PCR Results of the transcriptomic analysis were validated by qRT PCR of 38 representative genes from various
metabolic pathways such as carbohydrate metabolism (cellulose synthase: CS, chitinase: CTN, and pectate lyase: PL), fatty acid metabolism (omega 3 fatty acyl desaturase: O3FAD, omega 6 fatty
acyl desaturase: O6FAD, glyceraldehyde-3-phosphate acyl transferase: G3PAT, alcohol dehydrogenase: ADH and long chain fatty acyl CoA ligase: LCFACL), terpene metabolism (mono-terpene
synthases: MTPS, sesqui-terpene synthases: STPS and di-terpene synthases: DTPS) and proteins such as ethylene responsive factors: ERF and disease resistance proteins: DRP. Various transcript
variants of these genes were selected wherever available to confirm the accuracy of assembly. qRT PCR analysis revealed similar differential expression pattern for almost all the
transcripts through all the eight stages with correlation coefficient _R_ ranging between 0.8 and 0.99. Few transcripts showed variation in the fold change of RNAseq and qRT PCR data at few
stages but still had good correlation (_R_ ≥ 0.7) (Figs 5–9 and Supplementary Table ST5). Transcript variants confirmed the accurate assembly and showed differential expression of these
variants from each other through various tissues analyzed (Figs 5–9). DISCUSSION Recently transcriptome studies on mango cultivars namely, Zill25, Langra26, Kent27 and Dashehari28 have put
forth important information regarding the fruit and leaf physiology of mango. These studies have identified genes encoding multiple enzymes involved in various pathways of primary and
secondary metabolism such as, citrate cycle, glycolysis and gluconeogenesis from carbohydrate metabolism; fatty acid biosynthesis, beta oxidation and salicylic acid biosynthesis from fatty
acid metabolism; biosynthesis and degradation of various amino acids as well as ethylene biosynthesis from methionine. Genes involved in the flavonoid biosynthesis, vitamin biosynthesis
(β-carotene and α-tocopherols) as well as terpenoid backbone synthesis (mevalonate pathway) have also been well explored. In the present study genes involved in all these pathways were
identified and their differential expression was also evident through various stages of the Alphonso mango fruit development and ripening (Supplementary Table ST1). In addition the present
study revealed some novel findings highlighting better understanding of various processes involved in mango fruit development and ripening and some were unique to the most favoured Alphonso
mango fruit, which are discussed below. Quantitative abundance of terpenes in mangos is well known2, 35, Transcriptome and gene expression studies in mango have explored the terpene
biosynthesis pathway till GPP, FPP and GGPP synthesis9, 25,26,27,28. In this study, we identified six, five and three genes encoding mono-terpene synthases (MTPS), sesqui- terpene synthases
(STPS) and di-terpene synthases (DTPS), respectively that are involved in biosynthesis of specific terpene molecules (Supplementary Figure SF9). These genes were abundant in the flower
tissue followed by 30 DAP stage. Further, the transcript abundance of many of these terpene synthase genes in the present study has been depicted to be idiosyncratic to the developing
stages, leading to their least expression in the ripening stages of Alphonso fruit (Figs 8 and 9, Supplementary Figure SF7 and Supplementary Table ST2). Previous aroma volatile analysis from
Alphonso mango supports this observation wherein flower had the highest concentration of mono-terpenes, oxygenated mono-terpenes and sesqui-terpenes which decreased through the fruit
development35. Another flavor related pathway is lipoxygenase (LOX) followed by HPL pathway37,38,39, which produces C6 GLVs and lactones through peroxygenase pathway15. The transcriptomes
analyzed from Kent and Dashehari mangos reported the presence of six and five genes coding for the LOX family, respectively27, 28. Here, we report detailed annotation of Alphonso mango _LOX_
genes wherein, two genes encode the _9-LOX_ and six genes encode the _13-LOX_. Likewise, _Peroxygenase_ and _epoxide hydrolase_ (_EH_) genes have been well-studied for biosynthesis of cutin
biopolymer40 and defense related compounds41, 42; while our recent study has shown involvement of these genes in the production of lactones15. In spite of their biological significance,
none of the previous transcriptome studies on mango could determine the presence of peroxygenase and epoxide hydrolase genes. On the other hand in the current study various contigs encoding
novel EH (three contigs), EH2 (four contigs) and peroxygenase (three contigs) were detected. Similarly, multiple transcripts encoding _enone oxidoreductase_ and _O-methyltransferase_ having
role in furanone biosynthesis10, 11 were also identified and expression profiles of many of them were shown to be ripening specific (Supplementary Figure SF7). Abundance and ripening related
expression of large number of these unique flavor related genes in Alphonso mango (Supplementary Figure SF7) signifies their role in synthesis of diverse aroma volatiles and their unique
blend giving sweet and fruity flavor in Alphonso as shown by our previous studies34, 35. Fruit ripening is a complex physiological process and can be characterized by means of fruit
softening due to changes in the cell wall structure43,44,45, increased sugar content by polysaccharide hydrolysis16 and changes in the aroma volatiles35. Starch and pectin are the major
storage and structural polysaccharides in the mango fruit, respectively. Various hydrolases and lyases are known to carry out polysaccharide and cell wall hydrolysis43, 46,47,48,49,50, while
amylases degrade the starch in to soluble sugars during ripening. We identified four α-amylase, three isoamylase and 13 β-amylase coding transcripts. Only one transcript coding for
α-amylase (contig_1439) was down-regulated through flower to 30 DAP fruit transition while others were expressed throughout the fruit developing and ripening stages. On the other hand,
transcriptome analysis of Kent mango revealed identification of four β-amylase and three α-amylase transcripts out of which only two β-amylase coding transcripts were found to be
up-regulated in ripe tissue, none of the other showed differential expression27. In addition, we also detected large number of PL, PG and PE encoding transcripts known to be responsible for
degradation of complex hetero-polysaccharide, pectin48 were detected (Supplementary Table ST4). Most of these had stable expression and only few were differentially expressed in Alphonso
mango. These results are in contrast to the observations from Kent27 and Dashehari28 mango transcriptomic data in terms of the number of unigenes detected and their differential regulation.
In case of Dashehari mango four PL and none of the PE or PG encoding transcripts were up-regulated; whereas in Kent, four PL, six PE and six PG encoding unigenes were up-regulated. These
results signify controlled and steady activity of pectin degradation leading to slow and balanced transitions during Alphonso fruit ripening and this might be one of the reasons for its
longer shelf life. Another interesting observation in our study was, the identification of 79 contigs encoding 20 different inhibitor classes from Alphonso mango (Supplementary Figure SF8).
Previously, only three unigenes coding for the cysteine proteinase inhibitors were reported from Langra leaf transcriptome, while no such inhibitors were reported from Zill25, Kent27 and
Dashehari28 mango transcriptomes. The present study suggests Alphonso mango transcriptome to be rich in these inhibitors throughout the fruit development and ripening stages. Thus, the
presence of large numbers of amylase, PL, PE and PG transcripts with very few of them differentially regulated, perpetual expression of most of the starch and cell wall hydrolyzing enzymes
along with the persistent presence of inhibitors for amylase, pectinesterase, polygalacturonase and ethylene sensitivity can be cumulatively suggested to play a crucial role in controlled
and slow ripening and longer fruit shelf life of Alphonso mango. As a representative example the balance between pectinesterases and their inhibitors during Alphonso fruit development and
ripening has been demonstrated in Fig. 10 as heatmap and area chart of expression of PE and PEI. We further observed that phosphate metabolism related gene ontologies were enriched at
complete ripe stage (15 DAH) of Alphonso mango. Among these some transcripts were uniquely found at 15 DAH stage (Supplementary Table ST2) suggesting accelerated primary metabolism.
Moreover, contig 6946 encoding cytokinin riboside 5 -monophosphate phosphoribohydrolase was uniquely observed at 15 DAH stage. This enzyme plays important role in converting endogenous
inactive cytokinin nucleotides to the biologically active free cytokinin responsible for delayed ripening51, 52. On the other hand contig_6770 encoding phospholipase-D (PLD) involved in
degradation of important cell membrane component i.e. phospholipids was observed to be upregulated (3.4fold). A key role of PLD in softening was well explained in tomato plants transformed
with antisense PLD cDNA which showed delayed ripening and increased firmness in tomato fruits53. This counter play at molecular level might be responsible for fine tuning of ripening process
in Alphonso mango providing longer shelf life to the fruits. Additionally oxidative burst, oxidoreductase activities and oxidative stress related gene ontologies were observed during
ripening. These factors can generate reactive oxygen species and lead to cell death and fruit damage. Such reactive oxygen species induced cell death is suppressed by Bax inhibitor, which
was well studied in _Arabidopsis thaliana_ 54. Interestingly, four contigs coding for Bax inhibitor in Alphonso mango showed ripening specific expression probably responsible for preventing
cell death in ripening fruits and thus longer shelf life. Further detailed study on these inhibitors might help to understand jelly formation in Dashehari mangos due to excessive ripening
and spongy tissue formation in Alphonso mangos due to uneven ripening55, 56. It is known that ripened fruits are prone to attack by various pathogens57. A well distinct defense mechanism was
observed in Alphonso mango wherein various defense related proteins (227 contigs) and chitinases (19 contigs) acting on fungal cell wall58 were differentially regulated (Supplementary Table
ST1). Chitinases were found to be accumulated in the flower and in the early fruit developing stages. Insect driven pollination has the risk of fungal infection to flower and further spore
accumulation around ovary causing internal infection to the fruit. This might be restricted by the presence of various chitinases in Alphonso mango. Similarly, various disease resistance
proteins might play role during fruit development and ripening process to defend infections. CONCLUSIONS The transcriptome of Alphonso mango analyzed through eight stages of flower to fruit
development and ripening transitions revealed various differentially regulated and stage specific genes. Unique transcript profiles probably responsible for distinct and favorable
characteristics of Alphonso mango fruit such as flavor, color, ripening duration, skin to stone ripening pattern and longer shelf life were identified and analyzed. This study provides large
data sets for further functional validation of fruit ripening process. METHODS PLANT MATERIAL Flower and fruits of cv. Alphonso were collected from three independent trees as biological
replicates from the Mango Research Sub Centre, Deogad (16.528336 N, 73.344790 E) affiliated to Dr. Balasaheb Sawant Konkan Agricultural University, Dapoli, Maharashtra, India. Flowers from
inflorescence were collected and snap frozen. Fruits from developing stages were collected at 30, 60 and 90 days after pollination (DAP). Fruits from 30 and 60 DAP were analyzed as whole
fruit in the present study after removing fruit stone; whereas at mature raw stage (90 DAP) pulp (mesocarp) and skin (exocarp) were separated, snap frozen in liquid nitrogen and stored at
−80 °C until further analysis. A set of fruits were additionally harvested at their mature raw stage and kept in the hay containing boxes at ambient temperature for ripening and only pulp
tissue for ripening stages as table green, mid ripe and ripe were collected at 5, 10 and 15 days after harvest (DAH), respectively. At each ripening stage fruits were removed from the box,
pulp and skin were separated and pulp was frozen in liquid nitrogen and stored at −80 °C till further use. RNA ISOLATION AND CDNA SYNTHESIS Total RNA isolation was carried out for all the
tissues sampled for current study using RNeasy Plus mini kit (Quiagen, Hilden, Germany). RNA quality as 260 nm/ 280 nm ratio was evaluated using Nanodrop 1000 (Thermo Fisher Scientific,
Waltham, Massachusetts, USA) and RNA integrity was checked using Bioanalyzer 2100 (Agilent Technologies, Santa Clara, USA). Two microgram of total RNA was used to carry out reverse
transcription for synthesis of cDNA using High Capacity cDNA reverse transcription kit (Applied Biosystem, Carlsbad, CA, USA). LIBRARY PREPARATION AND SEQUENCING High quality RNAs from seven
progressive stages of fruit development and ripening as well as flower tissue from single representative biological replicate were sent to the Next Generation Genomics Facility (NGGF) at
the Centre for Cellular and Molecular Platforms (C-CAMP), Bangalore for performing transcriptome sequencing. Briefly, 1 ug of total RNA from each sample was used to prepare eight individual
libraries and mRNA was purified using polyT oligo beads. The purified mRNA was fragmented in the range of 100 to 140 bases with optimum at around 120 bases and the cDNA was synthesized. End
repair, A-Tailing, Adapter ligation and the library preparation were performed using Tru Seq RNA sample preparation kit v2 (Illumina, San Diego, USA) as per manufacturer’s instructions. PCR
enrichment was performed for 15 cycles and the sample was validated on the Bioanalyzer 2100. Libraries were sequenced in a Paired End 100 base run, using TruSeq SBS Kit v3-HS (Catalog No.:
FC-401-3001) for sequencing on the Illumina HiSeq. 1000 platform according to the manufacturer’s recommended protocols.
(http://www.illumina.com/systems/hiseq_systems/hiseq_2000_1000/kits.ilmn). BIOINFORMATICS DATA ANALYSIS Paired end RNA sequencing was performed using Illumina Hiseq2000. Quality check on raw
data was performed using FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Adapter free, good quality reads (Q >= 30; min read length = 85) were obtained using
Cutadapt59. Alphonso transcriptome for each stage was assembled using Velvet-Oases60 with K-mers 67, 75, 83 and merging them at 27 k-mer. Additionally, a merged transcriptome was also
generated using k-mer 55. Further, for all the merged assembled transcripts, we used Transdecoder (https://transdecoder.github.io/) to extract potential candidate coding regions within
transcripts. Partial cds were discarded and only those transcripts with start and stop codon were made non redundant based upon sequence identity cut-off of 90% using CD-HIT-est61 and used
for downstream analyses. Further, merged full length transcripts (from merged assembly) were used as reference to map back all the raw reads from each stage using default parameters of
Bowtie62. DESeq2 was used to identify differentially expressed transcripts63 and were filtered based upon p-value ≤ 0.05 and expression value >0. All those transcripts having mapping
count zero were excluded from further analysis such as in identification of uniquely expressed transcripts in a particular stage or a specific set of stages (e.g. developing and ripening
stages). Unique and common list of transcripts were represented using Venny (http://bioinfogp.cnb.csic.es/tools/venny/) and provided in Supplementary Table ST2. Full length transcripts (from
merged assembly) were used as reference for differential expression analysis using DESeq263. Annotation, enzyme code distribution and GO mapping and InterProScan analyses were carried using
BLAST2GO 3.1.3 workbench (Biobam Bioinformatics S.L., Valencia, Spain) as described in the user manual64. GO enrichment analysis was carried out in given test and reference sets by Fisher’s
exact test in BLAST2GO with p-value (0.001) and FDR filters. QUANTITATIVE REVERSE-TRANSCRIPTASE PCR Quantitative reverse-transcriptase PCR was performed using the Fast Start Universal SYBR
Green master mix (Roche Inc. Indianapolis, Indiana, USA) and _elongation factor 1α_ (_EF1α_) as an endogenous reference gene employing the primers reported earlier7. Various transcripts
selected from transcriptome data were amplified using gene specific primers (Supplementary Table ST5) and quantification was performed using 7900HT Fast Real-Time PCR System (Applied
Biosystems, California, USA) having thermal cycle program of initial denaturation at 95 °C for 10 min with subsequent 40 cycles of 95 °C for 3 sec and 60 °C for 30 sec followed by a
dissociation curve analysis of transcripts. Relative quantification (ΔΔCT method) and statistical analysis was carried out using DataAssist™ v3.01 software (Applied Biosystems, California,
USA). Eight tissue samples as described above from three independent biological replicates were used for this analysis. For individual transcript the highest expression at a particular fruit
development or ripening stage was considered as 1 and expression in other tissues including flower was normalized to that for better graphical presentation. DATA AVAILABILITY The raw data
files generated during the current study will be available in the NCBI repository under the BioProject - PRJNA391381. REFERENCES * Gil, A. M. _et al_. Study of the compositional changes of
mango during ripening by use of nuclear magnetic resonance spectroscopy. _Journal of Agricultural and Food Chemistry_ 48, 1524 (2000). Article CAS PubMed Google Scholar * Pandit, S. S.
_et al_. Cultivar relationships in mango based on fruit volatile profiles. _Food Chemistry_ 114, 363, doi:10.1016/j.foodchem.2008.09.107 (2009). Article CAS Google Scholar * Deshpande, A.
B. _et al_. Data on changes in the fatty acid composition during fruit development and ripening of three mango cultivars (Alphonso, Pairi and Kent) varying in lactone content. _Data in
Brief_ 9, 480, doi:10.1016/j.dib.2016.09.018 (2016). Article PubMed PubMed Central Google Scholar * de Magalhães Andrade, J. _et al_. 2D-DIGE analysis of mango (_Mangifera indica_ L.)
fruit reveals major proteomic changes associated with ripening. _Journal of proteomics_ 75, 3331 (2012). Article Google Scholar * Fasoli, E. & Righetti, P. G. The peel and pulp of
mango fruit: A proteomic samba. _Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics_ 1834, 2539 (2013). Article CAS Google Scholar * Renuse, S. _et al_. Proteomic analysis of an
unsequenced plant-_Mangifera indica_. _Journal of proteomics_ 75, 5793 (2012). Article CAS PubMed Google Scholar * Pandit, S. S. _et al_. Expression profiling of various genes during the
fruit development and ripening of mango. _Plant Physiology and Biochemistry_ 48, 426, doi:10.1016/j.plaphy.2010.02.012 (2010). Article CAS PubMed Google Scholar * Pandit, S. S. _et al_.
Genetic diversity analysis of mango cultivars using inter simple sequence repeat markers. _Current science_ 93, 1135 (2007). Google Scholar * Kulkarni, R. _et al_. Characterization of
three novel isoprenyl diphosphate synthases from the terpenoid rich mango fruit. _Plant physiology and biochemistry_ 71, 121 (2013). Article CAS PubMed Google Scholar * Kulkarni, R. _et
al_. An oxidoreductase from ‘Alphonso’mango catalyzing biosynthesis of furaneol and reduction of reactive carbonyls. _SpringerPlus_ 2, 494 (2013). Article PubMed PubMed Central Google
Scholar * Chidley, H. G. _et al_. Molecular cloning and characterization of _o_-methyltransferase from mango fruit (_Mangifera indica_ cv. Alphonso). _Molecular Biotechnology_ 58, 340,
doi:10.1007/s12033-016-9933-2 (2016). Article CAS PubMed Google Scholar * Martínez, P. G., Gómez, R. L. & Gómez-Lim, M. A. Identification of an ETR1-homologue from mango fruit
expressing during fruit ripening and wounding. _Journal of Plant Physiology_ 158, 101 (2001). Article Google Scholar * Sane, V. A., Chourasia, A. & Nath, P. Softening in mango
(_Mangifera indica_ cv. Dashehari) is correlated with the expression of an early ethylene responsive, ripening related expansin gene, _MiExpA1_. _Postharvest biology and technology_ 38, 223
(2005). Article CAS Google Scholar * Singh, R. K., Ali, S. A., Nath, P. & Sane, V. A. Activation of ethylene-responsive p-hydroxyphenylpyruvate dioxygenase leads to increased
tocopherol levels during ripening in mango. _Journal of experimental botany_ 62, 3375 (2011). Article CAS PubMed PubMed Central Google Scholar * Deshpande, A. B. _et al_. Isolation and
characterization of 9-lipoxygenase and epoxide hydrolase 2 genes: Insight into lactone biosynthesis in mango fruit (_Mangifera indica_ L.). _Phytochemistry_ 138, 65,
doi:10.1016/j.phytochem.2017.03.002 (2017). Article CAS PubMed Google Scholar * Chidley, H. G. _et al_. Effect of postharvest ethylene treatment on sugar content, glycosidase activity
and its gene expression in mango fruit. _Journal of the Science of Food and Agriculture_ 97, 1624 (2016). Article PubMed Google Scholar * Chidley, H. G., Kulkarni, R. S., Pujari, K. H.,
Giri, A. P. & Gupta, V. S. Spatial and temporal changes in the volatile profile of Alphonso mango upon exogenous ethylene treatment. _Food Chemistry_ 136, 585,
doi:10.1016/j.foodchem.2012.08.029 (2013). Article CAS PubMed Google Scholar * González-Aguilar, G., Zavaleta-Gatica, R. & Tiznado-Hernández, M. Improving postharvest quality of
mango ‘Haden’by UV-C treatment. _Postharvest Biology and Technology_ 45, 108 (2007). Article Google Scholar * Hofman, P. J., Smith, L. G., Joyce, D. C., Johnson, G. I. & Meiburg, G. F.
Bagging of mango (_Mangifera indica_ cv.Keitt’) fruit influences fruit quality and mineral composition. _Postharvest Biology and Technology_ 12, 83 (1997). Article CAS Google Scholar *
Jiang, Y. & Joyce, D. Effects of 1‐methylcyclopropene alone and in combination with polyethylene bags on the postharvest life of mango fruit. _Annals of Applied Biology_ 137, 321 (2000).
Article CAS Google Scholar * Wu, G. A. _et al_. Sequencing of diverse mandarin, pummelo and orange genomes reveals complex history of admixture during citrus domestication. _Nature
biotechnology_ 32, 656 (2014). Article CAS PubMed PubMed Central Google Scholar * Consortium, T. G. The tomato genome sequence provides insights into fleshy fruit evolution. _Nature_
485, 635 (2012). Article ADS Google Scholar * Shulaev, V. _et al_. The genome of woodland strawberry (_Fragaria vesca_). _Nature genetics_ 43, 109 (2011). Article CAS PubMed Google
Scholar * Martinelli, F. _et al_. Transcriptome profiling of citrus fruit response to huanglongbing disease. _PloS one_ 7, 1 (2012). Google Scholar * Wu, H.-x _et al_. Transcriptome and
proteomic analysis of mango (_Mangifera indica_ Linn) fruits. _Journal of proteomics_ 105, 19 (2014). Article ADS CAS PubMed Google Scholar * Azim, M. K., Khan, I. A. & Zhang, Y.
Characterization of mango (_Mangifera indica_ L.) transcriptome and chloroplast genome. _Plant molecular biology_ 85, 193 (2014). Article CAS PubMed Google Scholar * Dautt-Castro, M. _et
al_. Mango (_Mangifera indica_ L.) cv. Kent fruit mesocarp de novo transcriptome assembly identifies gene families important for ripening. _Frontiers in plant science_ 6, 62 (2015). Article
PubMed PubMed Central Google Scholar * Srivastava, S. _et al_. Comparative transcriptome analysis of unripe and mid-ripe fruit of _Mangifera indica_ (var.“Dashehari”) unravels ripening
associated genes. _Scientific Reports_ 6, 1 (2016). Article ADS Google Scholar * Veda, S., Platel, K. & Srinivasan, K. Varietal differences in the bioaccessibility of β-carotene from
mango (_Mangifera indica_) and papaya (_Carica papaya_) fruits. _Journal of agricultural and food chemistry_ 55, 7931 (2007). Article CAS PubMed Google Scholar * Tharanathan, R.,
Yashoda, H. & Prabha, T. Mango (_Mangifera indica_ L.),“The king of fruits”—An overview. _Food Reviews International_ 22, 95 (2006). Article CAS Google Scholar * Kanehisa, M.,
Furumichi, M., Tanabe, M., Sato, Y. & Morishima, K. KEGG: new perspectives on genomes, pathways, diseases and drugs. _Nucleic acids research_ 45, D353, doi:10.1093/nar/gkw1092 (2017).
Article PubMed Google Scholar * Kanehisa, M. & Goto, S. KEGG: kyoto encyclopedia of genes and genomes. _Nucleic acids research_ 28, 27 (2000). Article CAS PubMed PubMed Central
Google Scholar * Kanehisa, M., Sato, Y., Kawashima, M., Furumichi, M. & Tanabe, M. KEGG as a reference resource for gene and protein annotation. _Nucleic acids research_ 44, D457,
doi:10.1093/nar/gkv1070 (2016). Article CAS PubMed Google Scholar * Kulkarni, R. S., Chidley, H. G., Pujari, K. H., Giri, A. P. & Gupta, V. S. Geographic variation in the flavour
volatiles of Alphonso mango. _Food Chemistry_ 130, 58, doi:10.1016/j.foodchem.2011.06.053 (2012). Article CAS Google Scholar * Pandit, S. S. _et al_. Changes in volatile composition
during fruit development and ripening of ‘Alphonso’ mango. _Journal of the Science of Food and Agriculture_ 89, 2071, doi:10.1002/jsfa.3692 (2009). Article CAS Google Scholar * Sallaud,
C. _et al_. A novel pathway for sesquiterpene biosynthesis from Z, Z-farnesyl pyrophosphate in the wild tomato Solanum habrochaites. _The Plant Cell_ 21, 301 (2009). Article CAS PubMed
PubMed Central Google Scholar * Huang, F.-C. & Schwab, W. Cloning and characterization of a _9-lipoxygenase_ gene induced by pathogen attack from _Nicotiana benthamiana_ for
biotechnological application. _BMC Biotechnology_ 11, 1, doi:10.1186/1472-6750-11-30 (2011). * Huang, F.-C. & Schwab, W. Overexpression of hydroperoxide lyase, peroxygenase and epoxide
hydrolase in tobacco for the biotechnological production of flavours and polymer precursors. _Plant Biotechnology Journal_ 10, 1099, doi:10.1111/j.1467-7652.2012.00739.x (2012). Article CAS
PubMed Google Scholar * Baysal, T. & Demirdöven, A. Lipoxygenase in fruits and vegetables: A review. _Enzyme and Microbial Technology_ 40, 491, doi:10.1016/j.enzmictec.2006.11.025
(2007). Article CAS Google Scholar * Blee, E. & Schuber, F. Biosynthesis of cutin monomers: involvement of a lipoxygenase/peroxygenase pathway. _The Plant Journal_ 4, 113 (1993).
Article CAS Google Scholar * Masui, H., Kondo, T. & Kojima, M. An antifungal compound, 9, 12, 13-trihydroxy-(E)-10-octadecenoic acid, from _Colocasia antiquorum_ inoculated with
_Ceratocystis fimbriata_. _Phytochemistry_ 28, 2613 (1989). Article CAS Google Scholar * Ohta, H. _et al_. The occurrence of lipid hydroperoxide-decomposing activities in rice and the
relationship of such activities to the formation of antifungal substances. _Plant and cell physiology_ 31, 1117 (1990). CAS Google Scholar * Atkinson, R. G. _et al_. Down-regulation of
polygalacturonase1 alters firmness, tensile strength and water loss in apple (_Malus x domestica_) fruit. _BMC plant biology_ 12, 129 (2012). Article CAS PubMed PubMed Central Google
Scholar * Gross, K. C. & Sams, C. E. Changes in cell wall neutral sugar composition during fruit ripening: a species survey. _Phytochemistry_ 23, 2457 (1984). Article CAS Google
Scholar * Lunn, D., Phan, T. D., Tucker, G. A. & Lycett, G. W. Cell wall composition of tomato fruit changes during development and inhibition of vesicle trafficking is associated with
reduced pectin levels and reduced softening. _Plant Physiology and Biochemistry_ 66, 91 (2013). Article CAS PubMed Google Scholar * Peroni, F. H. Ga _et al_. Mango starch degradation.
II. _The binding of α-amylase and β-amylase to the starch granule. Journal of agricultural and food chemistry_ 56, 7416 (2008). PubMed Google Scholar * Lizada, C. In _Biochemistry of fruit
ripening_ (eds G. Seymour, J. Taylor & G. Tucker) 255 (Chapman and Hall, 1993). * Ali, Z. M., Chin, L.-H. & Lazan, H. A comparative study on wall degrading enzymes, pectin
modifications and softening during ripening of selected tropical fruits. _Plant Science_ 167, 317 (2004). Article CAS Google Scholar * Chourasia, A., Sane, V. A. & Nath, P.
Differential expression of pectate lyase during ethylene‐induced postharvest softening of mango (_Mangifera indica_ var. Dashehari). _Physiologia Plantarum_ 128, 546 (2006). Article CAS
Google Scholar * Fischer, R. L. & Bennett, A. B. Role of cell wall hydrolases in fruit ripening. _Annual review of plant biology_ 42, 675 (1991). Article CAS Google Scholar *
Kurakawa, T. _et al_. Direct control of shoot meristem activity by a cytokinin-activating enzyme. _Nature_ 445, 652, doi:10.1038/nature05504 (2007). Article CAS PubMed Google Scholar *
Ludford, P. In Postharvest Physiology and Pathology of Vegetables (CRC Press, 2002). * Grittle Pinhero, R., Almquist, K. C., Novotna, Z. & Paliyath, G. Developmental regulation of
phospholipase D in tomato fruits. _Plant Physiology and Biochemistry_ 41, 223, doi:10.1016/S0981-9428(03)00014-7 (2003). Article CAS Google Scholar * Kawai-Yamada, M., Ohori, Y. &
Uchimiya, H. Dissection of Arabidopsis Bax inhibitor-1 suppressing Bax–, hydrogen peroxide–, and salicylic acid–induced cell death. _The Plant Cell_ 16, 21 (2004). Article CAS PubMed
PubMed Central Google Scholar * Shivashankar, S., Ravindra, V. & Louis, H. Biochemical changes in seed and mesocarp of mango (_Mangifera indica_ L.) cv.‘Alphonso’and their significance
during the development of spongy tissue. _The Journal of Horticultural Science and Biotechnology_ 82, 35 (2007). Article CAS Google Scholar * Srivastav, M., Singh, S. & Ajang, M.
Evaluation of mango genotypes for jelly seed disorder. _Indian Journal of Horticulture_ 72, 408 (2015). Article Google Scholar * Coates, L. & Johnson, G. Postharvest diseases of fruit
and vegetables. _Plant pathogens and plant diseases_ 533 (1997). * Daulagala, P. Expression of chitinase with antifungal activities in ripening Banana fruit. _Tropical Plant Research_ 1, 72
(2014). Google Scholar * Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. _EMBnet. journal_ 17, 10 (2011). Article Google Scholar * Schulz, M. H.,
Zerbino, D. R., Vingron, M. & Birney, E. Oases: robust de novo RNA-seq assembly across the dynamic range of expression levels. _Bioinformatics_ 28, 1086 (2012). Article CAS PubMed
PubMed Central Google Scholar * Li, W. & Godzik, A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. _Bioinformatics_ 22, 1658 (2006).
Article CAS PubMed Google Scholar * Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome.
_Genome biology_ 10, R25.1 (2009). Google Scholar * Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq. 2. _Genome
biology_ 15, 1 (2014). Article Google Scholar * Conesa, A. _et al_. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. _Bioinformatics_
21, 3674 (2005). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS The authors would like to thank Dr. Aarti Desai for helpful discussion and Dr. Abhay Jere
(Persistent Systems Ltd., Pune, India) for his critical suggestions, guidance and support. The authors acknowledge the Next Generation Genomics Facility (NGGF) at the Centre for Cellular and
Molecular Platforms (C-CAMP), Bangalore (Project ID: BT/PR3481/INF/22/140/2011) for performing transcriptome sequencing of the eight mango tissues. Authors would like to thank Dr. Sanjiv
Galande, Dr. Ram Kulkarni and Mr. Saurabh Pradhan (Indian Institute of Science Education and Research, Pune, India) for their help in evaluation of RNA quality by Bioanalyzer 2100. This
research was funded by the Council of Scientific and Industrial Research, New Delhi, India under the project CSC0133 (FUNHEALTH) to CSIR-NCL and 21(0997)/16/EMR-II grant as Emeritus
Scientist scheme to VSG. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Plant Molecular Biology Group, Biochemical Sciences Division, CSIR-National Chemical Laboratory, Pune, 411008,
Maharashtra, India Ashish B. Deshpande, Hemangi G. Chidley, Pranjali S. Oak, Narendra Y. Kadoo, Ashok P. Giri & Vidya S. Gupta * Labs, Persistent Systems Limited, Pingala-Aryabhata,
Erandwane, Pune, 411004, India Krishanpal Anamika & Vineet Jha * Dr. Balasaheb Sawant Konkan Agriculture University, Dapoli, 415712, Maharashtra, India Keshav H. Pujari Authors * Ashish
B. Deshpande View author publications You can also search for this author inPubMed Google Scholar * Krishanpal Anamika View author publications You can also search for this author inPubMed
Google Scholar * Vineet Jha View author publications You can also search for this author inPubMed Google Scholar * Hemangi G. Chidley View author publications You can also search for this
author inPubMed Google Scholar * Pranjali S. Oak View author publications You can also search for this author inPubMed Google Scholar * Narendra Y. Kadoo View author publications You can
also search for this author inPubMed Google Scholar * Keshav H. Pujari View author publications You can also search for this author inPubMed Google Scholar * Ashok P. Giri View author
publications You can also search for this author inPubMed Google Scholar * Vidya S. Gupta View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
A.B.D. designed, standardized and performed majority of the experiments, analyzed the data and wrote the manuscript. K.A. and V.Z. carried out bioinformatics analysis. H.G.C. and P.S.O.
helped with high quality RNA isolation, cDNA synthesis and qRT PCR studies. K.H.P. contributed by providing the experimental tissue. A.P.G. contributed with suggestions for the work and for
manuscript planning, while NYK contributed in bioinformatic analysis and manuscript writing. V.S.G. designed the study, participated in all parts of the work and contributed to the
preparation of the manuscript. All authors read and approved the final manuscript. CORRESPONDING AUTHOR Correspondence to Vidya S. Gupta. ETHICS DECLARATIONS COMPETING INTERESTS The authors
declare that they have no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION SUPPLEMENTARY TABLE ST1 SUPPLEMENTARY TABLE ST2 SUPPLEMENTARY TABLE ST3 SUPPLEMENTARY TABLE ST4
SUPPLEMENTARY TABLE ST5 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Deshpande, A.B., Anamika, K., Jha, V. _et al._ Transcriptional transitions in Alphonso mango (_Mangifera indica_ L.) during fruit development and ripening explain its
distinct aroma and shelf life characteristics. _Sci Rep_ 7, 8711 (2017). https://doi.org/10.1038/s41598-017-08499-5 Download citation * Received: 04 May 2017 * Accepted: 10 July 2017 *
Published: 18 August 2017 * DOI: https://doi.org/10.1038/s41598-017-08499-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative