Play all audios:
ABSTRACT Amyloid-β (Aβ) and tau pathologies are intertwined in Alzheimer’s disease, and various immunotherapies targeting these hallmarks are in clinical trials. To determine if tau
pathology influences Aβ burden and to assess prophylactic benefits, 3xTg and wild-type mice received tau immunization from 2–6 months of age. The mice developed a high IgG titer that was
maintained at 22 months of age. Pronounced tau and Aβ pathologies were primarily detected in the subiculum/CA1 region, which was therefore the focus of analysis. The therapy reduced
histopathological tau aggregates by 70–74% overall (68% in males and 78–86% in females), compared to 3xTg controls. Likewise, western blot analysis revealed a 41% clearance of soluble tau
(38–76% in males and 48% in females) and 42–47% clearance of insoluble tau (47–58% in males and 49% in females) in the immunized mice. Furthermore, Aβ burden was reduced by 84% overall (61%
in males and 97% in females). These benefits were associated with reductions in microgliosis and microhemorrhages. In summary, prophylactic tau immunization not only prevents tau pathology
but also Aβ deposition and related pathologies in a sustained manner, indicating that tau pathology can promote Aβ deposition, and that a short immunization regimen can have a long-lasting
beneficial effect. SIMILAR CONTENT BEING VIEWED BY OTHERS A MULTI-TARGETING IMMUNOTHERAPY AMELIORATES MULTIPLE FACETS OF ALZHEIMER’S DISEASE IN 3XTG MICE Article Open access 20 August 2024
ADAMANT: A PLACEBO-CONTROLLED RANDOMIZED PHASE 2 STUDY OF AADVAC1, AN ACTIVE IMMUNOTHERAPY AGAINST PATHOLOGICAL TAU IN ALZHEIMER’S DISEASE Article 14 June 2021 DEVELOPMENT OF AN
ANTI-TAUOPATHY MUCOSAL VACCINE SPECIFICALLY TARGETING PATHOLOGIC CONFORMERS Article Open access 15 June 2024 INTRODUCTION Alzheimer’s disease (AD) is characterized by accumulation of
extracellular amyloid-β (Aβ) plaques, intracellular neurofibrillary tangles (NFT), and extensive synaptic loss leading to progressive cognitive impairment and eventually dementia. NFT are
primarily composed of filaments of aggregated hyperphosphorylated tau protein1. Extensive work by numerous investigators suggests that Aβ pathology may lead to tau pathology2. However, it is
interesting to note that reanalysis of a large number of human AD and control brains of various ages with phospho-specific tau antibodies revealed that phospho-tau immunoreactivity is
generally detected in control brains prior to Aβ deposition3. This unexpected finding suggests that tau pathology may precede Aβ pathology in AD, at least in certain individuals, although it
is of course unclear if these subjects would ever have developed the disease. Importantly, Aβ plaque clearance has had limited effect on tau pathology in the Aβ immunotherapy trials (for
review see4), which emphasizes the need for therapy that specifically targets this other major hallmark of the disease. Numerous reports by us and others have shown the feasibility of tau
immunotherapy5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35, and several clinical trials have been initiated (for review see36). However, all these
studies were conducted in mice or related culture models that have only tau pathology but no Aβ pathology. Tau antibody immunization in Aβ plaque models has been reported to improve
cognition and clear certain Aβ species while increasing Aβ plaque burden37, or when using a different tau antibody in a different Aβ model provided no cognitive benefits and increased
mortality38. A few prior studies have reported toxic effects of active tau immunization in mice when very strong adjuvants are being used39,40. One of the few models with both Aβ and tau
deposits, is the triple transgenic (3xTg) mouse model, which harbors a presenilin 1 mutation (PS1/M146V) knock in allele, as well as the Swedish mutation of the amyloid precursor protein
(APPSwe), and tau P301L mutation transgenes41. The PS1 and APP mutations individually cause familial AD and the tau mutation leads to frontotemporal dementia, which is an Aβ negative
tauopathy. The 3xTg mice develop age-dependent and region-specific Aβ and tau deposits that mimic the disease progression in humans. They have previously received Aβ immunotherapy, which
cleared Aβ plaques and rescued early but not late hyperphosphorylated tau aggregates42. More recently, a couple of studies have reported on the effect of tau antibodies on tau and Aβ burden
in 3xTg mice43,44. A single injection of AT8, a phospho-tau antibody was shown to transiently reduce tau pathology without affecting Aβ pathology43. More recently, multiple injections of
another tau antibody acutely reduced tau and Aβ pathology in their early stages44. Here, we report that immunization of this 3xTg model with Tau379–408[P-Ser396, 404] from 3–6 months of age,
with animals killed at 22 months for analysis, resulted in a robust tau antibody response and long-term clearance of not only tau aggregates but also associated Aβ plaques. MATERIALS AND
METHODS PEPTIDES Phosphorylated tau peptide, Tau379–408[P-Ser396,404], was synthesized and purified at the Keck facility (Yale University) as described previously5. TRANSGENIC MICE The
treatment was performed in 3xTg transgenic mice expressing knock-in mutation PS1/M146V combined with APP/K670N, M671L and MAPT/P301L transgenes41. A breeding pair of homozygous mice and
another pair of wild-type (wt) mice of the same mixed strain background (C57BL6/129 SVJ) were graciously donated by Frank LaFerla (University of California at Irvine). These mice develop
plaque and tangle pathology in AD relevant brain regions (hippocampus, cortex and amygdala). Wt mice from the same background were used as a control. The mice were housed in Association for
Assessment and Accreditation of Laboratory Animal Care (AAALAC) approved facilities. All mouse care and experimental procedures were compliant with guidelines of animal experimentation and
were approved by the Institutional Animal Care and Use committee at New York University School of Medicine. VACCINE ADMINISTRATION At the start of the study, the 3xTg and wt mice were split
into treatment groups that received the tau vaccine and control groups that received adjuvant alone (Table 1). The immunogen Tau 379–408 [P-Ser396, 404] was added to Adju-Phos adjuvant
(Brenntag Biosector, Denmark) (1 mg/mL) and mixed overnight at 4 °C the day before injection for the peptide to adsorb onto the aluminum phosphate particles. The vaccine was injected
subcutaneously (100 μl) with a second injection administered two weeks later and subsequent injections monthly thereafter. The treatment period was from 3-6 months of age (four injections).
The mice were bled before the first immunization (T0) and then periodically thereafter to monitor their tau antibody response (T1: 1 week after the 3rd immunization; T2-T5: 2, 5, 8 and 11
months after the 4th immunization, respectively; Tf: 16 months after the 4th immunization). At 22 months, their brains were extracted for analyses. The mice went through several behavioral
tests in the two months prior to killing. At the end of study, a few mice had died in each of the four groups except in the immunized 3xTg groups, in which all the mice survived the
experimental period (Table 1). Upon western blot analysis, 3 Tg mice (2 control males and 1 control female) were eliminated from the study because they did not express human tau, although
the transgene was present. We have observed this at a similar rate in other Tg tauopathy models over the years. These three mice are not included in Table 1 or in any of the analyses.
BEHAVIOR Each instrument was wiped clean with 30% ethanol between animals. LOCOMOTOR ACTIVITY A circular open field activity chamber (70 cm in diameter) was used to measure exploratory
locomotor activity over 15 min45. A camera placed above the field recorded animal movements (San Diego Instruments). Measured parameters were distance traveled (in centimeters), mean resting
time, and velocity [mean (_V_mean) and maximum (_V_max)] of the mouse. TRAVERSE BEAM This test measures balance, motor coordination and function integration45. Mice were evaluated by
examining their ability to traverse a narrow beam to enter a goal box. The animals were placed on a wooden beam (1.1 cm wide, 50.8 cm long) that was suspended 30 cm above a soft foam cushion
by two identical columns. At each end of the beam was attached a shaded goal box. Habituation consisted of placing the mouse on the middle of the beam for 60 s. Subsequently, in four
successive trials, the number of foot slips before falling or reaching the goal box was recorded for each mouse. Errors were defined as foot slips and their numbers counted. A mouse that
fell off the beam, was placed back on it at the location it fell from. ROTAROD This test was conducted to measure potential differences in forelimb and hindlimb motor coordination and
balance without a practice confound (ref.45 Rotarod 7650 accelerating model; Ugo Basile). Habituation consisted of two trial training sessions to allow the animals to perform at a baseline
level. Subsequently, the mice went through three test trials, with 15 min break between sessions. The rotarod was set 1.0 rpm and was gradually raised every 30 s until the mouse fell off or
inverted (by clinging) from the rotating rod. The rpm at that point was recorded. To prevent injury, a soft foam cushion was beneath the rod. COGNITIVE TESTS Before each test, the mice were
adapted to the room with lights on for 15 min. RADIAL ARM MAZE An eight–arm radial maze with a water well at the end of each arm was used to evaluate spatial learning45. Guillotine doors
made from clear Plexiglas and operated by a remote pulley system, controlled access to the arms from the central circle from which the mouse entered and exited the maze. Following adaptation
for 3–4 days, water-restricted mice (2 h daily access to water) went through one training session per day for ten consecutive days. In each session, all the arms of the maze were baited
with saccharine flavored water, and the mouse was allowed to enter and explore all arms until the eight sugar rewards had been consumed. Spatial learning was assessed by recording the number
of errors (entries to previously visited arms) and the time needed to complete each session. CLOSED FIELD SYMMETRICAL MAZE This maze consists of a rectangular field ( 63.5 cm square with 9
cm high walls divided into 36, 9.5 cm squares) that is covered by a clear Plexiglas top. Two boxes (each 15 × 20 × 9 cm), for the mice to enter or exit are situated at diagonal corners of
the maze45. This symmetrical maze46 is based on the Hebb-Williams47 and Rabinovitch-Rosvold48 tests. Briefly, the key difference is that each end compartment serves as both a start box and a
goal box, and the mouse navigates in opposite direction on alternate trials. An advantage of this setup is that it eliminates intertrial handling, which reduces animal stress and thereby
gives more reliable data. The barriers are positioned symmetrically in the maze, so that the mouse faces the same turns going in either direction within a given setup. Before testing, the
mice were adapted to a water restriction schedule, with 2 h daily access to water. Habituation consisted of two adaptation sessions before the first testing period. In the first such
session, all the mice had access to saccharine flavored water in the goal box for 10 min. In the second adaptation session, the mouse was put in the start chamber and allowed to explore the
field and enter the goal box, which contained saccharin water reward (0.05 mL). When the mice were consistently traveling between the start and goal boxes, they went through three practice
sessions on simple navigational problems, in which one or two barriers were positioned in different regions of the maze to prevent direct route to the goal box. Formal testing consisted of
three problems of varying difficulty, starting with the easiest one and ending with the most difficult one. The mice tackled one problem per day and the mice went through five trials to
solve it with an inter-trial interval of 2 min. Their performance was scored by the same person for the number of errors (i.e. entries and reentries into designated error zones) and time to
complete each trial. ANTIBODY RESPONSE The antibody response was determined in a 1:200 dilution of plasma using an ELISA assay as detailed previously49,50, in which the immunogen was coated
on to microtiter wells (Immulon 2HB,Thermo Electron). The binding of plasma antibodies was detected by a goat anti-mouse IgG or IgM linked to a horseradish peroxidase (Pierce), with the
enzyme catalyzing a color reaction in the substrate (tetramethyl benzidine; Pierce). TISSUE PROCESSING AND HISTOLOGY After the behavioral testing, the mice were deeply anesthetized with
ketamine/xylazine (250 mg/50 mg per kg body weight, i.p.). The brain was then extracted without perfusion and processed as detailed previously51,52. The left hemisphere was snap frozen on
CO2 pellets and stored at −80 °C until processed for western blots. The right hemisphere was sectioned coronally (40 µm) from the frontal pole to the cerebellum, and sections were saved into
5 serial series for histological staining with about 40 sections per series. For each stain/antibody, at least half a series (20 sections), spaced equally apart (400 μm) were reacted.
Staining was performed at room temperature as described previously49,50,52. Briefly, sections were placed in 0.3% H2O2 for 15 min to block endogenous peroxidase activity, and then incubated
in mouse-on-mouse (MOM) blocking reagent (Vector Laboratories, Burlingame, CA) to block nonspecific binding for 1 h. Following washes in TBS-Tx, the sections were stained with PHF1 (1:2000
dilution of cell culture supernatant) and MC1 (1:100 dilution of cell culture supernatant) tau antibodies (generously provided by Dr. Peter Davies, Albert Einstein College of Medicine,
Bronx, NY). Adjacent sections were also stained with 6E10/4G8 (1:2000 dilution of 1 mg/ml stock of each antibody) for Aβ deposits. To assess glial response, brain sections were stained with
1) rabbit polyclonal antibody against glial fibrillary acidic protein (GFAP) in astrocytes (1:500; Dako, Carpinteria, CA), and 2) Iba1 (10 µg/mL; Wako, Richmond, VA) to detect microglia.
Neurons were stained with cresyl violet using standard procedure as described previously53. For staining of microhemorrhages, serial coronal sections of experimental and control mouse brains
were mounted on gelatin coated slides and stained with Prussian blue working solution as described previously54. Briefly, the brain sections were incubated in a mixture of equal volumes of
10% potassium ferrocyanide (K4Fe(CN)6 trihydrate) in distilled H2O and 20% hydrochloric acid (HCl) for 30 min. The sections were subsequently washed with H2O, counterstained with nuclear
fast red solution for 10 min, washed again with H2O, dehydrated and coverslipped using Depex mounting media (BDH Laboratory Supplies, England). IMAGE ANALYSIS Tau and Aβ deposition was
analyzed in the subiculum of the brain because of its prominent and consistent such pathology. Tau pathology was quantified blindly in 5 sections per brain spaced 200 μm apart similar to as
described previously5,7,8. The measurement was the percentage of area in the measurement field (200X) that was occupied by the reaction product (ImageJ, NIH). Aβ burden was analyzed as per
our standard procedure54, using the StereoInvestigator Program (Area Fraction Fractionator; MBF Biosciences, Burlington, VT). The area of the grid was 800 μm2 × 800 μm2 and Aβ burden was
measured in one frame per section (640 × 480 μm2 each) chosen randomly within the subiculum region, and five sections spaced 200 μm apart were analyzed. The Aβ burden is defined as the
percentage of area in the measurement field (subiculum) that is occupied by the reaction product. The assessment of the Iba1 (microglia) stained sections was based on a semi-quantitative
analysis of microgliosis in the subiculum (0, predominantly resting microglia; 1+, a few ramified and/or phagocytic microglia; 2+, moderate number of ramified/phagocytic microglia; 3+,
numerous ramified/phagocytic microglia). The rating of the GFAP sections was based on the complexity of astrocytic branching in the subiculum (1+, resting astrocytes, few processes; 2+,
reactive astrocytes, moderate branching; 3+, reactive astrocytes, extensive branching). The haemorrhage profiles (hemosiderin stain) were counted, and the average number of Prussian
blue-positive deposits in the subiculum was calculated for each brain section. All procedures were performed by an individual blinded to the experimental condition of the study. The accuracy
of the findings was verified by two independent observers. WESTERN BLOTTING Brains were weighed and homogenized in modified RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM
NaF, 1 mM Na3VO4, 1 μg/ml complete protease inhibitor cocktail (Roche) and subjected to a low speed spin (14,000 rpm) to remove the membrane fraction. For sarkosyl extraction, 1% sarkosyl
solution was added to 300 μL supernatant for a final concentration of 1% and then incubated for 1 h at 37 °C. Sarkosyl extracted supernatant and supernatant without sarkosyl were then
centrifuged at 100,000 × _g_ for 1 h at 4 °C in Beckman TL-100 ultracentrifuge, and the high-speed supernatants were collected and used for western blot analysis. Sarkosyl extraction results
in dissociation of insoluble proteins including aggregated tau proteins. For the insoluble fraction, the pellet was re-suspended in the same volume of buffer without protease and
phosphatase inhibitors, but that contained 1% (v/v) Triton X-100 and 0.25% (w/v) deoxycholate sodium. It was then ultracentrifuged at 50,000 × _g_ for 30 min to retrieve a detergent
extracted supernatant that was analyzed as an insoluble fraction17,18. The supernatants from these three fractions were heated at 100 °C for 5 min and the same amount of protein was
electrophoresed on a 12% (w/v) polyacrylamide gel. The proteins were then transferred to a nitrocellulose membrane that was subsequently blocked in 5% nonfat milk with 0.1% Tween-20 in TBS,
and incubated with different antibodies for at least 3 h at room temperature or at 4 °C overnight (PHF1, CP27 generously provided by Peter Davies). Following washes, the membranes were then
incubated for 2 h with 1:2000 horseradish-peroxidase (HRP) conjugated goat anti-mouse antibody (Pierce), developed in ECL (Pierce), imaged with Fuji LAS-4000, and the signal quantified with
Multigauge. EXPERIMENTAL DESIGN AND STATISTICAL ANALYSES The experimental design was as detailed above. Briefly, transgenic and wt mice of both sexes received short-term prophylactic active
tau vaccination at a young age (3–6 months). Control mice received only adjuvant. The mice were bled periodically to determine their antibody titers and they went through behavioral testing
at the end of the study (20–22 months). Subsequently, their brains were removed for histological and biochemical analyses of the effects of the vaccination on AD related pathologies, and to
monitor potential adverse effects. All data were analyzed with GraphPad Prism 7. Unless specified below, the analysis was performed with an unpaired t-test, two-tailed. Welch correction was
used if the data failed a test of equal variance. When the data failed at least two of three normality tests (Kolmogorov–Smirnov, D’Agostino and Pearson omnibus, and Shapiro–Wilk normality
tests), nonparametric Mann–Whitney test was used. That test was also used for analyzing the astro- and microgliosis. Behavioral data was analyzed with one or two-way ANOVA, depending on the
number of factors. When the data failed at least two of three normality tests (Kolmogorov–Smirnov, D’Agostino and Pearson omnibus, and Shapiro–Wilk normality tests), nonparametric Kruskal
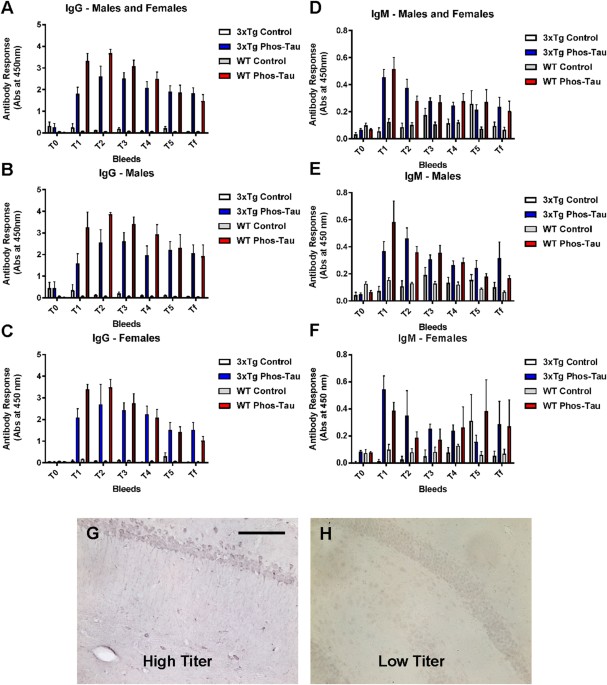
Wallis test was used. Radial arm maze test was analyzed with two-way ANOVA, repeated measures. RESULTS TAU IMMUNIZATION ELICITS A ROBUST ANTIBODY RESPONSE Mice immunized with the Tau
379–408[P-Ser396, 404] immunogen in Adju-Phos adjuvant developed a robust IgG response in both male and female 3xTg as well as wt mice compared to controls that received adjuvant alone.
(Fig. 1A–C). IgM response was less pronounced in the same groups (Fig. 1D–F). Notably, the mice maintained high antibody levels, after the fourth and last immunization at 6 months of age,
until the end of the study when the mice were 22 months of age. This was evident both in Tg and wt mice. Plasma from a high titer mouse stained intraneuronal tau aggregates, whereas plasma
from a control mouse did not (Fig. 1G,H), which confirms our previous findings that polyclonal antibodies elicited to this vaccine recognize pathological tau protein5,10. In a pilot study,
we noticed substantial mortality in the immunized mice after the 5th immunization, with surviving mice maintaining high antibody titer for more than a year. Hence, the enrolled mice received
only four vaccine injections. That short vaccination paradigm did not appear to elicit side effects. As noted in Table 1, only a few mice died in three of the groups and none in the
immunized 3xTg mice. Based on prior work with this vaccine in other mouse models, the mortality in the pilot study is likely related to more robust immune response to this vaccine in mice on
this particular mixed strain background. COGNITIVE BENEFITS CANNOT BE DETECTED WITH THE IMMUNOTHERAPY AS THE TG MICE ARE NOT IMPAIRED COMPARED TO WT MICE The tau immunotherapy did not lead
to any significant cognitive improvement in the immunized 3xTg mice, compared to adjuvant treated controls (data not shown). Our analysis however, did not detect cognitive impairments in the
3xTg controls compared to wt controls. Hence no therapeutic behavioral benefits were observed with the immunotherapy as the mice were not impaired. We have used these same tests extensively
in other tangle models5,7,8,34, and observed that Tg tau immunized mice performed better than the Tg control mice. These prior studies include the use of the same tau immunogen. The
immunized 3xTg mice and wt mice did not differ significantly from their non-immunized identical control mice in any of the cognitive tasks, and all the groups appeared to have normal motor
functions based on our experience with wt mice in these tests (data not shown). TAU IMMUNIZATION DECREASES TAU PATHOLOGY To determine the consequences of the active tau immunization on
hyperphosphorylated tau and pathological tau conformers, brain sections were stained with PHF1 and MC1 antibody, respectively. Pronounced tau pathology was revealed with both antibodies,
primarily in the subiculum/CA1 region, which was therefore the focus of analysis (Figs 2 and 3A–D). Only background staining was seen in the wt mice (Figs 2 and 3E–H). It may relate to their
old age and because the mice were not perfused. The therapy reduced PHF1-reactive tau aggregates by 74% in the combined 3xTg male and female group (Fig. 2I, p = 0.0008), which was also
significantly reduced in males (Fig. 2J; 68%; p = 0.0437) and females (Fig. 2K, 78%; p = 0.0120), compared to identical controls. MC1 immunoreactive tau aggregates were also reduced in the
immunized 3xTg male and female combined group (Fig. 3I, 70%, p = 0.0057), and in the separate female group (Fig. 3K, 86%; p = 0.0070), compared to identical controls. Likewise, western blot
analysis revealed a similar clearance of tau in the immunized 3xTg mice. Significant reductions were observed in soluble and insoluble human tau (CP27) in the immunized overall group (Fig.
4A,B, soluble tau: 41%, p = 0.0103; insoluble tau: 47%, p = 0.0008), and in males (Fig. 4C,D, soluble tau: 38%, p = 0.0322; insoluble tau: 47%, p = 0.0165), and females (Fig. 4E,F, soluble
tau: 48%, p = 0.0616; insoluble tau: 49%, p = 0.0477), analyzed separately compared to Tg controls. For PHF1 reactive phospho-tau, significant reductions were detected in the insoluble tau
fraction in the immunized combined group (Fig. 5B, 42%, p = 0.0003), and in males in soluble (Fig. 5C, 76%, p = 0.0001) and insoluble tau (Fig. 5D, 58%, p = 0.0018). The immunization did not
affect endogenous tau in the wt mice (Tau-5: wt controls = 456,740 ± 76,011 AUC/mm2 (average ± SEM); wt immunized = 376,469 ± 98,775 AUC/mm2). TAU IMMUNIZATION REDUCES AΒ PLAQUE BURDEN Aβ
plaque burden in the subiculum region of the 3xTg mice was analyzed by immunohistochemistry using a combination of 6E10 and 4G8 antibodies (Fig. 6A–D). Only background staining was seen in
the wt mice (Fig. 6E–H). Aβ deposits in tau immunized 3xTg mice were decreased significantly as compared to control vaccinated mice (Fig. 6I–K, 84% in combined group, p < 0.0001; 61% in
males, p = 0.0033, and; 97% in females, p < 0.0001). These results indicate that prophylactic tau immunization reduces the formation of Aβ plaque deposits. TAU IMMUNIZATION DOES NOT
APPEAR TO AFFECT NEURONAL DENSITY Like many other AD models, 3xTg mice do not have extensive neuronal loss41,55. We stained brain sections with cresyl violet from Tg mice that had a robust
therapeutic response to the vaccine, and compared those to sections from adjuvant control 3xTg mice. Neuronal density/numbers appeared to be similar in these two groups (data not shown),
which fits with the previously reported limited effect of tau or Aβ pathology on this parameter in this model41,55. TAU IMMUNIZATION REDUCES MICROGLIOSIS AND MICROBLEEDS BUT DOES NOT AFFECT
ASTROCYTES To assess the potential involvement of activated astrocytes, microglia and microhemorrhage following immunization, histological analysis was performed focusing on the subiculum of
the hippocampus, the region with the highest Aβ plaque burden and associated tauopathy. GFAP immunoreactivity was greater in 3xTg mice compared to wt mice (males and females: p = 0.0139;
males: p = 0.0286; females: 0.2286) but the immunotherapy did not affect astrogliosis (Figs 7 and 8). Likewise, microgliosis was more pronounced in 3xTg mice than in wt mice (males and
females: p = 0.0013; males: p = 0.0699; females: 0.0294) but the immunotherapy reduced it significantly in the Tg mice (males and females: p = 0.0056; males: p = 0.0699; females: p = 0.0294,
Figs 9 and 10). For microhemorrhages, those were also seen more often in 3xTg mice compared to wt mice (males and females: p = 0.0088; males: p = 0.5714; females: p = 0.0165), and were
reduced significantly in the treated Tg mice compared to their Tg controls (males and females: p = 0.0113; males: p = 0.4725; females: p = 0.0078, Fig. 11). These results suggest that
gradual removal of tau aggregates, and an indirect clearance of Aβ deposits, are not associated with extensive gliosis or microhemorrhages, and actually reduce microgliosis and
microhemorrhages. This lack of treatment associated gliosis is in accordance with our prior results with this immunogen or tau antibody in other tauopathy models5,7,8. Overall, these results
support the view that Aβ and tau pathologies are synergistic. Clearing tau leads indirectly to clearance of Aβ and associated pathologies such as microgliosis and microhemorrhages.
DISCUSSION Our findings indicate that tau immunotherapy can not only lead to clearance of tau pathology but also of Aβ deposits. Importantly as well, the benefits are sustained, lasting at
least 16 months following the last immunization. Together, these findings may have major implications for clinical use of this approach. Aβ immunotherapy has previously been shown to reduce
tau pathology to a modest extent in mouse models and humans (reviewed in4). Also, a single intrahippocampal injection of a tau antibody reduces early and late tau pathology in 3xTg mice
without affecting Aβ deposits43. In that particular study, clearance of tau pathology was acute and transient, observed at 7 and 14 days post-injection but was no longer evident 21 days
after antibody administration. It is, therefore, not surprising that Aβ pathology was not affected within such a short timeframe. More recently, tau passive immunization was shown to inhibit
not only tau but also Aβ pathology in 3xTg mice that received 6 weekly tau antibody injections at the early stages (12 months) of tau pathology in this model44. In our study, the mice
received their first vaccine injection at 3 months, and the last one at 6 months, at which age the mice have minimal if any tau or Aβ pathology. The tau antibodies elicited by the vaccine
then prophylactically prevented the development of intraneuronal tau aggregates, which then indirectly attenuated Aβ deposition. Further support for the synergistic effects of these two
pathologies can be obtained from their regional colocalization in the 3xTg model. The Aβ deposits are most prominent in the subiculum region of the hippocampus and are surrounded by
dystrophic neurites positive for pathological tau protein. It can be inferred that neurons with tau lesions generate more Aβ than healthy neurons that is then deposited near their synaptic
terminals. This scenario would then further promote tau pathology and enhanced Aβ deposition resulting in a vicious cycle. Long-term antibody-mediated removal of tau aggregates would
therefore be expected to attenuate the progression of the intertwined tau and Aβ pathologies as confirmed by our findings. The tau immunotherapy reduced tau and Aβ burden in both sexes but
the therapeutic benefits were generally more pronounced in females, although they have more pronounced pathology, which fits our prior findings in a different tauopathy model7. Associated
pathology, microgliosis and microbleeds were also reduced more significantly in females than males following the immunotherapy, which would be expected as these are closely linked to Aβ
deposits. Cognitive impairment has been previously documented in 3xTg mice (for review see56). We were not able to detect such impairments in two different cognitive tests compared to
age-matched littermate wt mice. The performance of the wt controls in this study was comparable to our prior reports using these tasks in wt controls of a different strain background but of
a similar age50,54. This further supports lack of cognitive deficits of the 3xTg mice in these tasks. However, studies reporting memory issues in this model used different tests. Also, the
3xTg mice we used may have had less severe pathology than in reported studies. Finally, the mice in our study were older than in the prior report. It is conceivable that age-related memory
deficits in wt mice may catch up with Tg deficits at the 21–22 months of age when our mice were tested. We are not aware of other reports assessing cognition in this model in mice older than
15–18 months of age56. Most of these studies report deficits at various ages ranging from 3–5 months to 15–18 months with a few showing no impairment compared to controls at 1–2 months, 3–5
months and 9–11 months. None of the tests in the prior studies were similar to ours, which further complicates comparison. Regardless of the lack of a pronounced behavioral phenotype in the
3xTg mice compared to wt mice of the same strain background, the histological effects of the tau immunotherapy were pronounced and highly significant. These findings suggest that prevention
of the development of tau pathology can robustly diminish associated Aβ deposition in mice that are prone to develop such deposits because of APP and PS1 mutations. Remarkably, the
therapeutic benefits of the prophylactic immunization were long-lasting, up to 16 months after the fourth and last immunization. Prior active tau immunization studies have not assessed such
sustained benefits following the vaccination paradigm. Typically, the final immunization in those studies has been within a month prior to brain analysis. What led us down the path of
determining possible long-term benefits of active tau immunization was the unexpected high mortality in this model following the fifth immunization in a pilot study that preceded this
comprehensive study. Such catastrophic adverse effects most likely relate to the strong immune response in this particular hybrid strain because 3xTg and wt mice on the same strain
background were affected to a similar extent. In our prior studies using this tau immunogen and the same alum adjuvant, we did not observe adverse reactions let alone death in two different
tauopathy mouse models that received five or more immunizations5,7. Here, the four immunizations did not appear to lead to side effects. All the 3xTg immunized mice survived until the end of
the study, and in each of the other three groups (3xTg and wt controls as well as wt immunized) only a few mice died during the long experimental period (Table 1). The immunization did not
affect tau levels in wt mice. This is as expected because of the phospho-tau immunogen whose epitope is primarily found in pathological tau. Also, the normal tau in wt mice is primarily
cytosolic, whereas tau antibodies within neurons typically bind to pathological tau in endosomal-lysosomal vesicles 10,17,18,21,34,57. It remains to be seen if such prolonged prophylactic
benefits, not only for blocking tau pathology but also for diminishing Aβ burden, will hold up in humans receiving active tau immunizations. It is unlikely that this will be clarified in
ongoing active tau immunization trials as the enrolled subjects are likely to have already substantial Aβ deposits, whose development may have plateaued because of synaptic loss. As in our
study, prophylactic therapy will likely be required, which could be assessed first in individuals with genetic mutations that are known to cause AD or related tauopathies. However, because
the extent of tau pathology correlates much better with cognitive deficits than Aβ burden, tau immunotherapies are likely to provide clinical benefits at later stages of AD than treatments
that directly target Aβ. REFERENCES * Grundke-Iqbal, I. _et al_. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. _Proc. Natl.
Acad. Sci. USA_ 83, 4913–4917 (1986). Article ADS CAS PubMed PubMed Central Google Scholar * Selkoe, D. J. & Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years.
_EMBO Mol. Med._ 8, 595–608 (2016). Article CAS PubMed PubMed Central Google Scholar * Braak, H., Thal, D. R., Ghebremedhin, E. & Del, T. K. Stages of the pathologic process in
Alzheimer disease: age categories from 1 to 100 years. _J. Neuropathol. Exp. Neurol._ 70, 960–969 (2011). Article CAS PubMed Google Scholar * Congdon, E. E., Krishnaswamy, S. &
Sigurdsson, E. M. Harnessing the immune system for treatment and detection of tau pathology. _J. Alzheimers. Dis._ 40, S113–S121 (2014). PubMed PubMed Central Google Scholar * Asuni, A.
A., Boutajangout, A., Quartermain, D. & Sigurdsson, E. M. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional
improvements. _J. Neurosci._ 27, 9115–9129 (2007). Article CAS PubMed Google Scholar * Boimel, M. _et al_. _E_fficacy and safety of immunization with phosphorylated tau against
neurofibrillary tangles in mice. _Exp. Neurol._ 224, 472–485 (2010). Article CAS PubMed Google Scholar * Boutajangout, A., Quartermain, D. & Sigurdsson, E. M. Immunotherapy targeting
pathological tau prevents cognitive decline in a new tangle mouse model. _J. Neurosci._ 30, 16559–16566 (2010). Article CAS PubMed PubMed Central Google Scholar * Boutajangout, A.,
Ingadottir, J., Davies, P. & Sigurdsson, E. M. Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from
the brain. _J. Neurochem._ 118, 658–667 (2011). Article CAS PubMed PubMed Central Google Scholar * Chai, X. _et al_. _P_assive immunization with anti-Tau antibodies in two transgenic
models: reduction of Tau pathology and delay of disease progression. _J. Biol Chem._ 286, 34457–34467 (2011). Article CAS PubMed PubMed Central Google Scholar * Krishnamurthy, P. K.,
Deng, Y. & Sigurdsson, E. M. Mechanistic Studies of Antibody-Mediated Clearance of Tau Aggregates Using an _ex vivo_ Brain Slice Model. _Front Psychiatry_ 2, 59–65 (2011). Article CAS
PubMed PubMed Central Google Scholar * Bi, M., Ittner, A., Ke, Y. D., Gotz, J. & Ittner, L. M. Tau-Targeted Immunization Impedes Progression of Neurofibrillary Histopathology in Aged
P301L Tau Transgenic Mice. _PLoS. One._ 6, e26860 (2011). Article ADS CAS PubMed PubMed Central Google Scholar * Troquier, L. _et al_. _T_argeting phospho-Ser422 by active Tau
immunotherapy in the THY-Tau22 mouse model: a suitable therapeutic approach. _Curr. Alzheimer Res._ 9, 397–405 (2012). Article PubMed PubMed Central Google Scholar * Kfoury, N., Holmes,
B. B., Jiang, H., Holtzman, D. M. & Diamond, M. I. Trans-cellular Propagation of Tau Aggregation by Fibrillar Species. _J. Biol Chem._ 287, 19440–19451 (2012). Article CAS PubMed
PubMed Central Google Scholar * d’Abramo, C., Acker, C. M., Jimenez, H. T. & Davies, P. Tau Passive Immunotherapy in Mutant P301L Mice: Antibody Affinity versus Specificity. _PLoS ONE_
8, e62402 (2013). Article ADS PubMed PubMed Central Google Scholar * Theunis, C. _et al_. _E_fficacy and safety of a liposome-based vaccine against protein Tau, assessed in tau P301L
mice that model tauopathy. _PLoS. One._ 8, e72301 (2013). Article ADS PubMed PubMed Central Google Scholar * Yanamandra, K. _et al_. _A_nti-tau antibodies that block tau aggregate
seeding _in vitro_ markedly decrease pathology and improve cognition _in vivo_. _Neuron_ 80, 402–414 (2013). Article CAS PubMed PubMed Central Google Scholar * Gu, J., Congdon, E. E.
& Sigurdsson, E. M. Two novel Tau antibodies targeting the 396/404 region are primarily taken up by neurons and reduce Tau protein pathology. _J. Biol Chem._ 288, 33081–33095 (2013).
Article CAS PubMed PubMed Central Google Scholar * Congdon, E. E., Gu, J., Sait, H. B. & Sigurdsson, E. M. Antibody Uptake into Neurons Occurs Primarily via Clathrin-dependent
Fcgamma Receptor Endocytosis and Is a Prerequisite for Acute Tau Protein Clearance. _J. Biol Chem._ 288, 35452–35465 (2013). Article CAS PubMed PubMed Central Google Scholar *
Castillo-Carranza, D. L. _et al_. _S_pecific targeting of tau oligomers in htau mice prevents cognitive impairment and tau toxicity following injection with brain-derived tau oligomeric
seeds. _J. Alzheimers. Dis._ 40, S97–S111 (2014). Article PubMed Google Scholar * Castillo-Carranza, D. L. _et al_. _P_assive Immunization with Tau Oligomer Monoclonal Antibody Reverses
Tauopathy Phenotypes without Affecting Hyperphosphorylated Neurofibrillary Tangles. _J. Neurosci._ 34, 4260–4272 (2014). Article PubMed Google Scholar * Collin,L. _et al_. Neuronal uptake
of tau/pS422 antibody and reduced progression of tau pathology in a mouse model of Alzheimer’s disease. _Brain_ 137, 2834-2846 (2014). * Ittner, A. _et al_. _T_au-targeting passive
immunization modulates aspects of pathology in tau transgenic mice. _J. Neurochem._ 132, 135–145 (2015). Article CAS PubMed Google Scholar * Kontsekova, E., Zilka, N., Kovacech, B.,
Novak, P. & Novak, M. First-in-man tau vaccine targeting structural determinants essential for pathological tau-tau interaction reduces tau oligomerisation and neurofibrillary
degeneration in an Alzheimer’s disease model. _Alzheimers. Res. Ther._ 6, 44 (2014). Article PubMed PubMed Central Google Scholar * Selenica, M. L. _et al_. _E_pitope analysis following
active immunization with tau proteins reveals immunogens implicated in tau pathogenesis. _J. Neuroinflammation._ 11, 152 (2014). Article PubMed PubMed Central Google Scholar * Ando, K.
_et al_. _V_accination with Sarkosyl insoluble PHF-tau decrease neurofibrillary tangles formation in aged tau transgenic mouse model: a pilot study. _J. Alzheimers. Dis._ 40(Suppl 1),
S135–S145 (2014). PubMed Google Scholar * Castillo-Carranza, D. L. _et al_. _S_pecific targeting of tau oligomers in Htau mice prevents cognitive impairment and tau toxicity following
injection with brain-derived tau oligomeric seeds. _J. Alzheimers. Dis._ 40(Suppl 1), S97–S111 (2014). Article PubMed Google Scholar * Umeda, T. _et al_. _P_assive immunotherapy of
tauopathy targeting pSer413-tau: a pilot study in mice. _Ann. Clin. Transl. Neurol._ 2, 241–255 (2015). Article CAS PubMed PubMed Central Google Scholar * Sankaranarayanan, S. _et al_.
_P_assive immunization with phospho-tau antibodies reduces tau pathology and functional deficits in two distinct mouse tauopathy models. _PLoS. One._ 10, e0125614 (2015). Article PubMed
PubMed Central Google Scholar * Yanamandra, K. _et al_. _A_nti-tau antibody reduces insoluble tau and decreases brain atrophy. _Ann. Clin. Transl. Neurol._ 2, 278–288 (2015). Article CAS
PubMed PubMed Central Google Scholar * d’Abramo, C., Acker, C. M., Jimenez, H. & Davies, P. Passive Immunization in JNPL3 Transgenic Mice Using an Array of Phospho-Tau Specific
Antibodies. _PLoS. One._ 10, e0135774 (2015). Article PubMed PubMed Central Google Scholar * Luo, W. _et al_. _M_icroglial internalization and degradation of pathological tau is enhanced
by an anti-tau monoclonal antibody. _Sci. Rep._ 5, 11161 (2015). Article ADS PubMed PubMed Central Google Scholar * Funk, K. E., Mirbaha, H., Jiang, H., Holtzman, D. M. & Diamond,
M. I. Distinct Therapeutic Mechanisms of Tau Antibodies: Promoting Microglial Clearance Versus Blocking Neuronal Uptake. _J. Biol. Chem._ 290, 21652–21662 (2015). Article CAS PubMed
PubMed Central Google Scholar * Schroeder, S. K., Joly-Amado, A., Gordon, M. N. & Morgan, D. Tau-Directed Immunotherapy: A Promising Strategy for Treating Alzheimer’s Disease and Other
Tauopathies. _J._ _Neuroimmune_ _Pharmacol._ 11, 9-25 (2016). * Congdon, E. E. _et al_. _A_ffinity of Tau antibodies for solubilized pathological Tau species but not their immunogen or
insoluble Tau aggregates predicts _in vivo_ and _ex vivo_ efficacy. _Mol. Neurodegener._ 11, 62–85 (2016). Article PubMed PubMed Central Google Scholar * Liu, W. _et al_. _V_ectored
Intracerebral Immunization with the Anti-Tau Monoclonal Antibody PHF1 Markedly Reduces Tau Pathology in Mutant Tau Transgenic Mice. _J. Neurosci._ 36, 12425–12435 (2016). Article CAS
PubMed Google Scholar * Pedersen, J. T. & Sigurdsson, E. M. Tau immunotherapy for Alzheimer’s disease. _Trends Mol. Med._ 21, 394–402 (2015). Article CAS PubMed Google Scholar *
Castillo-Carranza, D. L. _et al_. _T_au immunotherapy modulates both pathological tau and upstream amyloid pathology in an Alzheimer’s disease mouse model. _J. Neurosci._ 35, 4857–4868
(2015). Article CAS PubMed Google Scholar * Mably, A. J. _et al_. _T_au immunization: a cautionary tale? _Neurobiol. Aging_ 36, 1316–1332 (2015). Article CAS PubMed Google Scholar *
Rosenmann, H. _et al_. _T_auopathy-like abnormalities and neurologic deficits in mice immunized with neuronal tau protein. _Archives of Neurology_ 63, 1459–1467 (2006). Article PubMed
Google Scholar * Rozenstein-Tsalkovich, L. _et al_. _R_epeated immunization of mice with phosphorylated-tau peptides causes neuroinflammation. _Exp. Neurol._ 248, 451–456 (2013). Article
CAS PubMed Google Scholar * Oddo, S. _et al_. _T_riple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Aβ and synaptic dysfunction. _Neuron_ 39, 409–421
(2003). Article CAS PubMed Google Scholar * Oddo, S., Billings, L., Kesslak, J. P., Cribbs, D. H. & LaFerla, F. M. Aβ immunotherapy leads to clearance of early, but not late,
hyperphosphorylated tau aggregates via the proteasome. _Neuron_ 43, 321–332 (2004). Article CAS PubMed Google Scholar * Walls, K. C. _et al_. _p_-Tau immunotherapy reduces soluble and
insoluble tau in aged 3xTg-AD mice. _Neurosci. Lett._ 575, 96–100 (2014). Article CAS PubMed PubMed Central Google Scholar * Dai, C. L., Tung, Y. C., Liu, F., Gong, C. X. & Iqbal,
K. Tau passive immunization inhibits not only tau but also Aβ pathology. _Alzheimers. Res. Ther._ 9, 1 (2017). Article PubMed PubMed Central Google Scholar * Boutajangout, A., Li, Y. S.,
Quartermain, D. & Sigurdsson, E. M. Cognitive and sensorimotor tasks for assessing functional impairments in mouse models of Alzheimer’s disease and related disorders. _Methods Mol
Biol_ 849, 529–540 (2012). Article CAS PubMed Google Scholar * Davenport, J. W., Hagquist, W. W. & Rankin, G. R. Symmetrical Maze - An Automated Closed-Field Test Series for Rats.
_Behavior Research Methods & Instrumentation_ 2, 112–118 (1970). Article Google Scholar * Hebb, D. O. & Williams, K. A. A method of rating animal intelligence. _J. Gen. Psychol._
34, 59–65 (1946). Article CAS PubMed Google Scholar * Rabinovitch, M. S. & Rosvold, H. E. A closed-field intelligence test for rats. _Can. J psychol._ 5, 122–128 (1951). Article CAS
PubMed Google Scholar * Sigurdsson, E. M., Scholtzova, H., Mehta, P. D., Frangione, B. & Wisniewski, T. Immunization with a non-toxic/non-fibrillar amyloid-β homologous peptide
reduces Alzheimer’s disease associated pathology in transgenic mice. _Am. J. Pathol._ 159, 439–447 (2001). Article CAS PubMed PubMed Central Google Scholar * Sigurdsson, E. M. _et al_.
_A_n attenuated immune response is sufficient to enhance cognition in an Alzheimer’s disease mouse model immunized with amyloid-β derivatives. _J Neurosci._ 24, 6277–6282 (2004). Article
CAS PubMed Google Scholar * Sigurdsson, E. M., Lorens, S. A., Hejna, M. J., Dong, X. W. & Lee, J. M. Local and distant histopathological effects of unilateral amyloid-β 25-35
injections into the amygdala of young F344 rats. _Neurobiol. Aging_ 17, 893–901 (1996). Article CAS PubMed Google Scholar * Rajamohamedsait, H. B. & Sigurdsson, E. M. Histological
Staining of Amyloid and Pre-amyloid Peptides and Proteins in Mouse Tissue. _Methods Mol Biol_ 849, 411–424 (2012). Article CAS PubMed Google Scholar * Sigurdsson, E. M., Lee, J. M.,
Dong, X. W., Hejna, M. J. & Lorens, S. A. Bilateral injections of amyloid-β 25-35 into the amygdala of young Fischer rats: Behavioral, neurochemical, and time dependent histopathological
effects. _Neurobiology of Aging_ 18, 591–608 (1997). Article CAS PubMed Google Scholar * Asuni, A. A. _et al_. _V_accination of Alzheimer’s model mice with Aβ derivative in alum
adjuvant reduces Aβ burden without microhemorrhages. _Eur. J Neurosci._ 24, 2530–2542 (2006). Article PubMed PubMed Central Google Scholar * Billings, L. M., Oddo, S., Green, K. N.,
McGaugh, J. L. & LaFerla, F. M. Intraneuronal Aβ causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. _Neuron_ 45, 675–688 (2005). Article CAS
PubMed Google Scholar * Webster, S. J., Bachstetter, A. D., Nelson, P. T., Schmitt, F. A. & Van Eldik, L. J. Using mice to model Alzheimer’s dementia: an overview of the clinical
disease and the preclinical behavioral changes in 10 mouse models. _Front Genet._ 5, 88 (2014). Article PubMed PubMed Central Google Scholar * Krishnaswamy, S. _et al_.
_A_ntibody-derived _in vivo_ imaging of tau pathology. _J. Neurosci._ 34, 16835–16850 (2014). Article PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS EMS was
supported by NIH R01 grants NS077239, AG032611 and AG020197 and in part by R24OD18340 and R24OD018339 during these experiments. We thank Frank LaFerla and Salvatore Oddo for providing
breeding pairs of the 3xTg and wt mice, and Peter Davies for the tau antibodies, CP27, PHF1 and MC1. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Departments of Neuroscience and Physiology,
New York University School of Medicine, 550 First Avenue, New York, NY, 10016, United States Hameetha Rajamohamedsait, Suhail Rasool, Wajitha Rajamohamedsait, Yan Lin & Einar M.
Sigurdsson * Departments of Psychiatry, New York University School of Medicine, 550 First Avenue, New York, NY, 10016, United States Einar M. Sigurdsson Authors * Hameetha Rajamohamedsait
View author publications You can also search for this author inPubMed Google Scholar * Suhail Rasool View author publications You can also search for this author inPubMed Google Scholar *
Wajitha Rajamohamedsait View author publications You can also search for this author inPubMed Google Scholar * Yan Lin View author publications You can also search for this author inPubMed
Google Scholar * Einar M. Sigurdsson View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS E.M.S. designed the experiments, analyzed the data and
wrote the paper; H.R. performed the immunizations, antibody measurements, behavioral analysis, brain sectioning and immunohistochemistry. S.R. performed the western blots and related
analysis, and participated in the histological analysis. W.R. assisted H.R. and S.R. Y.L. performed immunohistochemistry, western blots and related analyses. CORRESPONDING AUTHOR
Correspondence to Einar M. Sigurdsson. ETHICS DECLARATIONS COMPETING INTERESTS E.M.S. is an inventor on patents on tau immunotherapy and related diagnostics that are assigned to New York
University. This technology is licensed to and is being co-developed with H. Lundbeck A/S. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY FIGURES - ORIGINAL BLOTS RIGHTS AND PERMISSIONS OPEN ACCESS This
article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as
you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party
material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s
Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Rajamohamedsait, H., Rasool, S.,
Rajamohamedsait, W. _et al._ Prophylactic Active Tau Immunization Leads to Sustained Reduction in Both Tau and Amyloid-β Pathologies in 3xTg Mice. _Sci Rep_ 7, 17034 (2017).
https://doi.org/10.1038/s41598-017-17313-1 Download citation * Received: 10 July 2017 * Accepted: 07 November 2017 * Published: 06 December 2017 * DOI:
https://doi.org/10.1038/s41598-017-17313-1 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative