Play all audios:
ABSTRACT The composition of the maturing coffee bean determines the processing performance and ultimate quality of the coffee produced from the bean. Analysis of differences in gene
expression during bean maturation may explain the basis of genetic and environmental variation in coffee quality. The transcriptome of the coffee bean was analyzed at three stages of
development, immature (green), intermediate (yellow) and mature (red). A total of more than 120 million 150 bp paired-end reads were collected by sequencing of transcripts of triplicate
samples at each developmental stage. A greater number of transcripts were expressed at the yellow stage. As the beans matured the types of highly expressed transcripts changed from
transcripts predominantly associated with galactomannan, triacylglycerol (TAG), TAG lipase, 11 S and 7S-like storage protein and Fasciclin-like arabinogalactan protein 17 (FLA17) in green
beans to transcripts related to FLA1 at the yellow stage and TAG storage lipase SDP1, and SDP1-like in red beans. This study provides a genomic resource that can be used to investigate the
impact of environment and genotype on the bean transcriptome and develop coffee varieties and production systems that are better adapted to deliver quality coffee despite climate variations.
SIMILAR CONTENT BEING VIEWED BY OTHERS THE BHLH TRANSCRIPTION FACTOR VFTT8 UNDERLIES _ZT2_, THE LOCUS DETERMINING ZERO TANNIN CONTENT IN FABA BEAN (_VICIA FABA_ L.) Article Open access 31
August 2020 TRANSCRIPTOME ANALYSIS OF GENES INVOLVED IN STARCH BIOSYNTHESIS IN DEVELOPING CHINESE CHESTNUT (_CASTANEA MOLLISSIMA_ BLUME) SEED KERNELS Article Open access 11 February 2021
GENOME-WIDE IDENTIFICATION AND EXPRESSION ANALYSIS OF _ACS_ AND _ACO_ GENE FAMILY IN _ZIZIPHUS JUJUBA_ MILL DURING FRUIT RIPENING Article Open access 24 May 2025 INTRODUCTION Arabica coffee
(around 70% of world coffee consumed), is one of the most important and valuable commodities traded internationally1. The coffee bean is a tropical dicotyledonous albuminous seed, with a
copious endosperm and a tiny embryo. Generally, seeds have evolved intricate strategies to reserve nutrients and take advantage of the pericarp and pulp to encourage dispersal by animals2.
The composition of the coffee bean determines the quality of the coffee. An improved understanding of the molecular basis of determination of bean composition is required to support enhanced
coffee production and genetic improvement especially in response to climate change. Numerous biochemical studies have been conducted on the genetic and environment factors influencing the
accumulation of key components of the bean3,4. Genetic control of these processes can be investigated by the study of changes in gene expression through bean ripening, including transcripts
regulating bean filling as well as in response to stress2,5,6. Early studies applied RT-PCR (coffee beans) or microarrays to mainly coffee leaves or seedlings5,7,8. More recently, different
tissues of Arabica coffee including flowers, leaves and fruit pericarp have been subjected to transcriptome analysis. A genome wide study recently reported identification of key genes
regulating the lipid and diterpene contents of Arabica coffee bean4. However, analysis of the genetics of other essential components of the bean contributing to coffee quality was not
included in these studies9. Importantly, the absence of a reference genome or transcriptome further limited previous studies. Additionally, gaps remain in knowledge of gene interactions and
how expression varies at the global scale at specific ripening stages and finally determines the quality and regeneration capability of the bean. In this study, different development stages,
green, yellow and red coffee beans (other than exocarp or mesocarp) were collected and the RNA was sequenced for transcriptome analysis. A recent long read sequencing full-length (LRS)
coffee bean transcriptome was used as a reference to facilitate transcriptome analysis in the ripening coffee bean10. The aim was to understand the progress of accumulation of the key
component in the coffee bean through ripening and the molecular basis of genetic regulation of coffee quality and deliver a platform for the study of genotypic and environmental influences
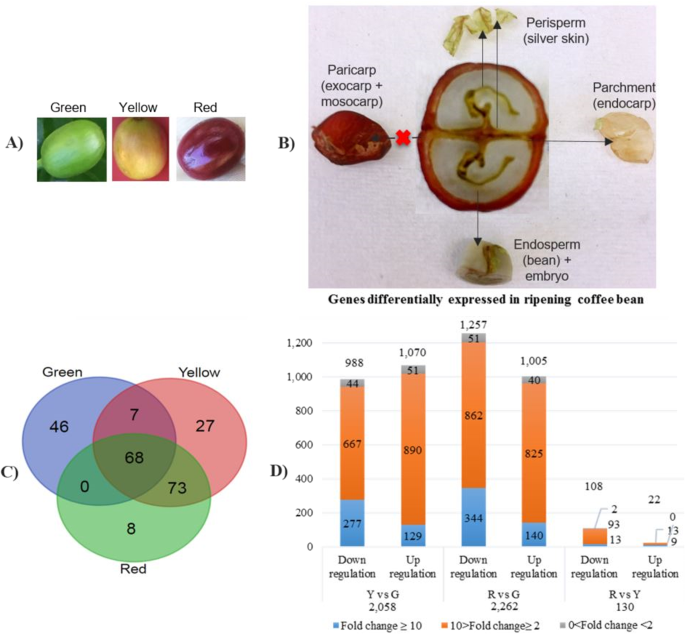
on the coffee transcriptome and coffee quality. RESULTS Nine coffee bean transcriptome datasets were generated in this study, including green, yellow and red stages, all in triplicate (Fig.
1a). As pericarp (including exocarp and mesocarp) was removed before RNA extraction, the coffee bean in this study refers to endocarp, perisperm (seed coat), endosperm and embryo
(encapsulated by endosperm) (Fig. 1b). RAW READ PROCESSING A total of 120,784,786 reads were generated from the nine coffee bean transcriptome datasets (Supporting Information Table S1).
This number was slightly reduced (117,539,360) after trimming. For individual samples, the number of trimmed reads were between 4,602,943 and 23,905,608. A range of 48.37% and 64.05% of
reads were mapped to the long-read sequencing coffee bean transcriptome (coffee LRS transcriptome). The yellow stage has the highest number of genes expressed (43,552 transcripts, TPM >
1), compared to red and green stages (43,257 and 36,388 transcripts) (Supporting Information Fig. S1). Functional annotation of the expressed transcripts revealed that GO terms associated
with the metabolic process, catalytic activity and cell part, ranked as the most abundant in “biological process”, “molecular function” and “cellular components” respectively (Supporting
Information 1 Fig. S2). The lower number of transcript expression was observed at green stage. The top three pathways with the most transcripts expressed were purine pathway, thiamine
metabolism, etc (Supporting Information 1 Table S2). Starch and sucrose metabolism and phenylpropanoid biosynthesis were the sixth and ninth most enriched pathway. The top 30 pathways with
the highest number of transcripts expressed were from 11 parent-pathway groups. The dominant parent-pathway group was carbohydrate metabolism, enriched with the highest number of pathways
(seven pathways), including starch and sucrose metabolism and galactose metabolism. This is followed by the amino acid and lipid metabolism parent-group, which relating to five and three
metabolism pathways, individually. HIGHLY EXPRESSED TRANSCRIPTS THROUGH COFFEE BEAN RIPENING OVERVIEW Of all the HEGs, more unique transcripts were expressed over TPM500 at the green stage
(46 transcripts) other than yellow and red stages (27 and 8) (Fig. 1c). A total of 68 common transcripts were expressed at all the three development stages. A significantly higher number of
common transcripts (73) were expressed at both the yellow and red stages, while the number of common transcripts (seven) expressed at the green and the red stages were the same as that of
the first two stages. INTENSIVE LIPIDS FORMATION AT THE GREEN STAGE The top ten HEGs in green, yellow and red coffee beans were extracted individually for further analysis (Table 1). Other
than three unnamed protein products, a ncRNA was one the most abundant transcript at all stages, peaking at the green stage and decreasing at the last two stages. Two non-specific lipid
transfer (LTP) A-like transcripts, showed extremely high expression in green coffee beans (33,227 and 18,563) but decreased dramatically in yellow stage (5,488 and 1,446) until maturity
(1,260, 378 and 1,165). This suggested a high level of lipids may accumulate and transported from this stage. A similar drop was also identified in alpha-galactosidase 2, from 10,120 in the
green stage to more than ten times lower in yellow stage (830) and red stage (351). Fewer changes were characterized in transcripts encoding 11S storage globulin (bean storage protein) and
metallothionein type 3 (important for bean development), presenting maximum expression in green coffee beans (9,994 and 7,709 respectively), while gradually dropped until red coffee beans
(4,059 and 6,829 respectively). Transcripts encoding for kirola-like protein and dehydrin DH1a (response to desiccation), peaked at the yellow stage and decreased slightly in red stage. MORE
CHANGES IN THE COMPARISON OF THE RED VS GREEN STAGE Three comparisons between developmental stages were conducted in this study of the ripening Arabica coffee bean transcriptome, yellow vs
green (Y vs G), red vs green (R vs G) and red vs yellow (R vs Y) included. The highest number of differentially expressed transcripts (DEGs) were shown in the R vs G comparison (2,262),
including the most downregulated DEGs (1,257) (Fig. 1d). Only 130 DEGs were seen in R vs Y, including 108 downregulated genes and 22 upregulated genes. A total of 2,058 DEGs were
characterized in Y vs G, with the most up-regulated DEGs (1,070). The majority of DEGs were distributed within the range of two to ten times fold change, while only a few varied less than
two times fold change. The top ten up and down-regulated DEGs from individual comparisons were extracted in Table 2 to understand the most significant changes in coffee bean ripening. These
transcripts included pathogen relevant, cell function and key chemical biosynthesis transcripts. In the comparison of Y vs G, two class III chitinase (chi2) transcripts were highly expressed
in green coffee beans and were absent (TPM < 1) in yellow stage. Similarly, a dramatic decrease was shown in a lipid degradation transcript (controls GDSL esterase lipase APG-like
protein), an O-fucosyltransferase family transcript (relating to mannan biosynthesis and galactomannan accumulation), an amino acid transport transcript (_WAT1-related protein-like_), and a
cellular function transcript (regulating ubiquitin 40S protein S27a). The ubiquitin 40S protein S27a related transcript also demonstrated a significant change in the comparison of R vs G.
Additionally, a significant decrease from green to red coffee beans was characterized in a cell wall vascular inhibitor of fructosidase 1-like (_vacuole invertase inhibitor_). In the
comparison of R vs Y, E3 ubiquitin- ligase SHPRH isoform X1, Luminal-binding 5-like, cinnamoyl- reductase 2-like etc. degraded in red stage. Numerous MYB transcription factors (_MYB90)_ were
identified as upregulated DEGs in top ten DEGs of all comparisons, increasing from green to maturity (maximum expression was TPM: 686). A trans-resveratrol di-O-methyltransferase-like
transcript, catalyzing pterostilbene (antifungal and pharmacological function) biosynthesis and a transposon elements variant gene (_retrotransposon Ty1-copia subclass_) were upregulated in
both comparison of Y vs G and R vs G as they increased sharply from green to red stage. From the green stage, cell wall degradation DEGs, such as probable rhamnogalacturonate lyase B
(probably pectin degradation) and probable xyloglucan endotransglucosylase hydrolase B (_XTHB_, cleaving primary cell wall xyloglucan polymers and contributing to the construction of the
growing tissues) were upregulated at the yellow stage. Meanwhile, transcript expression of the pectinesterase inhibitor 11 transcript, maintaining the integrity of cell walls, was increased
and peaked at the yellow stage. Steady growth was detected in beta-glucosidase 44-like, lipid transfer and pathogenesis-related 1 transcripts. Moreover, the comparison of R vs Y included
cell function transcripts, like _Luminal-binding_ 5_-like_, _endonuclease V_ and three transcripts probably related to phenolic compounds, _cinnamoyl- reductase 2-like_. STORAGE COMPOUNDS
ACCUMULATED THROUGH BEAN MATURITY An association study of the key storage component related DEGs was conducted with lipid, cell wall component, storage protein and phenylpropanoid related
DEGs in ripening coffee bean (Fig. 2). Major changes were observed in the comparison of Y vs G and R vs G. More DEGs were assigned in the comparison of R vs G, while only a few shown in a
comparison of the last two stages (R vs Y). MAJOR LIPIDS ACCUMULATION AT THE GREEN STAGE The main lipid-related DEGs went through a decrease in expression compared to levels in green coffee
beans, especially fatty acid desaturation (omega-6-desaturase, critical for biosynthesis of linoleic acid), TAG synthase (oil body oleosin family proteins) and lipase (for TAG degradation)
(Supporting Dataset). One omega-6-desaturase declined dramatically (log2 ratio: −6.10) at the yellow stage in comparison with the green stage (Supporting Dataset). Similarly, non-specific
LTP protein decreased sharply (maximum log2 ratio: −8.16 in R vs G) compared to the green stage, supporting the idea of lipids predominantly accumulating in green beans. Only a limited
number of DEGs remained in the comparison of the last two stages, and they were all down-regulated. This includes three non-specific lipid transfer proteins (phospholipid transfer protein)
and four GDSL-like Lipases. Therefore, TAG is likely to be synthesized in green beans, which accompanies its degradation by lipase. Linoleic acid was also apparently formed in green bean.
THE FLOW OF CELL WALL COMPONENTS Most down-regulated DEGs in the comparison of Y vs G were found to relate to cell wall precursor synthase, hemicellulose synthase (only downregulation),
cellulose synthase and arabinogalactan protein (AGPs) DEGs (Supporting Dataset). Only one UDP-XYL synthase (_USX_, UDP-D-xylose biosynthesis) transcript 2-like was upregulated in Y vs G and
R vs G in cell wall precursor DEGs. The others were downregulated either in Y vs G or R vs G. Mannose-1-phosphate guanylyltransferase (_CYT_, involved in GDP-mannose biosynthesis) was
downregulated in the last two stages. Downregulations include nucleotide-rhamnose synthase/epimerase-reductase, _USX6_ and _USX6-like_, UDP-glucose 6-dehydrogenase 5 (provides nucleotide
sugars for cell-wall polymers). Decreased cellulose synthase DEGs are transcript 1 and 2 (_CESA1, 2_), COBRA-like protein 1, _COBRA-like_ and CESA-like transcript (mannan synthase 1-_MS1_,
which peaks in the green stage), while upregulation was seen in two CESA-like transcripts with upregulation observed in _MS2_ (peaking at the yellow stage). AGP related DEGs were
downregulated in _FLA17_ and upregulated as was seen in _FLA1_ (top expression in yellow stage). Hemicellulose related DEG, regulating hydroxyproline O-galactosyltransferase 6 (translocates
galactose from UDP-galactose to the residues of AGPs), declined in the yellow and red stages compared to the green stage. However, the only increases in transcript expression were shown in
the comparison of Y vs G and R vs G in _cellulase_ (_CEL3_ and 5) and _beta 1,4-glucanase_ (such as transcript 6), with a peak expression in the yellow stage. Leucine-rich protein (LRR)
associated DEGs were also seen to rise in Y vs G and R vs G. A large number of DEGs were found in pectin degradation (pectin esterases, lyses and pectinases), which were mainly upregulated
in Y vs G and R vs G. A major rise of transcript expression was also observed in other cell wall modification DEGs including numerous expansin (and expansin-like) transcripts (isoform 6, 8,
10, 11 and 15) and XTH transcripts (isoform B, 2, 6, 12, 23, 30). Expression of _BGAL-like_, probable beta-D-xylosidase 7 and _mannan endo-1,4-beta-mannosidase 5-like_ (one transcript
variant was upregulated) DEGs declined at the yellow stage. Similar patterns continued in the second comparison, R vs G. A higher number of DEGs were associated with cell wall precursor
synthases, cellulose synthases, cell wall modifications and mannan degradation. However, in the last comparison, R vs Y, all DEGs were downregulated. Altogether, cellular precursors,
hemicellulose, _FLA17_, _CESA1_ and _CESA2_ and _MS1_ increased relative to the highest expression at the green stage. Peak expression shifted to cellular degradation (_CEL3_ and _5_) and
_MS2_ at the yellow stage and pectin degradation and LRR in the last two stages. MAJOR STORAGE PROTEIN ACCUMULATION AT THE GREEN STAGE In terms of storage protein related DEGs, both up and
down regulations were identified in both Y vs G and R vs G comparisons, while no DEGs were assigned in the comparison of R vs Y (Supporting Dataset). Altogether, significant decreases were
identified in eight storage protein related DEGs in the yellow and red stage compared to green coffee beans. They were four 11 s globulin (_11S_), three 7S-like globulin transcripts
(_7S-like_) and glutelin type A2 (_GLUA2_) DEGs. In addition to these eight DEGs, one more _11S_ and a patatin (_Pat_) 2 also decreased in the red stage in contrast to green beans. In
contrast, upregulation was observed in three _Pat6_, _GLUA3_, TAG lipase _SDP1_, _SDP1-like_ (storage lipids degradation in bean germination) and _SDP1-like_ DEGs in the comparisons of Y vs
G and R vs G (larger fold change at the red stage). Transcripts regulating these proteins are likely to peak at maturity. One more _GLU_, type B5 (_GLUB5_), was also more highly expressed at
the red stage rather than in green coffee beans. Hence, _11S_, _7S-Like_ and _GLUA2_ storage probably occurred since the green stage, while _SDP1_, _SDP1-like_, GLUA3, _Pat6_, accumulated
at the end. PHENYLPROPANOIDS Most CGAs related transcripts peaked at the yellow stage, with a significant increase since the green stage and decreased dramatically in the red stage (FDR
corrected p-value < 0.001). Exceptions were phenylalanine ammonia-lyase (_PAL_) 4 and 4-coumarate CoA ligase (_4CL_) 7, which increased from green coffee beans till maturity. This
suggested that CGAs were likely to be mainly formed from the yellow stage and _4CL7_ probably contributes to further accumulation of CGAs or lignin in later stages. CO-EXPRESSION NETWORK OF
THE BEAN STORAGE GENES Four groups of transcripts were targeted for co-expression network analysis, including lipid, cell wall, storage protein and phenylpropanoids DEGs (Fig. 3). The aim
was to investigate the connections among key bean storage/quality attributed transcripts. A great number of the cell wall and phenylpropanoids DEGs were filtered out in the co-expression
module (weight ≥0.9), followed by lipid, other metabolites, and storage protein related transcripts. Different transcripts of alpha-expansin transcripts (_EXPA_) played a key role in this
network module, especially _EXPA6_3_ (_EXPA6_3_ transcript variant 3). _EXPA6_3_ was co-expressed with 22 transcripts from all five categories, mainly cell wall-related. The top five
connected transcripts to _EXPA6_3_ were _EXPA_ 8_2 (0.9709), probable xyloglucan endotransglucosylase hydrolase 30 (_XTH30_2_, 0.9681), probable pectin lyase 8 (_PL8_1_, 0.9402),
polygalacturonase-like (_PG_2_), _EXPA11-like_ (0.9399), etc. Another core transcript connected with all five transcript groups was _PG_2_. _PG_2_, identified as being connected to
cinnamoyl- reductase 2 (_CCR2_), beta-xylosidase/alpha-L-arabinofuranosidase 2-like (_Xyl2-like_2_) and shikimate O-hydroxycinnamoyltransferase transcript a (_HCTa_). This suggested _PG_2_
is essential in cell wall modification and probably interacts with phenylpropanoid and phenolic biosynthesis. A lipid DEG, _CP5_3_, functions as a membrane related protein and was one of the
pivotal transcripts in the co-expression network. The top five co-expression were from different groups, comprise of _CCR2_, _EXPA8_1_, _PG_2_, _11S_2_, etc. In addition, 3-ketoacyl-CoA
synthase 11-like (_KCS11-like_, biosynthesis of very long chain fatty acids), unanimously expressed with transcripts from all categories, was another central transcript from the module. The
co-expressed transcripts were _CP5_3_, non-specific phospholipase C2, cytochrome P450 71D8, EXPA8_1, _CCR2_, etc. The relationship of these diverse transcripts suggested that _CP5_3_ and
_KCS11-like_ are essential in bean ripening and nutrient reservation. _CCR2_ (biosynthesis of phenolics), was among the most important transcripts from the network. Other than the above
connections, _CCR2_ was also associated with diverse categories with the highest impact on _EXPA6_4_, _Xyl2-like_2_, and _EXPA8_1_. Hence, it is likely _CCR2_ is pivotal to cell wall
expansion and modification. The next important phenylpropanoids related DEGs was _HCTa_, correlating to _EXPA8_1_, _Pat6_3_, _MS2_1_, and _7S-like_1_, indicating a key role in cell wall
expansion and storage protein biosynthesis. Very few storage protein related transcripts were distributed in this co-expression network module and they were co-expressed with a lower number
of transcripts. Among them, _SDP1_ and _11S_ were relatively important. _SDP1_ expression was also found to be concurrent with _SKU5_ similar 5 (_sks5_1_), _XTH30_2_, _CCR2_ and _HCTa_,
while 11S correlated with phosphoinositide phosphatase SAC3 (_SAC3_1_), _CCR2_, _SDP1-like_, and _KCS11-like_. DISCUSSION As a storage tissue, nutrients are formed and stored through
complicated pathways in beans to support the embryo development and reproduction of the plant. Arabica coffee beans are composed of cell wall polysaccharides (CWP, 50% of the dry mass),
lipids (13–17%), proteins (11–15%), sucrose (7–11%), and CGAs (5–8%)3,4,11. The major CWP in the young coffee bean, comprise arabinogalactan (~50%), cellulose, pectic polymers (~20%) and
galactomannans (10%)5,11,12. However, at maturity, this has changed to arabinogalactan-proteins (~30%, AGPs), cellulose (15%), pectic polymers (~5%) and galactomannan (50%)12,13. Lipids were
reported to be mainly composed of triacylglycerol (TAG, 70–80% of lipids), while the major storage protein was identified as 11S globulin (45%)5,11,13. This study reveals the peak
expression of candidate transcripts related to major storage compounds was in green beans at the initiation of the storage phase, for example, galactomanan, FLA17, TAG, linoleic acid, 11S,
7S-like and glutelin A2. The main accumulation of FLA1 started at the yellow stage. Transcripts encoding SDP1, SDP1-like, Glueteline A3, Pat2, storage proteins reached a maximum expression
at the red stage. In addition, pectin was mainly degraded at the yellow and red stages. The lower number but wider range of HEGs from green coffee beans was probably associated with the
initiation of the storage phase, where a large number of key components were formed predominantly at this stage. Galactomannan is a typical storage compound for legumes (>30% of the bean
dry weight), coffee, and palms14. Galactomannan can react with proteins through Millard reactions to produce volatile components, contributing to the coffee flavour15. The high viscosity of
the remaining galactomannan accounts for the texture, namely “body”, of the coffee beverage16. For the plant itself, the low solubility and high viscosity galactomannans are a stable storage
reserve that provides strong cells to prevent osmotic stress, microbial attack or mechanical damage14,17. In seed germination, galactomannans were degraded by α-galactosidase (encoded by
_gal_), endo-β-mannanase and β-mannosidase to provide carbon and energy for embryo development14,18. Golublins such as 11S and 7S are major storage proteins in legumes19,20. Glutelins and
prolamins are the predominant proteins in cereals, such as rice (>60%)21. 11S globulin was found to be the major storage protein in coffee11. In addition, this study suggested the
presence of other potential storage proteins of 7S-like, SDP1, SDP1-like, glutelinA2, A3, and Patatin 6. In contrast, the major storage proteins of the exalbuminous seed of Arabidopsis,
mainly 2S and 12S protein (one-third of the total protein) were mainly formed late in bean development22. Decreased accumulation through ripening was also shown in the TAG and linoleic acid
(fatty acids desaturation) storage in coffee and Arabidopsis seeds (stored at the late stage)23. Different storage pattern may result from a different structure (copious endosperm in the
coffee bean) and function of bean tissues. In Arabidopsis, TAG storage lipase SDP1 and SDP1-like were found to catalyze more than 90% of the TAG in seed germination24. The accumulation of
these two lipase at the end of maturity is likely to avoid unnecessary degradation of storage reserves, TAG. The higher number of transcripts expressed at the yellow stage together with more
DEGs compared to the green stage indicated a great shift at the yellow stage. A slight decrease in the number of transcripts expressed at the red stage as well as the small number of DEGs
(mostly down-regulated) compared to the yellow stage, demonstrates bean maturity and fewer changes in coffee beans when the pericarp changed from yellow to red. This also indicates the end
of bean maturity. Fewer components accumulated in the last two stages suggesting a possible shift to modification, such as cell wall degradation (pectin and cellulose degradation). Some or
all of the coffee bean arabinogalactans were combined with proteins and present as AGPs25. This structure is typical in plant cell walls and has an important role in intercellular signaling
and wound sealing (as a glue)26. Different transcripts of AGPs accumulated (_FLA17_ at the early stage with _FLA1_ in later stages) indicating various function through ripening in various
tissues (endosperm, perisperm and embryo). Pectinesterase, lyase, and polygalacturonase catalyze pectin degradation, modifying cell walls through demethylesterification and depolymerization
of pectin27,28,29,30. The decrease in pectin in the ripening coffee bean reaches a peak after the yellow coffee bean stage. This parallels the transcripts expressed in fleshy fruit, such as
transcripts encoding polygalacturonase in Arabidopsis, which are highly expressed in ripe fruits (pericarp and mesocarp) as they soften31. In addition, dilution by the accumulation of other
key compounds during bean ripening is probably another reason for the decline of pectin content. Importantly, this study suggested expansin transcripts (especially _EXPA6_3_) were essential
in bean ripening and storage. The higher number of the cell wall and phenylpropanoids related transcripts filtered with the storage DEGs module reveals the close relationship of transcripts
from these two groups. Multiple alpha-expansin transcripts from the core co-expression network connected to transcripts from different transcript categories indicating their diverse
functions in bean storage. Expansins loosen cell walls during plant growth and allow responses to the plant growth hormone, auxin32. There were four expansin subfamily members, _EXPA_
(acid-induced), _EXPB_ (beta-expansin), _EXPA-like_ and _EXPB-like_33. It was proposed that wall loosening by expansins may be involved with a breakdown of non-covalent binding of cellulose
microfibrils. This results in turgor driven polymer movement that may be inhibited by some polysaccharide-binding proteins33,34. However, the exact mechanism of expansin action remains
unknown. In this study, expansin related DEGs were _EXPA_ or _EXPA-like_. Consistently, core transcripts from the co-expression network, _EXPA6_3_, were highly stimulated with probable
xyloglucan endotransglucosylase 30 (degradation of xyloglucan polymer). Co-expression was also shown with pectin and xylan degradation transcripts (_PL8_, _PG_2_ and _Xyl2-like_), suggesting
pectin and hemicellulose are potentially targeted by expansins or involved in the cell wall expansion phase. Other than cell wall-related transcripts, _CP5_, _SDP1_ and _HCTa_, were
concurrent with _EXPA6_3_ suggesting a diverse interaction of _EXPA6_3_. When cell walls expand, they became vulnerable. This may result in the co-expression of _HCTa_, SDP1 and _CP5_ (lipid
transfer). Plants cannot move; therefore, they have evolved numerous strategies to survive, mature and regeneration. As the bean grows to become a nutrition factory, other species like
predators (virus or insects) are also interested in consuming the storage compounds generated. However, plants have evolved multiple strategies to protect beans from damage and recover from
damage. For example, galactomannan and the lignified endocarp provide a physical barrier to prevent bean from being digested by predators but dispersed after consumption of the pulp35.
Different HEGs and DEGs characterize the complicated strategy evolved in the dicotyledonous albuminous coffee beans. Stress-related transcripts are more highly expressed in the green coffee
beans, which have the protection of a thick and firm lignified pericarp. However, to protect against pathogens such as fungi, specific transcripts were expressed in green coffee beans. This
was supported by the peak expression of chi2, galactomannan at this stage. The gradually hardening of endosperm then takes over the protection of the embryo and endosperm. Assorted
transcripts were expressed at the yellow stage in coffee beans. As the coffee pericarp became soft and its color changed to be distinguished at the yellow stage. The brighter color (red) of
the coffee pericarp attracts animals to consume the pulp but to disperse intact beans it requires more stress-responsive transcripts, which were evidenced in this research. This coincidence
with digressive metabolite biosynthesis upon maturity and fewer growth transcripts. The beta-glucosidase 44-like transcript is a typical example of a plant stress resistance gene, expressed
at maximum level in the ripe coffee beans. It was highly expressed in the Arabica coffee bean to activate chemical defense compounds once the cell is being attacked36,37. Another
representative case is _actin-7_, highly expressed in red bean, related to growth and required for callus formation and response to wounding38. In conclusion, this study provides insights
into the sequence of accumulation of the key components in the ripening of the bean (Fig. 4). Importantly, the co-expression network illustrated the importance in bean storage and ripening
of expansin A transcripts which have a wide interaction with other key components DEGs. This information will facilitate the genetic control of these components in coffee. This ripening bean
transcriptome will also provide a platform to determine the genotype and environment influences on coffee quality. Targeted analysis is now possible to characterize gene families of
interest (e.g. transcriptional factors). Phylotranscriptomic analysis is another approach now enabled for the study of evolutionary diversity features. METHODS RNA SAMPLE AND CDNA LIBRARY
PREPARATION Coffea arabica cv. K7 cherries of different ripening stages (green, yellow and red) were collected from the upper canopy only as described previously10. Total RNA isolated from
nine samples (three development stages in triplicates) was processed individually according to Furtado _et al_.39. The integrity of total RNA was accessed with an Agilent RNA 6000 nano kit
and chips through a Bioanalyzer 2100 (Agilent Technologies, California, USA). Thereafter, a standard 18 x Truseq total RNA library preparation was conducted with the use of an additional
Ribo-Zero kit. Samples were subsequently sequenced on an Illumina HiSeq4000 platform (2 × 150 bp paired-end reads). READS MINING AND RNA-SEQ ANALYSIS Raw reads were mainly processed with CLC
Genomic Workbench 10.0.1 (CLC Bio, Denmark) as following. (1) Adapters and indexes were trimmed. (2) Reads failed matching the PHRED score (<0.01) and length (≥40 bp) were removed. (3)
RNA-Seq analysis (read similarity 0.9, length similarity 0.8) was conducted with the processed reads. A recent published long-read sequencing coffee bean transcriptome was used as a
reference with Transcripts Per Kilobase Million (TPM) as the expression parameter3. Outlier expression values, classified with coefficient variation and standard deviation, were not
considered in this study. STATISTICAL ANALYSIS Transcripts expressed at each development stage were filtered with TPM (>1). Functional annotations were analyzed with BLAST2GO for GO terms
and KEGG pathway distribution40,41. Venn diagrams were built through an online tool42. Highly expressed transcripts were filtered with TPM (>500). Differential gene expression tool for
RNA-seq (CLC) was used for significant through ripening stages. DEGs were filtered with FDR p-value correction (<0.01) and maximum group means (TPM ≥ 10). Key storage component
association with DEGs was constructed through Mercator and Mapman 3.6.0RC143,44. Co-expression network was constructed with Mapman annotated storage DEGs and candidate genes from the
targeted analysis. Gene expression of these transcripts were log2(x) transformed before analysis through WGCNA build-in Web MEV package (cutoff_0.9) and Cytoscape 3.5.1.45,46 (Note: 0.0001
was assigned to transcripts with an expression value of 0 before log2 transformation). DATA AVAILABILITY The RNA-sequencing trimmed data used in this manuscript has been submitted to EMBL
database under accession number: PRJEB24137. Please note that the two subsets of the paired-end reads were trimmed together in the data mining process. Two sub-files were generated after
trimming, paired reads and orphans. These two sub-files were combined, compressed and submitted to EMBL as “one Fastq file (Single)”. REFERENCES * Fridell, G. Coffee. (John Wiley & Sons,
2014). * Giovannoni, J., Nguyen, C., Ampofo, B., Zhong, S. & Fei, Z. The Epigenome and Transcriptional Dynamics of Fruit Ripening. _Annual Review of Plant Biology_ 68, 61–84 (2017).
Article PubMed CAS Google Scholar * Cheng, B., Furtado, A., Smyth, H. E. & Henry, R. J. Influence of genotype and environment on coffee quality. _Trends in Food Science &
Technology_ 57, 20–30 (2016). Article CAS Google Scholar * Sant’Ana, G. C. _et al_. Genome-wide association study reveals candidate genes influencing lipids and diterpenes contents in
Coffea arabica L. _Scientific reports_ 8, 465 (2018). Article PubMed PubMed Central ADS CAS Google Scholar * Joët, T. _et al_. Metabolic pathways in tropical dicotyledonous albuminous
seeds: Coffea arabica as a case study. _New Phytologist_ 182, 146–162 (2009). Article PubMed CAS Google Scholar * Li, L. _et al_. The Association of Hormone Signaling Genes,
Transcription, and Changes in Shoot Anatomy during Moso Bamboo Growth. _Plant Biotechnology Journal_ (2017). * Combes, M. C., Dereeper, A., Severac, D., Bertrand, B. & Lashermes, P.
Contribution of subgenomes to the transcriptome and their intertwined regulation in the allopolyploid Coffea arabica grown at contrasted temperatures. _New phytologist_ 200, 251–260 (2013).
Article PubMed CAS Google Scholar * Yuyama, P. M. _et al_. Transcriptome analysis in Coffea eugenioides, an Arabica coffee ancestor, reveals differentially expressed genes in leaves and
fruits. _Molecular Genetics and Genomics_ 291, 323–336 (2016). Article PubMed CAS Google Scholar * Ivamoto, S. T. _et al_. Transcriptome Analysis of Leaves, Flowers and Fruits Perisperm
of Coffea arabica L. Reveals the Differential Expression of Genes Involved in Raffinose Biosynthesis. _PloS one_ 12, e0169595 (2017). Article PubMed PubMed Central Google Scholar *
Cheng, B., Furtado, A. & Henry, R. J. Long-read sequencing of the coffee bean transcriptome reveals the diversity of full-length transcripts. _Giga Science_ 6, 1–13 (2017). Article
PubMed Google Scholar * De Castro, R. D. & Marraccini, P. Cytology, biochemistry and molecular changes during coffee fruit development. _Brazilian Journal of Plant Physiology_ 18,
175–199 (2006). Article Google Scholar * Redgwell, R. & Fischer, M. Coffee carbohydrates. _Brazilian Journal of Plant Physiology_ 18, 165–174 (2006). Article CAS Google Scholar *
Marraccini, P. _et al_. Biochemical and molecular characterization of α-D-galactosidase from coffee beans. _Plant Physiology and Biochemistry_ 43, 909–920 (2005). Article PubMed CAS
Google Scholar * Buckeridge, M. S. Seed cell wall storage polysaccharides: models to understand cell wall biosynthesis and degradation. _Plant Physiology_ 154, 1017–1023 (2010). Article
PubMed PubMed Central CAS Google Scholar * Nunes, F. M., Reis, A., Domingues, M. R. M. & Coimbra, M. A. Characterization of galactomannan derivatives in roasted coffee beverages.
_Journal of agricultural and food chemistry_ 54, 3428–3439 (2006). Article PubMed CAS Google Scholar * Viani, R. Espresso coffee: the science of quality. (Elsevier, 2004). * Morant, A.
V. _et al_. β-Glucosidases as detonators of plant chemical defense. _Phytochemistry_ 69, 1795–1813 (2008). Article PubMed CAS Google Scholar * Buckeridge, M. S. & Dietrich, S. M.
Mobilisation of the raffinose family oligosaccharides and galactomannan in germinating seeds of Sesbania marginata Benth. (Leguminosae-Faboideae). _Plant Science_ 117, 33–43 (1996). Article
CAS Google Scholar * Orruno, E. & Morgan, M. Purification and characterisation of the 7S globulin storage protein from sesame (Sesamum indicum L.). _Food Chemistry_ 100, 926–934
(2007). Article CAS Google Scholar * Rogers, W. J. _et al_. Biochemical and molecular characterization and expression of the 11S-type storage protein from Coffea arabica endosperm. _Plant
Physiology and Biochemistry_ 37, 261–272 (1999). Article CAS Google Scholar * Kusaba, M. _et al_. Low glutelincontent1: a dominant mutation that suppresses the glutelin multigene family
via RNA silencing in rice. _The Plant Cell_ 15, 1455–1467 (2003). Article PubMed PubMed Central CAS Google Scholar * Kroj, T., Savino, G., Valon, C., Giraudat, J. & Parcy, F.
Regulation of storage protein gene expression in Arabidopsis. _Development_ 130, 6065–6073 (2003). Article PubMed CAS Google Scholar * Santos‐Mendoza, M. _et al_. Deciphering gene
regulatory networks that control seed development and maturation in Arabidopsis. _The Plant Journal_ 54, 608–620 (2008). Article PubMed CAS Google Scholar * Eastmond, P. J.
SUGAR-DEPENDENT1 encodes a patatin domain triacylglycerol lipase that initiates storage oil breakdown in germinating Arabidopsis seeds. _The Plant Cell Online_ 18, 665–675 (2006). Article
CAS Google Scholar * Redgwell, R. J., Curti, D., Fischer, M., Nicolas, P. & Fay, L. B. Coffee bean arabinogalactans: acidic polymers covalently linked to protein. _Carbohydrate
Research_ 337, 239–253 (2002). Article PubMed CAS Google Scholar * Nothnagel, E. A., Bacic, A. & Clarke, A. E. Cell and developmental biology of arabinogalactan-proteins. (Springer
Science & Business Media, 2012). * Jiang, L. _et al_. VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract. _The
Plant Cell_ 17, 584–596 (2005). Article PubMed PubMed Central CAS Google Scholar * Micheli, F. Pectin methylesterases: cell wall enzymes with important roles in plant physiology.
_Trends in plant science_ 6, 414–419 (2001). Article PubMed CAS Google Scholar * Maríd‐Rodríds, M. C., Orchard, J. & Seymour, G. B. Pectate lyases, cell wall degradation and fruit
softening. _Journal of experimental botany_ 53, 2115–2119 (2002). Article Google Scholar * González-Carranza, Z. H., Elliott, K. A. & Roberts, J. A. Expression of polygalacturonases
and evidence to support their role during cell separation processes in Arabidopsis thaliana. _Journal of experimental botany_ 58, 3719–3730 (2007). Article PubMed CAS Google Scholar *
Rose, J. K., Catalá, C., Gonzalez-Carranza, Z. H. & Roberts, J. A. Cell wall disassembly. _Annual Plant Reviews_ 8, 264–324 (2003). CAS Google Scholar * Ding, X. _et al_. Activation of
the indole-3-acetic acid–amido synthetase GH3-8 suppresses expansin expression and promotes salicylate-and jasmonate-independent basal immunity in rice. _The Plant Cell_ 20, 228–240 (2008).
Article PubMed PubMed Central CAS Google Scholar * Cosgrove, D. J. Plant expansins: diversity and interactions with plant cell walls. _Current opinion in plant biology_ 25, 162–172
(2015). Article PubMed PubMed Central CAS Google Scholar * Cosgrove, D. J. Loosening of plant cell walls by expansins. _Nature_ 407, 321–326 (2000). Article PubMed ADS CAS Google
Scholar * Urbaneja, A., Jacas, J., Verdú, M. & Garrido, A. Dinámica e impacto de los parasitoides autóctonos de Phyllocnistis citrella Stainton, en la Comunidad Valenciana. _Invest.
Agric. Prod. Prot. Veg_ 13, 787–796 (1998). Google Scholar * Nisius, A. The stromacentre inAvena plastids: An aggregation of β-glucosidase responsible for the activation of oat-leaf
saponins. _Planta_ 173, 474–481 (1988). Article PubMed CAS Google Scholar * Halkier, B. A. & Gershenzon, J. Biology and biochemistry of glucosinolates. _Annu. Rev. Plant Biol._ 57,
303–333 (2006). Article PubMed CAS Google Scholar * McDowell, J. M., An, Y., Huang, S., McKinney, E. C. & Meagher, R. B. The Arabidopsis ACT7 actin gene is expressed in rapidly
developing tissues and responds to several external stimuli. _Plant physiology_ 111, 699–711 (1996). Article PubMed PubMed Central CAS Google Scholar * Furtado, A. In Cereal Genomics
23–28 (Springer, 2014). * Conesa, A. & Götz, S. Blast2GO: A comprehensive suite for functional analysis in plant genomics. _International journal of plant genomics_ 2008 (2008). *
Kanehisa, M. _et al_. From genomics to chemical genomics: new developments in KEGG. _Nucleic acids research_ 34, D354–D357 (2006). Article PubMed CAS Google Scholar * Bioinformatics
& Evolutionary Genomics, G. U., Belgium. _Calculate and draw custom Venn diagrams_, http://bioinformatics.psb.ugent.be/webtools/Venn/. * Thimm, O. _et al_. mapman: a user‐driven tool to
display genomics data sets onto diagrams of metabolic pathways and other biological processes. _The Plant Journal_ 37, 914–939 (2004). Article PubMed CAS Google Scholar * Lohse, M. _et
al_. Mercator: a fast and simple web server for genome scale functional annotation of plant sequence data. _Plant, cell & environment_ 37, 1250–1258 (2014). Article CAS Google Scholar
* Franz, M. _et al_. Cytoscape. js: a graph theory library for visualisation and analysis. _Bioinformatics_ 32, 309–311 (2015). PubMed PubMed Central ADS Google Scholar * _Multiple
Experiment Viewer_, http://mev.tm4.org/#/welcome. Download references ACKNOWLEDGEMENTS The authors thank Green Cauldron Coffee, Australia for providing the coffee bean samples and Poss
Reading, Marta Brozynska, Adam Healey, Tiparat Tikapunya and Hayba Badro for help with sampling. This research is supported by Australian Research Council (PROJECT ID: LP130100376) and
Chinese Scholarship Council (2014–2018). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Queensland Alliance for Agriculture and Food Innovation, The University of Queensland, Brisbane, QLD,
4072, Australia Bing Cheng, Agnelo Furtado & Robert J. Henry Authors * Bing Cheng View author publications You can also search for this author inPubMed Google Scholar * Agnelo Furtado
View author publications You can also search for this author inPubMed Google Scholar * Robert J. Henry View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS B.C., A.F. and R.H. designed this study. B.C. did the analysis and manuscript drafting. A.F. and R.H. edited the manuscript. CORRESPONDING AUTHOR Correspondence to Robert J.
Henry. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION SUPPLEMENTARY DATASET RIGHTS AND PERMISSIONS OPEN ACCESS
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as
long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third
party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the
article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright
holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Cheng, B., Furtado, A. & Henry,
R.J. The coffee bean transcriptome explains the accumulation of the major bean components through ripening. _Sci Rep_ 8, 11414 (2018). https://doi.org/10.1038/s41598-018-29842-4 Download
citation * Received: 26 January 2018 * Accepted: 16 July 2018 * Published: 30 July 2018 * DOI: https://doi.org/10.1038/s41598-018-29842-4 SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative