Play all audios:
ABSTRACT The sorption mechanism of nickel (Ni) at the illite/water interface was investigated using batch, sorption modelling, extended X-ray absorption fine structure (EXAFS), and
extraction approaches. The results showed that Ni(II) sorption on illite was strongly dependent on pH, contact time, temperature, and initial Ni(II) concentration. At a low initial Ni(II)
concentration, the ion exchange species of ≡X2Ni° and the inner-sphere complexes including ≡SsONi+, ≡SwONi+ and ≡SwONiOH° species are observed on the sorption edges of Ni(II) on illite. As
the initial Ni(II) concentration increased to 1.7 × 10−3 mol/L, precipitates including surface-induced precipitation of s-Ni(OH)2 and amorphous Ni(OH)2 became more significant, especially
under neutral to alkaline conditions. EXAFS analysis confirmed that Ni-Al layered double hydroxide (LDH) can gradually form with an increase in the contact time. At pH 7.0, α-Ni(OH)2 was
produced in the initial stage and then transformed to the more stable form of Ni-Al LDH with increasing contact time because of the increased Al3+ dissolution. With an increase in
temperatures, α-Ni(OH)2 phase on illite transformed to Ni-Al LDH phase, indicating a lower thermodynamic stability compared to Ni-Al LDH phase. These results are important to understand the
geochemical behaviors to effectively remediate soil contaminated with Ni(II). SIMILAR CONTENT BEING VIEWED BY OTHERS U(VI) REMOVAL FROM DILUTED AQUEOUS SYSTEMS BY SORPTION–FLOTATION Article
Open access 10 October 2022 KINETICS, EQUILIBRIUM AND THERMODYNAMICS STUDIES ON NATURAL AND HEAT TREATED CLAYS FOR THE REMOVAL OF ARSENATE IONS FROM AQUEOUS SOLUTION Article Open access 03
May 2025 ARSENIC REMOVAL PERFORMANCE AND MECHANISM FROM WATER ON IRON HYDROXIDE NANOPETALINES Article Open access 14 October 2022 INTRODUCTION Nickel (Ni) is one of the most toxic and
widespread contaminants in the environment. There have been vast quantities of nickel released into the soil and water from industrial production such as mining, oil refining,
electroplating, and battery and accumulator manufacturing1, 2. Once released into the environment, nickel readily transports and migrates as a soluble form of Ni2+ or Ni(II)-ligand
complexes, which are very important and directly related to Ni(II) toxicity in environmental media. Therefore, Ni(II) can be easily assimilated by terrestrial and aquatic organisms and can
gradually accumulate in the biological food chain. Although trace nickel is one of the essential elements for many organisms, a high level of Ni(II) is toxic and can cause cancer, nausea,
vomiting, diarrhea, skin dermatitis, renal edema, and pulmonary fibrosis3,4,5. Therefore, the study of Ni(II) speciation and bioavailability at a solid/water interface is important for the
prediction and evaluation of its ecological and environmental risks and toxicity and to the remediation of Ni(II)-contaminated soil and water. Generally, the migration and retention of
Ni(II) in contaminated soil and groundwater is largely controlled by its sorption and desorption behaviors at the solid/water interface. Previous studies have demonstrated that Ni(II)
strongly and selectively interacted with phyllosilicate minerals that were widely distributed in the soil and sediments, such as montmorillonite6,7,8,9,10,11,12,13,14,15, kaolinite16, 17,
bentonite18, 19, attapulgite1, 20, diatomite21, and illite2, 22, 23. These studies showed that environmental factors such as pH, ionic strength, and temperature can affect the
sorption/desorption behaviors of Ni(II) to a large extent at both the micro- and macro-scales. Moreover, ion exchange (IE) or the outer-sphere complexes (OSCs) were the primary mechanisms of
Ni(II) sorption on clay minerals at a low pH, whereas the inner-sphere complexes (ISCs), especially surface precipitates, were possibly dominant mechanisms for Ni(II) retention in the
neutral to high pH range. Some studies found various surface precipitates, such as Ni(II) hydroxide, Ni-Al layered double hydroxides (LDHs) and Ni-phyllosilicate, under neutral to weak
alkaline conditions21, 24,25,26,27,28,29,30,31,32,33,34. The formation of these mixed hydroxide phases can significantly stabilize Ni(II) in environmental media and notably decrease the
Ni(II) mobility and bioavailability31. Therefore, it is crucial to understand the nature of these surface precipitates because their solubility depends on their structure and composition.
Extended X-ray absorption fine structure (EXAFS) was confirmed to be useful in discerning and providing important insights to these different surface species and precipitates by
investigating the different features of Ni-O and the Ni-Ni/Al/Si distance (_R_) and coordination number (_CN_). Previous studies have reported that Ni-phyllosilicate (co)precipitates on
solid surfaces with a Ni-Ni interatomic distance of 3.07 to 3.10 Å and a Ni-Si interatomic distance of ~3.28 Å21, α-Ni(OH)2 with a Ni-Ni interatomic distance of 3.07 to 3.09 Å27, 35, and
β-Ni(OH)2 with a Ni-Ni interatomic distance of 3.11–3.13 Å21, 25, 27, 32, 33. The Ni-Al LDH is quite stable and insoluble, resulting in a reduction of the Ni(II) bioavailability in the
environment31, where Ni is surrounded by several Ni atoms at distances ranging from 3.05 Å to 3.08 Å and Al atoms at distances ranging from 3.03 Å to 3.12 Å27, 30. However, whether Ni-Al LDH
can form on a clay surface strongly depends on the availability of Al3+ during the dissolution of Al-containing sorbents. Previous studies have confirmed that Ni-Al LDH can form very
quickly within several minutes, which is questionable given that mineral dissolution is generally slow. Illite, which is one of the most dominant clay minerals in soil and sediments, forms
from silicate weathering through the alteration of other clay minerals and the degradation of muscovite. The structure is a 2:1 clay with silica tetrahedron-alumina octahedron-silica
tetrahedron layers and has a large specific surface area. Its high sorption capacity for cations via ion exchange and surface complexation has a major impact on metal ion retention in the
environment. To the best of our knowledge, discussions of the relationship between Ni(II) speciation and availability at the illite/water interface is scarce, especially using the EXAFS
technique; however, this relationship is critical to understand the geochemical behaviors of Ni(II) in the environment. In addition, the primary focus of previous studies on Ni sorption at
low concentrations is unsuitable for significantly contaminated soils close to the metal ores. Therefore, the sorption mechanism and speciation of Ni(II) at the illite/water interface will
be explored by batch, sorption modelling and EXAFS approaches in this work. The results of the relationship between species and availability in this study will provide important insights
into the understanding and prediction of Ni(II) geochemical behaviors in soils enriched in phyllosilicate clays such as illite. RESULTS AND DISCUSSION NI(II) SORPTION ON ILLITE The uptake of
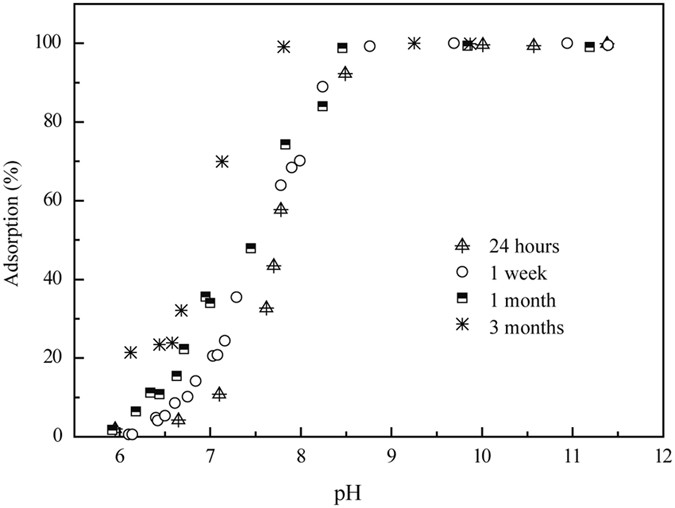
Ni(II) on illite as a function of pH was examined at different contact times of 24 hours, 1 week, 1 month and 3 months (Fig. 1). The sorption percentage of Ni(II) increased as the contact
time increased from 24 hours to 3 months. Fan _et al_.1, Hu _et al_.2, and Sheng _et al_.21 showed that the initial sorption of Ni(II) was very quick on attapulgite (2 h), illite (5 h) and
diatomite (4 h) and continued at much slower sorption rates, demonstrating that strong surface complexation or (co)precipitates contributed to the Ni(II) sorption on illite. Figure 1 shows
that Ni(II) sorption on illite was clearly affected by the pH values, which is a very important factor in the environment. From pH 6.0 to 7.0, the sorption of Ni(II) on illite was lower and
ranged from 1.0% to 10.0%. However, over 90% of the Ni(II) was adsorbed on illite above pH 8.0. The strong pH-dependence suggested that both OSCs and ISCs possibly control Ni(II) sorption
behaviors on illite. Echeverria _et al_.22 and Hu _et al_.2 concluded that the ISCs were the primary mechanism under neutral to weak alkaline conditions. Figure 2 shows the Ni(II) sorption
percentage on illite as a function of pH in 0.01 M and 0.1 M NaClO4 solutions. Ni(II) sorption decreased with the increase in ionic strength below pH 8.0. This suggested that IE or OSCs were
dominant mechanisms in the low pH range because both are very sensitive to the ionic strength36. No significant difference was observed at different ionic strength conditions above pH 8.0.
According to the solubility product of Ni(OH)2 (2.0 × 10−15)2, one can deduce that the formation of Ni(OH)2 can occur only above pH 8.0 at the concentration of 1.7 × 10−3 mol/L if no Ni(II)
is adsorbed on the illite surface. This indicated that, in theory, the formation of Ni(OH)2 did not contribute to the sorption of Ni(II) on illite below pH 8.0. However, recent studies have
confirmed the presence of Ni(OH)2 and Ni-Al or Si LDH on clay and oxide surfaces21, 24,25,26,27,28,29,30,31,32,33,34. For example, Scheidegger and Sparks25 observed the formation of
mixed-cation hydroxide phases produced by metal sorption on clays and aluminum oxides. These hydroxide surface phases formed far below the theoretical monolayer coverage and in a pH range
well below the pH where metal hydroxide precipitates would be expected according to the thermodynamic solubility product. Therefore, another possibility for the Ni(II) sorption mechanisms on
illite, i.e., surface-induced precipitation, cannot be excluded given the limited sorption data, which will be discussed in detail later by combining the sorption modelling and
spectroscopies analyses. SORPTION MODELLING OF NI(II) ON ILLITE The sorption edges of Ni(II) on illite were estimated in a wide range of initial Ni(II) concentrations from 1.7 × 10−5 to 1.7
× 10−3 mol/L (Fig. 3). Figure 3a shows that the sorption edge of Ni(II) on illite was significantly shifted forward to a high pH range (approximately 2.0 pH units) as the initial Ni(II)
concentration increased from 1.7 × 10−5 to 1.7 × 10−3 mol/L. To explore the sorption species and mechanism of Ni(II) on illite, the surface complexation model and MINTEQ 3.1 code were
combined to study the sorption edges at the different initial Ni(II) concentrations, and the fit results are shown in Fig. 3 and Table 1. For the sorption system with the initial Ni(II)
concentration of 1.7 × 10−5 mol/L (Fig. 3b), three surface species including ≡X2Ni°, ≡SsONi+, and ≡SwONi+ were dominant below pH ~6.5, and ≡SwONiOH° was primarily responsible for the
sorption above pH ~7.0. The results showed that IE was important to Ni(II) sorption on illite at a low pH range and that ISCs primarily controlled Ni(II) sorption over a high pH range. The
relative sorption reaction constants were comparable to the previous studies37,38,39, which indicated that the sorption model was reasonable. To further validate the sorption model under
different conditions, the model and the corrected parameters were directly extrapolated to the systems with higher initial Ni(II) concentrations. The fitting results showed that the sorption
model can reasonably predict the sorption edge of Ni(II) on illite at _C_ Ni = 1.7 × 10−4 mol/L, although the contributions of each surface species were changed in comparison with the
sorption system at _C_ Ni = 1.7 × 10−5 mol/L. However, the above sorption model cannot predict Ni(II) sorption behaviors at _C_ Ni = 1.7 × 10−3 mol/L. Conversely, the total capacities of the
sorption sites and cation exchange site were limited and far lower than the Ni(II) concentration in the illite phase at the highest Ni(II) concentration (i.e., _C_ Ni = 1.7 × 10−3 mol/L);
thus, it is necessary to consider other sorption mechanisms in this model. In this study, three cases were considered to tentatively describe the sorption edge of Ni(II) on illite as a
function of pH for the system with the highest Ni(II) concentration (_C_ Ni = 1.7 × 10−3 mol/L, Fig. 3d–f). Case 1: only IE and surface complexation; case 2: IE, surface complexation, and
amorphous Ni(OH)2; and case 3: IE, surface complexation, amorphous Ni(OH)2 and surface-induced precipitation of s-Ni(OH)2 were analyzed for their contributions to Ni(II) sorption over the
entire observed pH range. Figure 3d shows that all sorption sites and IE were saturated when the sorption was approximately 15% at pH ~7.0. However, the primary species of ≡X2Ni°, ≡SwONi+
and ≡SwONiOH° can explain the sorption trend below pH 6.5. The strong site contribution is negligible for Ni(II) sorption, due to its limited site capacity (approximately 4.0% that of the
weak site, see Table 1). Above pH 6.5, the fit data deviated far from the experimental data, indicating that some important sorption species were missed for case 1. As discussed in the
sorption process, amorphous Ni(OH)2 possibly played a very important role in the sorption of Ni(II), especially in the high Ni(II) concentration and pH ranges. Therefore, in case 2, the
amorphous precipitate Ni(OH)2 was included in the sorption model, and the fit data were plotted (Fig. 3e). Calculations show that the amorphous Ni(OH)2 began to form above pH 8.0. Case 2,
which included the amorphous Ni(OH)2 contribution to Ni(II) sorption on illite had improved results compared to case 1; however, there were still fitting vacancies in the pH range from 6.5
to 8.0. Attempting to improve the model in this region, one type of surface-induced precipitate (s-Ni(OH)2) was considered in case 3. These surface-induced precipitates, which were reported
in previous studies and observed in the EXAFS spectra in this study, were formed and were possibly dominant for Ni(II) retention in the neutral to high pH range21,
24,25,26,27,28,29,30,31,32,33,34. Figure 3f shows that five different species were necessary to describe the sorption edge of Ni(II) on illite at _C_ Ni = 1.7 × 10−3 mol/L. Below pH 6.5, the
two species ≡X2Ni° and ≡SwONi+ were predominant, and the sorption were ~30% and 70%, respectively. In the pH range from 7.0 to 9.0, s-Ni(OH)2, amorphous Ni(OH)2 and the ISCs of ≡SwONiOH°
were responsible for Ni(II) sorption. Above pH 9.0, the ISCs of ≡SwONiOH° and amorphous Ni(OH)2 were predominant. The sorption species and precipitates of Ni(II) at the illite/water
interface were confirmed in the observed pH range by X-photoelectron spectroscopy in the supplemental information (SI). EXAFS ANALYSIS OF REFERENCE SAMPLES EXAFS spectra and the radial
structure functions (RSFs) of reference samples Ni(NO3)2, Ni-phyllosilicate, Ni-Al LDH, and β-Ni(OH)2 are shown in Fig. 4. Figure 4A shows a monotonous feature of Ni(NO3)2 in the _k_ 3χ(_k_)
function at k > 3.0 Å−1, indicating that Ni(NO3)2 possessed a single coordination environment surrounding the Ni atoms that was primarily the hydration shell. The _k_ 3χ(_k_) functions
of Ni-phyllosilicate, Ni-Al LDH and β-Ni(OH)2 have more complicated oscillation features. At 5.0 Å−1, there was a small beat in the _k_ 3χ(_k_) function; notably, there was a typical split
in the _k_-range of ~7.5 Å−1, which is characteristic of Ni precipitation and is due to the neighboring atoms. Therefore, the sorption speciation was accurately distinguished from these
characteristic oscillations in the _k_ 3χ(_k_) functions. Figure 4B shows the corresponding RSFs of the reference samples. For Ni(NO3)2, only one coordinated shell at approximately 2.0 Å
(phase shift uncorrected) was observed in the RSFs assigned to the Ni-O shell, which was the hydration shell. For the samples of Ni-phyllosilicate, Ni-Al LDH and β-Ni(OH)2, the second
coordinated shells were observed at approximately 3.08 Å and were assigned to the Ni-Ni, Ni-Si or Ni-Al scattering pairs25, 30, 33, 34. As shown in the RSFs (Fig. 4B), the intensities of the
second shells were different for each Ni precipitate, which was reflected in the _k_ 3χ(_k_) functions and could be an important criterion to identify the type of precipitates. The
structural parameters of the reference samples are specified in Table 2. Ni(NO3)2 was surrounded by 6.0 O atoms at _R_ Ni-O ≈ 2.04 Å. For the complex features of backscattering,
Ni-phyllosilicate was surrounded by 4.5 O atoms at _R_ Ni-O ≈ 2.03 Å in the first shell and by 2.7 Ni atoms at _R_ Ni-Ni ≈ 3.07 Å and 4.0 Si atoms at _R_ Ni-Si ≈ 3.27 Å. For Ni-Al LDH, Ni
was surrounded by 6.0 O atoms at _R_ Ni-O ≈ 2.03 Å in the first shell and by 3.5 Ni atoms at _R_ Ni-Ni ≈ 3.06 Å and 2.5 Al atoms at _R_ Ni-Al ≈ 3.12 Å. For Ni(OH)2, Ni was surrounded by 6.0
O atoms at _R_ Ni-O ≈ 2.05 Å and by 6.0 Ni atoms at _R_ Ni-Ni ≈ 3.11 Å. The reference samples can be simulated well by combining the _CN_ and _R_ parameters, which are well correlated with
previous studies25, 30, 33, 34. EXAFS ANALYSIS ON THE EFFECTS OF CONTACT TIME AND PH Figures 5A and 5B show the _k_ 3 _χ_(_k_) functions and the corresponding RSFs of the sorption samples
for different contact times and pH values. A complicated oscillation feature was observed, especially at k ≥ 5.0 Å−1, indicating the presence of higher coordinated shells surrounding the Ni
atoms, with an exception of the Ni-O shell. At different contact times, the _k_ 3 _χ_(_k_) functions were characterized by multi-frequency wave shapes with distinct features (Fig. 5A). The
splitting degree was enhanced with an increasing contact time from 1 day to 1 month at pH 6.0 and 7.0; however, no clear change was observed for the Ni(II)-adsorbed illite at pH 10.0.
Moreover, the oscillations for the samples at pH 10.0 were extremely similar to the β-Ni(OH)2 reference and were independent of the contact time, which suggested that β-Ni(OH)2 was the
dominant sorption mechanism for Ni(II) sorption on illite under strong alkaline conditions. As expected, two typical coordinated shells in the _R_ space were observed for all the samples at
different pH values and contact times (Fig. 5B). The second shell for the samples at pH 6.0 and 7.0 increased as the contact time increased, which indicated that the sorption mechanism
possibly changed completely from the IE and/or ISCs at pH 6.0 and α-Ni(OH)2 at pH 7.0 to surface precipitation such as Ni-Al LDH as the contact time increased. No changes in the RSFs for the
Ni(II)-adsorbed illite at pH 10.0 suggested that the primary sorption mechanism did not change. This is consistent with the XPS analysis, showing that IE and ISCs are dominant in a low pH
range and that the precipitates, including Ni(OH)2 and Ni-Al LDH, are contributing to Ni(II) sorption under alkaline conditions (Fig. SI-6). The structural parameters obtained using EXAFS
analysis are also summarized in Table 2. Ni was surrounded by ~6.0 O atoms at _R_ Ni-O ≈ 2.05–2.06 Å in the first shell for all the samples, indicating that Ni is in an octahedral
environment25, 30, 33, 34. For the samples at pH 6.0 with a contact time of 1 day, the second shell was fitted with the Ni-Si scattering pair rather than Ni-Ni and Ni-Al, possibly due to (i)
the limited availability of free Al3+ within a short contact time (Fig. 6) and (ii) the pH being too low to form any precipitates. Therefore, the Ni-Si scattering pair was considered for
the second shell in the RSFs. EXAFS analysis showed that _R_ Ni-Si was 3.23 Å with a _CN_ of ~3.0 Si atoms, which confirmed the more stable tridentate surface complexes of Ni(II) formed on
the illite surface. For the sample with a contact time over 1 month, the best fit of the second shell includes both the Ni-Ni and Ni-Al scattering pairs. From the _k_ 3 _χ_(_k_) function, a
distinctive beat pattern at ~7.5 Å−1 was observed that unequivocally identified the Ni-Al LDH33, 34. The central Ni was surrounded by 5.2 Ni atoms with _R_ Ni-Ni ≈ 3.08 Å and 0.8 Al atoms
with _R_ Ni-Al ≈ 3.14 Å. In comparison to the standard Ni-Al LDH sample parameters and the abovementioned features of _k_ 3 _χ_(_k_) function, the formation of Ni-Al LDH was identified at pH
6.0 with a contact time over 1 month. This phenomenon might be caused by the available Al dissolved by the sorbent substrate. With increasing time, surface precipitates might form on illite
and then capture the dissolved Al3+ to nucleate a small mass of Ni-Al LDH (Fig. 6). From the sorption edge (Fig. 2), the amount of Ni(II) uptake increased with increasing pH; thus, striking
oscillations were observed in the _k_ 3χ(_k_) functions and RSFs at pH 7.0. For samples at pH 7.0 with 1 day of contact time, the second shell fitting results showed only Ni-Ni and that the
number of second neighbor Ni atoms (_CN_ Ni-Ni) was 4.0 at _R_ Ni-Ni ~ 3.07 Å, which was shorter than the _R_ Ni-Ni of β-Ni(OH)2 (~3.11 Å) and close to the _R_ Ni-Ni of α-Ni(OH)2 (3.07–3.09
Å)27, 35. This suggested that the Ni precipitate in the early stage was α-Ni(OH)2 without available Al3+ due to the insufficient Al3+ dissolved from the sorbent substrate. After 1 month, Ni
was surrounded by 6.0 O atoms (_R_ Ni-O ≈ 2.06 Å) in the first shell and 5.2 Ni atoms (_R_ Ni-Ni ≈ 3.07 Å) as well as 1.8 Al atoms (_R_ Ni-Al ≈ 3.14 Å) in the second shell. This result
suggested that Ni-Al LDH was formed as the contact time increased. Scheidegger and Sparks25 reported that both adsorption and nucleation processes (mixed Ni/Al phase formation) can occur
simultaneously over time scales of only minutes; however, this is suspicious given that the formation process of Ni-Al LDH is related to the kinetics of Al3+ dissolution from the illite
surface. Unlike the previous studies, we thought that α-Ni(OH)2 was formed initially and then progressively converted to the more stable formation of Ni-Al LDH. For the samples at pH 10.0,
the contact time had no effect on the second shell in the RSFs, and the _CN_ Ni-Ni was 6.0 at _R_ Ni-Ni ≈ 3.13 Å, which suggested that β-Ni(OH)2 was the primary sorption species. Conversely,
the Ni-Al LDH was possibly formed as well at pH 10.0 with an increase in contact time; however, its contribution was too low to be distinguished using EXAFS analysis in this study. Based on
the sorption experiment, EXAFS analysis, and SCM, the ISCs and α-Ni(OH)2 were primarily responsible for Ni(II) sorption on illite at pH 6.0 and 7.0 with a short contact time, and as the
contact time increased from 1 day to 1 month, the sorption species tended to be the more stable form of Ni-Al LDH. However, β-Ni(OH)2 gradually became more ubiquitous as pH increased. EXAFS
ANALYSIS ON THE EFFECTS OF TEMPERATURE Figure 7 shows the _k_ 3χ(_k_) functions and RSFs at different temperatures. The oscillation of the _k_ 3χ(_k_) function was similar to the Ni
precipitate references, and no significant differences were observed at different temperatures (Fig. 7A); however, the corresponding RSFs exhibited an increased intensity for the second
shell (Fig. 7B). The structural parameters show that the interatomic distances between Ni and O (_R_ Ni-O ≈ 2.05 Å) and _CN_ Ni-O were not affected by the increasing temperature, while the
coordination environment in the second shell significantly changed with increasing temperature, which indicated that the Ni(II) species changed on the illite surface. At 298 K, the Ni-Ni
backscattering pair was dominant, and Ni was surrounded by 4.0 Ni atoms at _R_ Ni-Ni ≈ 3.07 Å, indicating the formation of α-Ni(OH)2 as discussed above. Moreover, the _CN_ Ni-Al at _R_ Ni-Al
≈ 3.14 Å increased from 1.5 to 3.2 and the ratio of _CN_ Ni-Ni/_CN_ Ni-Al reduced from 3.7 to 1.8 as the temperature increased from 303 to 313 K, demonstrating the growth of the Ni-Al LD
phase. Sheng _et al_.21 and Ren _et al_.33 also found that Ni(II) surface precipitates were favored at high temperatures. This is reasonable given that the dissolved Al3+ has higher
availability at high temperatures (Fig. 6), which benefits the formation of Ni-Al LDH; additionally, Ni-Al LDH is a more thermodynamically stable species compared to the other Ni
precipitates such as α- or β-Ni(OH)2. EXTRACTION EXPERIMENT Two desorption agents were used: 0.1 M CaCl2 (pH = 6.0) and HNO3 (pH = 4.0). A CaCl2 solution can remove any weakly held ion
exchange or surface complexes40. An HNO3 solution is the most likely to remove Ni proton competition and promote the dissolution of the precipitate phases31. Figure 8A shows that both
extraction tendencies resulted in a clear and sustained release of Ni(II) because the reversible sorption mechanisms, including the IE and ISCs, were predominant under acidic conditions.
Approximately 60% of the Ni(II) was extracted after six treatments with the 0.1 M CaCl2 solution, which approximated the contribution of ≡X2Ni0 to Ni(II) sorption under the observed
conditions as shown in Fig. 3. The HNO3 solution was significantly more effective for extracting Ni(II) from illite (~70%) compared to the CaCl2 solution because both IE and ISCs could be
extracted using H+. Figure 8B shows that CaCl2 released almost 25% of the Ni(II) from illite after six treatments, which was possibly related to the IE, and the remaining 75% of the Ni(II)
was possibly related to (co)precipitates (α-Ni(OH)2) or the hydrolyzed Ni(II) surface complexes (≡SwONiOH0). In the 1.0 M HNO3 solution, 63% of the Ni(II) was removed in the six total
treatments, indicating the release of IE, ISCs and the partial dissolution of α-Ni(OH)2. The remaining phase might be caused by (co)precipitates that have a proton-resistance and cannot be
completely dissolved by HNO3 (pH = 4.0). These results were consistent with sorption model and EXAFS analyses, suggesting that surface phases and surface complexes were the primary sorption
mechanisms at pH 7.0. ENVIRONMENTAL IMPLICATIONS The sorption mechanisms of heavy metals at the solid/water interface are crucial to their mobility, bioavailability and environmental
toxicity. Climatic and environmental conditions typically control heavy metal speciation in the contaminated soil (Fig. 9). For example, the ecologic risk and environmental toxicity of heavy
metals in acidic soil will be much higher compared to alkaline soil because the weak heavy metal bonds on soil particles, such as IE or OSCs, are prevalent under acidic conditions as shown
in Fig. 9. Similarly, acid rain can also increase the mobility and bioavailability of heavy metals in soil. The heavy metal content in soil is widespread depending on the surrounding
geological environment and anthropogenic and natural activities. At high concentrations, it is well documented that the typical heavy metals such as Zn(II), Co(II), Ni(II), and Fe(II) have
similar sorption mechanisms and can readily form mixed metal-Al-layered double hydroxide (LDH) precipitates during sorption with Al-rich soil clays under neutral to weak alkaline
conditions21, 24,25,26,27,28,29,30,31,32,33,34, 41,42,43,44,45. Kinetic studies on Zn(II)-, Co(II)-, Ni(II), and Fe(II)-Al LDH have shown increased stability of those precipitates on the
scale of 1 day. Conversely, these (co)precipitates have special features. For instance, Voegelin and Kretzschmar42 reported the formation of Zn-Ni LDH in the simultaneous presence of two
metal cations (e.g., Ni and Zn) in soil, and Starcher _et al_.43 showed the potential formation of the Fe(II)-Al/Fe(III) LDH phase with Fe(III) impurities in suboxic and anoxic geochemical
systems. The abovementioned (co)precipitates significantly decrease metal mobility and bioavailability in contaminated soils. Moreover, our results confirmed that temperature has an impact
on the sorption of heavy metals and the formation of (co)precipitates under neutral to weak alkaline conditions (Fig. 9). Several mentioned studies and this study observed that the initial
α-Ni(OH)2 might transform into Ni-Al LDH due to the increased loading of Ni(II) and the dissolution of Al3+ with an increase in temperature34. These findings provide a theoretical basis and
scientific guidance for the remediation and purification of heavy metal contaminants in soil; few previous studies considered this point. The precipitation reactions mentioned here may be
particularly significant to the speciation and mobility of heavy metals (e.g., Ni, Co, Zn and Fe) in contaminated soil. Additional characteristics and reactions under different conditions,
such as the initial concentration, particle size and _S/L_ ratio, are important to assess and predict the effects of these precipitate phases in environmental systems. MATERIALS AND METHODS
MATERIALS All chemicals used in the experiments were purchased at analytic purity and used directly without any further purification. The illite used in this study was obtained from the
Rochester Shale, crushed, and passed through a 200-mesh sieve prior to the experiments. The cation exchange capacity (_CEC_) and N2-BET specific surface area are approximately 20.0 meq/100 g
and 28.0 m2/g, respectively. Additionally, the XRD, FTIR and SEM analyses are shown in the SI. SORPTION PROCEDURE Ni(II) sorption was performed using batch technology in 10.0 mL
polyethylene test tubes, and all stock solutions were prepared in Milli-Q water. A 2.0 g/L slurry was created by mixing illite with deionized water containing 0.1 M NaClO4 as a background
electrolyte. The stock suspension of illite and NaClO4 were pre-equilibrated for 24 h, and the Ni(II) stock solution (_C_ _Ni_ = 1.7 × 10−2 mol/L) was spiked to achieve the desired
concentrations; the total volume of each sorption system was maintained at 6.0 mL. The pH was adjusted to the desired value by adding a negligible HClO4 or NaOH solution. After the
suspensions were shaken for 24 hours, 1 week, 1 month, or 3 months, the solid and liquid phases were separated by centrifugation at 12,000 rpm for 30 min. The concentration of Ni(II) in the
supernatant (_C_ e) was analyzed via spectrophotometry at 530 nm using a Ni butanedione dioxime complex. POTENTIOMETRIC TITRATION OF ILLITE Previous studies have confirmed three types of
sorption sites on illite surfaces, including a strong sorption site (≡SsOH) that has a very low density and high reactivity, a weak sorption site (≡SwOH) that has a high density and low
reactivity, and an IE site (≡XNa)37,38,39, 46. In this study, the site capacity and intrinsic complex constants (_K_) of the strong and weak sites were calculated by fitting the
potentiometric titration data (Fig. SI-3) using MINTEQ 3.1 code. The classical constant capacitance model (CCM) was generally applied to describe the surface amphoteric sites (i.e., surface
hydroxyl) and the fixed-charge site was used to describe the IE. The capacity of the weak site (≡SwOH) was 1.0 × 10−1 mol/kg and the strong site (≡SsOH) had a capacity that was approximately
4.0% of the weak site. The capacity of the IE site (≡XNa) listed in the fixed-charge site was 2.0 × 10−1 mol/kg and used the gt_std.cdb database combined with the MINTEQ 3.1 code. The fit
results showed that the titration curve can be adequately simulated and the relative parameters are comparable to previous studies37,38,39, which will be used as constants in the following
modelling for Ni(II) sorption edges on illite. EXAFS MEASUREMENT The procedures for the EXAFS samples and spectra were performed following the method of Qiang _et al_.47. The detailed
methods are shown in the SI-3. Nickel K-edge EXAFS spectra at 8333.0 eV were collected at the BL14W1 station of the Shanghai Synchrotron Radiation Facility (SSRF, Shanghai, China) and the
BL12C station of the KEK Photon Factory (Tsukuba, Japan). The EXAFS spectra of Ni(NO3)2, β-Ni(OH)2, Ni-phyllosilicate and Ni-Al LDH were collected under the transmission mode, whereas the
Ni(II)-adsorbed samples were measured under the fluorescence mode using a multi-element, high-purity Ge solid-state detector (32-element SSD for the BL14W1 at the SSRF and 19-element SSD for
the BL12C at the KEK-PF). The normalization of the EXAFS spectra and data analysis were reduced using standard procedures48 and were performed with the aid of the Athena and Artemis
interfaces compacted in the IFEFFIT49 and FEFF 7.050. During the optimization, the energy shift (Δ_E_ 0) was constrained to be equal, and the amplitude reduction factor, (_S_ 0 2), was fixed
at 0.85. A good fit was determined based on the minimum residual factor (_R_ f)40. EXTRACTION PROCEDURE Extraction experiments were performed using the modified sequential extraction
following the procedures by Nachtegaal _et al_.40, Peltier _et al_.31 and Fan _et al_.51. In general, two types of extraction solutions were chosen in this study: 0.1 M CaCl2 (pH = 6.0) (IE
fraction) and HNO3 (pH = 4.0) (proton-promoted dissolution). Two Ni(II)-adsorbed samples were prepared at pH 6.0 and 7.0, which were strictly identical to those used in the adsorption
experiments in Section 2.2. After the sorption experiment, the solid was rinsed rapidly using Milli-Q water for the subsequent extraction experiments. For each extraction experiment, 15.0 mL
of the extraction agent was added to the polyethylene test tubes and continuously shaken. For the first treatment, a contact time of 12 hours was used to minimize potential resorption of Ni
to the solid phases. Then, all subsequent treatments used a 24-hour contact time. Afterwards, the batches were centrifuged, and the supernatant was collected for Ni analysis. The remaining
solids were then washed with Milli-Q water and centrifuged prior to the addition of an extraction solution. REFERENCES * Fan, Q. H., Shao, D. D., Lu, Y., Wu, W. S. & Wang, X. K. Effect
of pH, ionic strength, temperature and humic substances on the sorption of Ni(II) to Na-attapulgite. _Chem. Eng. J._ 150, 188–195 (2009). Article CAS Google Scholar * Hu, B. W., Cheng,
W., Zhang, H. & Yang, S. T. Solution chemistry effects on sorption behavior of radionuclide Ni-63(II) in illite-water suspensions. _J. Nucl. Mat._ 406, 263–270 (2010). Article ADS CAS
Google Scholar * Akhtar, N., Iqbal, J. & Iqbal, M. Removal and recovery of nickel(II) from aqueous solution by loofa sponge-immobilized biomass of Chlorella sorokiniana:
characterization studies. _J. Hazard. Mater._ 108, 85–94 (2004). Article CAS PubMed Google Scholar * Patel, J. S., Patel, P. C. & Kalia, K. Isolation and characterization of nickel
uptake by nickel resistant bacterial isolate (NiRBI). _Biomed. Environ. Sci._ 19, 297–301 (2006). CAS PubMed Google Scholar * Saleem, M., Wongsrisujarit, N. & Boonyarattanakalin, S.
Removal of nickel (II) ion by adsorption on coconut copra meal biosorbent. _Desalin. Water Treat._ 57, 5623–5636 (2016). Article CAS Google Scholar * Baeyens, B. & Bradbury, M. H. A
mechanistic description of Ni and Zn sorption on Na-montmorillonite.1. Titration and sorption measurements. _J. Contam. Hydrol._ 27, 199–222 (1997). Article ADS CAS Google Scholar *
Bradbury, M. H. & Baeyens, B. A mechanistic description of Ni and Zn sorption on Na-montmorillonite 2. Modelling. _J. Contam. Hydrol._ 27, 223–248 (1997). Article ADS CAS Google
Scholar * Bradbury, M. H. & Baeyens, B. Modelling the sorption of Zn and Ni on Ca-montmorillonite. _Geochim. Cosmochim. Acta_ 63, 325–336 (1999). Article ADS CAS Google Scholar *
Elzinga, E. J. & Sparks, D. L. Nickel sorption mechanisms in a pyrophyllite-montmorillonite mixture. _J. Colloid Interf. Sci._ 213, 506–512 (1999). Article ADS CAS Google Scholar *
Green-Pedersen, H. & Pind, N. Preparation, characterization, and sorption properties for Ni(II) of iron oxyhydroxide-montmorillonite. _Colloid Surf., A_ 168, 133–145 (2000). Article CAS
Google Scholar * Dahn, R. Structural evidence for the sorption of Ni(II) atoms on the edges of montmorillonite clay minerals. A polarized X-ray absorption fine structure study. _Geochim.
Cosmochim. Acta_ 67, 2299–2299 (2003). Article ADS CAS Google Scholar * Nachtegaal, M., Scheidegger, A. M., Dahn, R., Chateigner, D. & Furrer, G. Immobilization of Ni by Al-modified
montmorillonite: A novel uptake mechanism. _Geochim. Cosmochim. Acta_ 69, 4211–4225 (2005). Article ADS CAS Google Scholar * Bradbury, M. H. & Baeyens, B. Experimental measurements
and modeling of sorption competition on montmorillonite. _Geochim. Cosmochim. Acta_ 69, 4187–4197 (2005). Article ADS CAS Google Scholar * Boonfueng, T., Axe, L., Xu, Y. & Tyson, T.
A. Nickel and lead sequestration in manganese oxide-coated montmorillonite. _J. Colloid Interf. Sci._ 303, 87–98 (2006). Article ADS CAS Google Scholar * Tertre, E., Berger, G., Castet,
S., Loubet, M. & Giffaut, E. Experimental sorption of Ni2+, Cs+ and Ln3+ onto a montmorillonite up to 150 degrees °C. _Geochim. Cosmochim. Acta_ 69, 4937–4948 (2005). Article ADS CAS
Google Scholar * Sen, T. K., Mahajan, S. P. & Khilar, K. C. Adsorption of Cu2+ and Ni2+ on iron oxide and kaolin and its importance on Ni2+ transport in porous media. _Colloid Surf., A_
211, 91–102 (2002). Article CAS Google Scholar * Yavuz, O., Altunkaynak, Y. & Guzel, F. Removal of copper, nickel, cobalt and manganese from aqueous solution by kaolinite. _Water
Res._ 37, 948–952 (2003). Article CAS PubMed Google Scholar * Tahir, S. S. & Rauf, N. Thermodynamic studies of Ni(II) adsorption onto bentonite from aqueous solution. _J. Chem.
Thermodyn._ 36, 2003–2009 (2003). Article Google Scholar * Donat, R., Akdogan, A., Erdem, E. & Cetisli, H. Thermodynamics of Pb2+ and Ni2+ adsorption onto natural bentonite from
aqueous solutions. _J. Colloid Interf. Sci._ 286, 43–52 (2005). Article ADS CAS Google Scholar * Fan, Q. H., Shao, D. D., Hu, J., Wu, W. S. & Wang, X. K. Comparison of Ni2+ sorption
to bare and ACT-graft attapulgites: Effect of pH, temperature and foreign ions. _Surf. Sci._ 602, 778–785 (2008). Article ADS CAS Google Scholar * Sheng, G. D. _et al_. Macroscopic and
Microscopic Investigation of Ni(II) Sequestration on Diatomite by Batch, XPS, and EXAFS Techniques. _Environ. Sci. Technol._ 45, 7718–7726 (2011). Article ADS CAS PubMed Google Scholar
* Echeverria, J., Indurain, J., Churio, E. & Garrido, J. Simultaneous effect of pH, temperature, ionic strength, and initial concentration on the retention of Ni on illite. _Colloid
Surf., A_ 218, 175–187 (2003). Article CAS Google Scholar * Marques Fernandes, M., Ver, N. & Baeyens, B. Predicting the uptake of Cs, Co, Ni, Eu, Th and U on argillaceous rocks using
sorption models for illite. _Appl. Geochem._ 59, 189–199 (2015). Article CAS Google Scholar * Elzinga, E. J. & Sparks, D. L. Reaction condition effects on nickel sorption mechanisms
in illite-water suspensions. _Soil Sci. Soc. Am. J._ 65, 94–101 (2001). Article CAS Google Scholar * Scheidegger, A. M., Strawn, D. G., Lamble, G. M. & Sparks, D. L. The kinetics of
mixed Ni-Al hydroxide formation on clay and aluminum oxide minerals: A time-resolved XAFS study. _Geochim. Cosmochim.Acta_ 62, 2233–2245 (1998). Article ADS CAS Google Scholar * Ford, R.
G., Scheinost, A. C., Scheckel, K. G. & Sparks, D. L. The link between clay mineral weathering and the stabilization of Ni surface precipitates. _Environ. Sci. Technol._ 33, 3140–3144
(1999). Article ADS CAS Google Scholar * Scheinost, A. C., Ford, R. G. & Sparks, D. L. The role of Al in the formation of secondary Ni precipitates on pyrophyllite, gibbsite, talc,
and amorphous silica: A DRS study. _Geochim. Cosmochim. Acta_ 63, 3193–3203 (1999). Article ADS CAS Google Scholar * Roberts, D. R., Scheidegger, A. M. & Sparks, D. L. Kinetics of
mixed Ni-Al precipitate formation on a soil clay fraction. _Environ. Sci. Technol._ 33, 3749–3754 (1999). Article ADS CAS Google Scholar * Scheckel, K. G., Scheinost, A. C., Ford, R. G.
& Sparks, D. L. Stability of layered Ni hydroxide surface precipitates - A dissolution kinetics study. _Geochim. Cosmochim.Acta_ 64, 2727–2736 (2000). Article ADS CAS Google Scholar
* Scheinost, A. C. & Sparks, D. L. Formation of layered single- and double-metal hydroxide precipitates at the mineral/water interface: A multiple-scattering XAFS analysis. _J. Colloid
Interf. Sci._ 223, 167–178 (2000). Article ADS CAS Google Scholar * Peltier, E., Van der Lelie, D. & Sparks, D. L. Formation and Stability of Ni-Al Hydroxide Phases in Soils.
_Environ. Sci. Technol._ 44, 302–308 (2010). Article ADS CAS PubMed Google Scholar * Yang, S. T. _et al_. Determination of Ni(II) uptake mechanisms on mordenite surfaces: A combined
macroscopic and microscopic approach. _Geochim. Cosmochim. Acta_ 75, 6520–6534 (2011). Article ADS CAS Google Scholar * Ren, X. M. _et al_. Microscopic level investigation of Ni(II)
sorption on Na-rectorite by EXAFS technique combined with statistical F-tests. _J. Hazard. Mater._ 252, 2–10 (2013). Article PubMed Google Scholar * Tan, X. L. _et al_. Effect of Silicate
on the Formation and Stability of Ni-Al LDH at the gamma-Al2O3 Surface. _Environ. Sci. Technol._ 48, 13138–13145 (2014). Article ADS CAS PubMed Google Scholar * Pandya, K. I., O’Grady,
W. E., Corrigan, D. A., McBreen, J. & Hoffman, R. W. Extenden x-ray absorption fine structure investigation of nickel hydroxides. _J. Phys. Chem._ 94, 21–26 (1990). Article CAS Google
Scholar * Fan, Q. H. _et al_. Comparative adsorption of Eu(III) and Am(III) on TPD. _Environ. Sci. Proc. Impacts_ 17, 1634–1640 (2015). Article CAS Google Scholar * Bradbury, M. H.
& Baeyens, B. Sorption modelling on illite Part I: Titration measurements and the sorption of Ni, Co, Eu and Sn. _Geochim. Cosmochim.Acta_ 73, 990–10dd03 (2009). Article ADS CAS
Google Scholar * Benedicto, A., Degueldre, C. & Missana, T. Gallium sorption on montmorillonite and illite colloids: Experimental study and modelling by ionic exchange and surface
complexation. _Appl. Geochem._ 40, 43–50 (2014). Article CAS Google Scholar * Marsac, R. _et al_. Neptunium redox speciation at the illite surface. _Geochim. Cosmochim. Acta_ 152, 39–51
(2015). Article ADS CAS Google Scholar * Nachtegaal, M. & Sparks, D. L. Nickel sequestration in a kaolinite-humic acid complex. _Environ. Sci. Technol._ 37, 529–534 (2003). Article
ADS CAS PubMed Google Scholar * Li, W. _et al_. Formation of crystalline Zn-Al LDH precipitates on γ-alumina: The role of mineral dissolution. _Environ. Sci. Technol._ 46, 11670–11677
(2012). Article ADS CAS PubMed Google Scholar * Voegeliin, A. & Kretzschmar, R. Formation and dissolution of single and mixed Zn and Ni precipitates in soil: evidence from column
experiments and extended X-ray absorption fine structure spectroscopy. _Environ. Sci. Technol._ 39, 5311–5318 (2005). Article ADS Google Scholar * Starcher, A. N., Li, W., Kukkadapu, R.
K., Elzinga, E. J. & Sparks, D. Fe(II) sorption on pyrophyllite: Effect of structural Fe(III) (impurity) in pyrophyllite on nature of layered double hydroxide (LDH) secondary mineral
formation. _Chem. Geol._ 439, 152–160 (2016). Article ADS CAS Google Scholar * Zhu, Y. & Elzinga, E. J. Formation of layered Fe(II)-hydroxides during Fe(II) sorption onto clay and
metal-oxide Substrates. _Environ. Sci. Technol._ 48, 4937–4945 (2014). Article ADS CAS PubMed Google Scholar * Siebecker, M., Li, W., Khalid, S. & Sparks, D. L. Real-time QEXAFS
spectroscopy measures rapid precipitate formation at the mineral-water interface. _Nature Communications._ 5, 5003 (2014). Article ADS CAS PubMed Google Scholar * Missana, T., Alonso,
U. & Garcia-Gutierrez, M. Experimental study and modelling of selenite sorption onto illite and smectite clays. _J. Colloid Interf. Sci._ 334, 132–138 (2009). Article ADS CAS Google
Scholar * Qiang, S. R. _et al_. Uptake of Nickel(II) on an Aridosol calcareous soil, China: an EXAFS spectroscopic investigation. _Sci. Rep._ 7, 1–10 (2017). Article ADS Google Scholar *
Teo, B. K. EXAFS: Basic Principles and Data Analysis. _Springer-Verlag_, _Berlin_ (1986). * Ravel, B. & Newville, M. ATHEAN, ARTERMIS, HEPHAESTUS; data analysis for X-ray absorption
spectroscopy using IFEFFIT. _J. Synchrotron. Radiat._ 12, 537–541 (2005). Article CAS PubMed Google Scholar * Zabinsky, S. I., Rehr, J. J. & Ankudinov, A. Multiple-scattering
calculations of X-ray-absorption spectra. _Phys. Rev. B_ 52, 2995–3009 (1995). Article ADS CAS Google Scholar * Fan, Q. H., Tanaka, M., Tanaka, K., Sakaguchi, A. & Takahashi, Y. An
EXAFS study on the effects of natural organic matter and the expandability of clay minerals on cesium adsorption and mobility. _Geochim. Cosmochim. Acta_ 136, 49–65 (2014). Article ADS
Google Scholar Download references ACKNOWLEDGEMENTS This study was supported by the Natural National Science Foundation of China (Grant no. 41573128, 21601179, 21601169, and 21647009), the
Key Laboratory Project of Gansu Province (Grant No. 1309RTSA041), and by the “100-Talent” Program from the Chinese Academy of Sciences in Lanzhou Center for Oil and Gas Resources, Institute
of Geology and Geophysics, CAS. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Key Laboratory of Petroleum Resources Research, Institute of Geology and Geophysics, Chinese Academy of
Sciences, Lanzhou, Gansu Region, 730000, China Xiaolan Zhao, Hanyu Wu, Yunbo Yang, Jianjun Liang, Ping Li & Qiaohui Fan * Key Laboratory of Preclinical Study for New Drugs of Gansu
Province, and Institute of Physiology, School of Basic Medical Sciences, Lanzhou University, 199 Donggang West Road, Lanzhou, 73000, China Shirong Qiang * Institute of Plasma Physics,
Chinese Academy of Sciences, Hefei, 230031, China Dadong Shao * State Key Laboratory of Soil Erosion and Dryland Farming on the Loess Plateau, Northwest A&F University, Yangling, 712100,
China Linchuan Fang * University of Chinese Academy of Sciences, Beijing, 100049, China Xiaolan Zhao & Yunbo Yang Authors * Xiaolan Zhao View author publications You can also search for
this author inPubMed Google Scholar * Shirong Qiang View author publications You can also search for this author inPubMed Google Scholar * Hanyu Wu View author publications You can also
search for this author inPubMed Google Scholar * Yunbo Yang View author publications You can also search for this author inPubMed Google Scholar * Dadong Shao View author publications You
can also search for this author inPubMed Google Scholar * Linchuan Fang View author publications You can also search for this author inPubMed Google Scholar * Jianjun Liang View author
publications You can also search for this author inPubMed Google Scholar * Ping Li View author publications You can also search for this author inPubMed Google Scholar * Qiaohui Fan View
author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Fan Q.H. and Zhao X.L. conceived the study. Fan Q.H., Liang J.J. and Li P. supervised the study.
Zhao X.L., Qiang S.R. and Wu H.Y. did the experiments. Yang Y.B. and Zhao X.L. prepared the figures and wrote the main manuscript. Shao D.D. and Fang L.C. analyzed the EXAFS results. All
authors reviewed the manuscript. CORRESPONDING AUTHOR Correspondence to Qiaohui Fan. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare that they have no competing interests.
ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY
MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,
adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Zhao, X., Qiang, S., Wu, H. _et al._ Exploring the Sorption Mechanism of Ni(II) on Illite: Batch Sorption, Modelling, EXAFS and Extraction Investigations. _Sci
Rep_ 7, 8495 (2017). https://doi.org/10.1038/s41598-017-09188-z Download citation * Received: 10 February 2017 * Accepted: 24 July 2017 * Published: 17 August 2017 * DOI:
https://doi.org/10.1038/s41598-017-09188-z SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative