Play all audios:
ABSTRACT With the increasing recognition of biofilms in human disease, the development of novel antimicrobial therapies is of critical importance. For example, in patients with cystic
fibrosis (CF), the acquisition of host-adapted, chronic _Pseudomonas aeruginosa_ infection is associated with a decline in lung function and increased mortality. Our objective was to test
the _in vitro_ efficacy of a membrane-active antimicrobial peptide we designed, termed 6K-F17 (sequence: KKKKKK-AAFAAWAAFAA-NH2), against multidrug resistant _P_. _aeruginosa_ biofilms. This
peptide displays high antimicrobial activity against a range of pathogenic bacteria, yet is non-hemolytic to human erythrocytes and non-toxic to human bronchial epithelial cells. In the
present work, _P_. _aeruginosa_ strain PAO1, and four multidrug resistant (MDR) isolates from chronically infected CF individuals, were grown as 48-hour biofilms in a static biofilm slide
chamber model. These biofilms were then exposed to varying concentrations of 6K-F17 alone, or in the presence of tobramycin, prior to confocal imaging. Biofilm biovolume and viability were
assessed. 6K-F17 was able to kill biofilms – even in the presence of sputum – and greatly reduce biofilm biovolume in PAO1 and MDR isolates. Strikingly, when used in conjunction with
tobramycin, low doses of 6K-F17 significantly potentiated tobramycin killing, leading to biofilm destruction. SIMILAR CONTENT BEING VIEWED BY OTHERS DJK-5, AN ANTI-BIOFILM PEPTIDE, INCREASES
_STAPHYLOCOCCUS AUREUS_ SENSITIVITY TO COLISTIN KILLING IN CO-BIOFILMS WITH _PSEUDOMONAS AERUGINOSA_ Article Open access 08 January 2025 SELECTIVE TOXICITY OF A NOVEL ANTIMICROBIAL PEPTIDE
ACIDOCIN 4356 AGAINST _PSEUDOMONAS AERUGINOSA_ AND _ACINETOBACTER BAUMANNII_ IN HUMAN CELL-BASED IN VITRO INFECTION MODELS Article Open access 19 January 2025 THE ROLE OF PSL IN THE FAILURE
TO ERADICATE _PSEUDOMONAS AERUGINOSA_ BIOFILMS IN CHILDREN WITH CYSTIC FIBROSIS Article Open access 04 August 2021 INTRODUCTION Cystic fibrosis (CF) is a genetic disease arising from
mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene that encodes a chloride ion transporter1. Impaired trans-epithelial chloride transport leads to dehydrated
airway secretions and a lack of airway mucus clearance2,3. As a result, individuals with CF are prone to repeated bacterial infections by different pathogens, leading to bronchiectasis and
respiratory failure3,4. In particular, chronic infection with _Pseudomonas aeruginosa_ has been shown to lead to more rapid lung function decline and premature death5,6. Once _P_.
_aeruginosa_ establishes chronic infection, it undergoes a number of adaptations, including the formation of biofilms and the expression of multidrug resistance pumps, making infections
difficult to treat with standard antibiotic therapy7,8,9,10,11,12,13,14. Progress toward developing treatments for biofilm infections has been made through the use of cationic antimicrobial
peptides (CAPs), which are found naturally in a wide variety of organisms and constitute a major component of the innate immune system15,16,17. There are a number of possible mechanisms of
action of antimicrobial peptides in inhibiting or eradicating bacterial biofilms. The anti-biofilm activity of antimicrobial peptides may be the result of a classic microbicidal effect
(usually occurring at concentrations equal or higher than minimum inhibitory concentration (MIC)) as well as through non-classical mechanisms of action (at concentrations much lower than
MIC)18. The microbicidal effect is due primarily to the permeabilization of the bacterial cell membrane of either detached planktonic or biofilm embedded bacteria19,20,21. Antimicrobial
peptides can also target the biofilm mode of growth by inhibiting bacterial adhesion (to surfaces or other bacteria)22,23, interfering with gene expression (including genes related to
motility, matrix synthesis and quorum sensing)24,25 and modulating the host response (such as the activity of host immune cells and the release of inflammatory cytokines)26. Given their
mechanism of action, CAPs may effectively target biofilms, as their activity is independent of growth rate, metabolic activity or the presence of persister cells27,28. Naturally occurring
peptides have adapted to perform in a specific environmental niche, and thus are limited in their ability to function in settings such as the CF lung15,16,17. In contrast, synthetically
engineered CAPs can often be developed to overcome these limitations29. In the present study we report the ability of 6K-F17 – a peptide we designed from first principles of hydrophobicity,
net positive charge, and sequence patterning30,31 – to effectively kill mature biofilms of _P_. _aeruginosa_ from chronically infected CF patients, grown in static culture with and without
CF sputum, along with the power of the peptide to potentiate the bactericidal activity of tobramycin, a commonly used inhaled antibiotic in CF _P_. _aeruginosa_ infections32. RESULTS
PROPERTIES OF THE ANTIMICROBIAL PEPTIDE 6K-F17: SAFETY TOWARD MAMMALIAN CELLS Essentially all natural CAPs consist of cationic amphipathic sequences with Lys (and/or Arg) residues
distributed throughout the sequence, which when folded into an α-helix upon membrane association, presents a lipid-interactive hydrophobic face33. In contrast, the synthetic CAP contains
positive (Lys) residues clustered at the peptide N-terminus, with the remainder of the sequence consisting of an uninterrupted hydrophobic segment (6K-F17; sequence KKKKKK-AAFAAWAAFAA-NH2)
which is designed to maximize insertion into, and cause physical disruption of, bacterial membranes34,35. The membrane-penetrating power of this peptide likely derives from this
implementation of charge segregation from the hydrophobic core30. As such, the designed CAPs operate by a non-specific bacterial (but not mammalian) membrane disruption mechanism that is
unlikely to evoke rapid resistance, suggesting that CAPs of the present design can prolong their therapeutic time frame. Yet 6K-F17 is remarkably selective for bacterial rather than host
membranes and, in contrast to most natural amphipathic CAPs, is non-hemolytic to human erythrocytes up to concentrations >500 μg/ml31. This selectivity devolves from the fact that once
attracted electrostatically to the anionic surface of a bacterial membrane, the effective hydrophobicity of 6K-F17 is above the threshold for spontaneous membrane penetration, while in the
absence of electrostatic attraction, this level of hydrophobicity _per se_ is below the threshold for penetration of the zwitterionic membranes of mammalian cells30. Because the 6K-F17
peptide consists of the natural L-isomers of common amino acids it can be efficiently synthesized and is rendered water-soluble by the six polar Lys residues, thereby facilitating
characterization and purification. Examples of 6K-F17 MICs against the _P_. _aeruginosa_ lab strain PAO1, and against several clinical _P_. _aeruginosa_ strains as studied herein (_vide
infra_), are presented in Table 1, and compared to corresponding MICs for the conventional antibiotic tobramycin; in several instances, the 6K-F17 MICs are equal or less than the tobramycin
values. The activity of CAPs may be limited by certain host factors, where specific _in vivo_ conditions such as high sodium chloride concentrations and/or low pH can impede their
performance36. To investigate these possibilities, we determined the inhibitory activity of 6K-F17 against planktonic _P_. _aeruginosa_ isolates as a function of salt concentration ([NaCl] =
0–200 mM; PAO1 strain), and of changes in pH (range = 6–8; 007E3–2 strain) (Fig. S1). We found that the peptide’s MIC values remained unaffected by both of these sets of conditions. In
addition, given the reality that much of the therapy for CF patients is administered through inhalation of antibiotics such as tobramycin, we undertook to assess a further aspect of 6K-F17
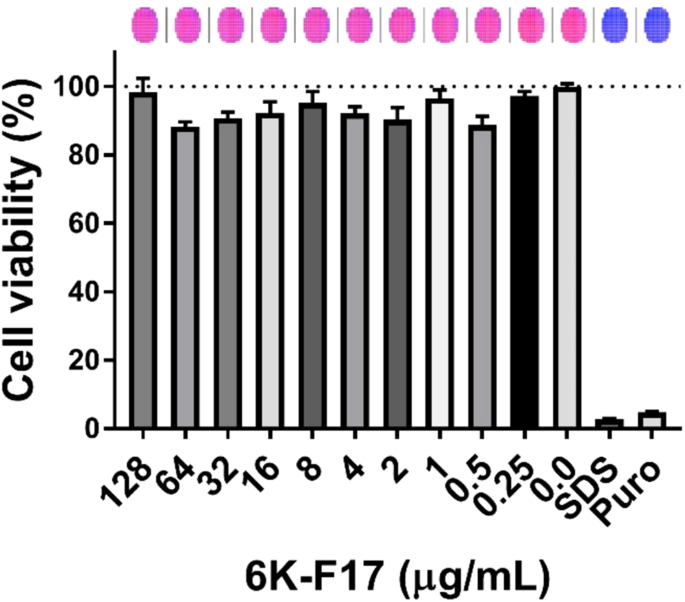
safety in a bronchial epithelial cell line derived from an individual homozygous for F508del (CFBE410−). Cell viability was assessed using the fluorescent live cell dye, Calcein-AM. As shown
in Fig. 1, we found that 6K-F17 was not cytotoxic when tested in the CF airway cells up to 128 μg/mL. 6K-F17 DISRUPTS _P_. _AERUGINOSA_ PAO1 BIOFILMS In order to determine the effect of the
cationic peptide 6K-F17 on biofilm disruption, we grew the laboratory strain PAO1 in slide chambers for 30 hours followed by exposure to different concentrations of 6K-F17. As seen in Fig.
2a, increasing concentrations of 6K-F17 had the ability to disrupt 30-hour biofilms leading to reduced biovolume (Fig. 2b) and decreased number of viable cells in these biofilms (Fig. 2c).
Increasing concentrations of the peptide (10–180 µg/mL) resulted in increasing disruption of biofilm (86% biovolume for 10 µg/mL to less than 3% biovolume for 180 µg/mL of the untreated
control, p < 0.001) and less viable cells (20% of the viable cells in control condition). Additionally, the number of viable cells as assessed by colony forming units per mL (CFU/mL) was
decreased in a dose dependent manner (Table 2). 6K-F17 POTENTIATES TOBRAMYCIN ACTIVITY AGAINST _P_. _AERUGINOSA_ PAO1 BIOFILMS We next tested the ability of our peptide to enhance tobramycin
activity against _P_. _aeruginosa_ biofilms. To do this, we grew PAO1 in slide chambers for 30 hours. Following initial growth, biofilms were subjected to various concentrations of
tobramycin with or without a fixed concentration of 6K-F17 (10 µg/mL) for 16 hours. Figure 3a demonstrates that while 10 µg/mL of 6K-F17 or low doses of tobramycin (10 µg/mL) had little
impact on biofilm biovolume alone (87% and 77% of untreated control respectively), the combination of 10 µg/mL 6K-F17 and 10 µg/mL tobramycin decreased overall biofilm biovolume to less than
20% of control conditions (p < 0.001). Subsequent increases in tobramycin concentration in the presence of 10 µg/mL of 6K-F17 further decreased biofilm biovolume to 4% of the control.
The addition of 6K-F17 to tobramycin also led to a reduction in the number of viable bacterial cells as assessed by the ATP assay (Fig. 3b)37,38,39 as well as CFU/mL (Table 2). There was no
detectable growth in the media fraction (planktonic fraction) of tobramycin alone, or of 6K-F17 + tobramycin, suggesting that the biofilm is efficiently destroyed (_data not shown_). The
time required for 6K-F17 to potentiate tobramycin kill of _P_. _aeruginosa_ biofilms was then investigated. Using a tobramycin concentration of 1000 µg/mL (reflecting the mean sputum
concentrations achievable with inhalation therapy40), the addition of 6K-F17 to tobramycin resulted in significant bacterial killing within 15 minutes, while very little killing was evident
with tobramycin alone (Fig. 4a). The presence of 6K-F17 increased the proportion of biofilm killed in as little as 15 minutes and resulted in significant reduction of biovolume by 3 hours
(Fig. 4b,c). Taken together, these data suggest that combination of 6K-F17 with tobramycin results in biofilm killing and ultimately reduces biofilm volume more rapidly than does tobramycin
alone. 6K-F17 POTENTIATES THE TOBRAMYCIN KILL OF PAO1 _P_. _AERUGINOSA_ IN THE PRESENCE OF CF SPUTUM Host-derived products can often inactivate cationic microbial peptides16 and the addition
of sputum supernatant can increase the thickness of biofilms, making them more drug resistant41,42. We thus tested the activity of 6K-F17 and tobramycin compared to tobramycin alone against
biofilms grown in the presence of sputum supernatant collected from CF patients. As shown in Fig. 5a, neither 100 µg/mL of tobramycin alone nor 10 µg/mL of 6K-F17 alone significantly
reduced the biovolume of PAO1 biofilms after 3 hours of incubation in media alone or in the presence of sputum supernatant. However, the combination of both compounds resulted in significant
reduction of biofilm biovolume compared to the untreated control even in the presence of CF sputum; biovolume reduced to 23% of untreated control in media alone (p < 0.001) and reduced
to 24% of untreated control in the presence of sputum (p < 0.001). Similarly, the combination of both compounds resulted in increased killing compared to untreated conditions, even in the
presence of CF sputum supernatant (p < 0.05) (Fig. 5b). 6K-F17 DISRUPTS BIOFILMS FROM MULTIDRUG RESISTANT _P_. _AERUGINOSA_ CLINICAL ISOLATES In addition to testing the activity against
the laboratory strain PAO1, we measured the ability of 6K-F17 to disrupt biofilms of multidrug resistant CF clinical isolates of _P_. _aeruginosa_. Four clinical isolates of _P_.
_aeruginosa_ were collected from CF patients with chronic _P_. _aeruginosa_ infection followed at the Hospital for Sick Children (Toronto, Canada). The antibiotic susceptibility results and
mucoidy status of these isolates are shown in Table S1. Figure 6 illustrates the biofilm biovolume and viability after 30 hours of growth once exposed to varying concentrations of 6K-F17 for
16 hours. Figure 6b (isolate 007E3-2) and Fig. 6d (isolate 035B7-2) demonstrate significant disruption and killing of the biofilm at 6K-F17 concentrations as low as 45 µg/mL, whereas Fig.
6a (isolate 005E3-2) and Fig. 6c (014B2-1) show a more modest response to the peptide. Finally, we examined the ability of 6K-F17 to potentiate tobramycin biofilm disruption and killing of
these multidrug resistant CF clinical isolates. Figure 7 demonstrates that 6K-F17 potentiated the effect of tobramycin in reducing the biovolume and viability of clinical isolate 007E3-2
(Fig. 7b), 014B2-1 (Fig. 7c) and 035B7-2 (Fig. 7d). For isolate 005E3-2, however, the addition of 6K-F17 significantly reduced the biovolume (compared to tobramycin alone) only at tobramycin
concentrations of 500 µg/mL (Fig. 7a). DISCUSSION Antimicrobial peptides have long been investigated as antimicrobial agents against a variety of different bacterial species43,44,45. Drug
development has been limited primarily by safety concerns and the ability of the compounds to function _in vivo_15. In the present study, we demonstrated that the novel designed peptide
6K-F17 can act both as an antimicrobial at higher concentrations as well as a potentiator of a conventional antibiotic (tobramycin) at concentrations 100-fold lower than the hemolytic dose,
providing a wide therapeutic window. A low dose (10 µg/mL) of 6K-F17 in combination with tobramycin killed _P_. _aeruginosa_ biofilms quickly (within 15 minutes) and for a prolonged period
(up to 24 hours), both desirable qualities for an antimicrobial agent. As a potentiator, 6K-F17 was able to decrease the effective required dose of tobramycin from 1,000 µg/mL to 10 µg/mL.
Although clinical trials of nebulized inhaled tobramycin in CF have reported mean sputum tobramycin concentrations of 1,000 µg/mL, there is a wide range of measured levels and
non-homogeneous ventilation that can lead to lower drug concentrations in certain regions of the lung40,46,47,48. Adding a potentiator such as 6K-F17 to tobramycin may thus improve the
latter’s clinical efficacy. The potentiating effect of 6K-F17 may also be useful when administering tobramycin intravenously, as systemic drug administration is known to result in lower
sputum concentrations compared to aerosolized treatment46. We additionally showed that 6K-F17 can kill _P_. _aeruginosa_ biofilms in the presence of sputum collected from CF patients, which
is known to increase biofilm thickness and contains potentially inactivating compounds such as proteases, elastases, and bacterial DNA41. To our knowledge, this has not been previously
demonstrated and suggests that 6K-F17 will be effective in the actual environment of the CF airways. Furthermore, we showed the efficacy of 6K-F17 against mature, established biofilms (48
hour growth) of multidrug resistant CF clinical isolates, to reflect a more accurate, clinical scenario49; Bomberger _et al_. similarly demonstrated the efficacy of their engineered CAP
against clinical _P_. _aeruginosa_ isolates grown as biofilms for 6 hours on airway epithelial cells, at low pH and in high salt concentrations29. The molecular origin of the _de facto_
synergism we observed for 6K-F17 in potentiating tobramycin activity remains a subject of current investigation. The primary mechanism of 6K-F17 antimicrobial action is believed to involve
bacterial plasma membrane damage – where no protein or polysaccharide target is specifically involved34. In this context, it is striking that 6K-F17 nevertheless displays a range of
anti-biofilm activity against the various _P_. _aeruginosa_ clinical isolates studied herein. For example, 6K-F17 disrupted the biofilms of isolates of strains 007-E3-2 and 035-B7-2 more
effectively than the biofilms of isolates 005-E3-2 and 014B2-1; of note, the latter two isolates were also resistant (or of intermediate resistance) to colistin, whereas the former two
isolates were colistin susceptible (Table S1). Colistin is a cationic, cyclic peptide antibiotic belonging to the polymyxin family that, in principle, has a similar mechanism of action as
antimicrobial peptides, by binding to bacterial cell membranes causing cell lysis and death50. _P_. _aeruginosa_ resistance to colistin has been described through the modification by the
bacteria of the lipid A component of lipopolysaccharides (LPS), the binding site of colistin51. Thus, the high affinity of CAPs for LPS may make them susceptible to LPS modifications
(_viz_., by rendering the bacterial surface less negatively-charged) - a well-known adaptive response of _P_. _aeruginosa_ to the CF lung environment - and ultimately explain the
strain-strain variability in effectiveness8,18,52. Indeed, lipid A modification in _P_. _aeruginosa_ has been shown to be associated with resistance to selected CAPs53. In any case, given
the energy expended in modifying bacterial cell membranes, the threshold for the development of resistance against CAPs is thought to be much higher than for small molecule antibiotics. As
an additional mechanistic consideration, the varying expression level(s) of membrane-embedded efflux pumps among clinical strains is likely to be a prime factor underlying the wide range of
effectiveness of both CAPs and tobramycin54 that we observed herein. And in a further aspect of this activity, we showed in previous _in vitro_ work that 6K-F17 can form insoluble
stereospecific complexes with the anionic polysaccharide alginate – a common component of the exopolysaccharide (EPS) matrix in biofilms – suggesting a ‘dual-action’ mechanism wherein 6K-F17
may disrupt not only the bacterial plasma membrane but also the biofilm EPS, thereby allowing increased access of both 6K-F17 and tobramycin (in combination experiments; Fig. 3) to the
bacterial membrane55. Although we sought to re-create the _in vivo_ CF environment using patient sputum, our model did not include elements of the host, such as airway epithelial (AECs) and
inflammatory cells. CAPs can act as immunomodulators, recruiting polymorphonuclear cells and modulating the release of pro- or anti-inflammatory cytokines, which may influence its ultimate
activity26. Previous studies have investigated the antimicrobial effects of CAPs against _P_. _aeruginosa_ biofilms grown on AECs, but these experiments are limited by the length of time one
can grow a biofilm on AECs without causing cell death (typically 6 hours), resulting in more immature biofilms that are easier to kill29. Furthermore, the cell viability assay may not
detect metabolically dormant, persister cells. It therefore remains of importance to develop adequate animal models of chronic respiratory biofilms in which to test the _in vivo_ efficacy of
CAPs. The spectrum of antimicrobial activity of 6K-F17 as well as its ability to potentiate other antibiotics (in addition to tobramycin) remains to be determined and is currently under
investigation. In summary, we have shown the ability of the designed CAP 6K-F17 to disrupt _P_. _aeruginosa_ biofilms – both PAO1 and several multidrug resistant clinical isolates obtained
from chronically infected CF patients – in a dose dependent manner. Importantly, the peptide was able to maintain its activity against _P_. _aeruginosa_ biofilms in the presence of CF sputum
supernatant. Low doses of 6K-F17 were able to potentiate tobramycin kill of _P_. _aeruginosa_ biofilms, suggesting the potential ability to co-administer the peptide to increase the utility
of tobramycin against resistant isolates. As well, 6K-F17 was able to reduce the overall biovolume of established _P_. _aeruginosa_ biofilms as well as reduce the number of viable cells
present. These findings portend the further development and ultimate therapeutic utility of peptide antibiotics such as 6K-F17 in the treatment of respiratory infections. METHODS BACTERIAL
ISOLATES AND SPUTUM SAMPLES PAO1 was used as a general laboratory strain for these experiments56. Clinical isolates of _P_. _aeruginosa_ [Research Ethics Board (REB) #1000019444] and sputum
samples (REB #100011132) were obtained with informed consent from CF patients with chronic infection followed at the Hospital for Sick Children (Toronto, Canada). Consent was obtained from a
parent or legal guardian if not of age. All methods were performed in accordance with the relevant guidelines and regulations for research involving human subjects at the Hospital for Sick
Children. Antimicrobial susceptibility testing was performed as per the Clinical Laboratory Standards Institute (CLSI)57. PEPTIDE SYNTHESIS AND PURIFICATION 6K-F17 was synthesized by the
continuous flow Fmoc solid-phase method on a Protein Technologies PS3 peptide synthesizer using the standard cycle58. (Fmoc-aminomethyl-3,5-dimethoxyphenoxy)-valeric acid-polyethylene
glycol-polystyrene resin was used to produce an amidated C-terminus. 2-(7-Aza-1_H_-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate and _N_,_N_-diisopropylethylamine were
used as the activation pair. Deprotection and peptide cleavage was performed in a mixture of 88% TFA, 5% phenol, 5% water, and 2% triisopropylsilane for 2 h in the dark at room temperature.
Crude peptide was purified on a reverse-phase C4 preparative HPLC column using a linear gradient of acetonitrile in 0.1% TFA. Purity and identity of the peptide were confirmed using
MALDI-MS. Peptide concentration was determined using amino acid analysis. MIC VALUES OF 6K-F17 AND TOBRAMYCIN AGAINST _P_. _AERUGINOSA_ CLINICAL ISOLATES The antibacterial activity was
tested in sterile 96-well plates in a final volume of 100 μL by following standard microtiter dilution protocols in Mueller-Hinton Broth/not cation-adjusted (MHB). _P_. _aeruginosa_ PAO1 or
clinical isolate cells were grown in MHB at 37 °C for overnight and were diluted in the same medium to a final concentration of 5 × 105 colony forming units (CFU/mL) as determined by optical
density at 600 nm. Aliquots of 10 μL of serial two-fold dilutions in water of the lyophilized peptide (or tobramycin) were added to microtiter plates followed by 90 μL of bacterial
suspension. Peptide (tobramycin) antibacterial activity was expressed as the MIC – the lowest concentrations that resulted in 100% prevention of bacterial growth after 24 hours incubation at
37 °C (Table 1). Optical density was measured at OD600 using a microplate autoreader Spectrophotometer. Positive controls contained no peptide and showed visible turbidity after 24 hours
incubation at 37 °C. All assays were carried out in triplicate; repeated tests were within one dilution (standard error of the test). PEPTIDE TOXICITY ASSAY IN HUMAN AIRWAY CELLS Peptide
toxicity to human cystic fibrosis bronchial epithelial cells (CFBE) containing the mutation F508del was tested using a Calcein-AM based fluorescence assay59. CFBE410− F508del cells (kind
gift from the Dieter Gruenert laboratory, California Pacific Medical Center Research Institute, San Francisco, CA) were grown on 96-well black clear bottom plates, as previously described
for this immortalized cell line60. Media used to culture the cells were EMEM (Wisent Bio Products, Saint-Jean-Baptiste, Canada) containing 10% Fetal Bovine Serum (Wisent Bio Products,
Saint-Jean-Baptiste, Canada), 300 µg/mL Hygromycin, and 1% penicillin/streptomycin. These cells were grown to confluence and then differentiated for 5 days. The cells were then treated with
6K-F17 (128 µg/mL to 0.0 µg/mL), or puromycin (20 µg/ml). On the day of the experiment, cells were washed with PBS, and then loaded with live cell marker Calcein-AM (10 µM) (Thermo Fischer
Scientific, MA, USA) along with either the 6K-F17 peptide, with puromycin, or with 1% (v/v) SDS, for 30 minutes. Plates were then read using a fluorescence multi-plate reader (Molecular
Devices i3x), at excitation maximum of 490 nm and emission maximum of 515 nm. Multiple points were read in the same well to account for heterogeneity across the well. The bar graph
represents average fluorescence across the well (n = 137 technical replicates per well of a 96 well plate, n = 3 wells per condition, n = 3 biological replicates from independent plates).
COLONY FORMING UNITS FROM BIOFILMS _Pseudomonas aeruginosa_ was subcultured 1/100 from an overnight culture and grown to an OD600 of 0.06 in cation adjusted Muller-Hinton broth (CAMHB) at 37
°C. 100 μL of this broth was then added to wells of a 96-well polystyrene microtiter plate (Fisher, non-tissue cultured) and grown for 16 hours without shaking at 37 °C. Following this
growth, media was removed, and wells were washed gently with PBS 2x prior to addition of peptide or tobramycin in CAMHB. Biofilms were grown in presence of antibiotics for 24 hours at 37 °C.
After growth period, media was removed, and wells were washed twice with PBS. 100 μL of fresh PBS was then added to the wells and the plate was vortexed for 5 min. Media from wells were
then serially diluted and plated on blood agar plates. Plates were grown for 24–48 hours at 37 °C and CFU were counted. CFU counts were log transformed as previously described, with the mean
and standard deviations reported61. BACTERIAL VIABILITY ASSAY Cell viability of biofilms was assessed using an adapted method of the ATP assay for biofilms62. Briefly, _P_. _aeruginosa_
isolates were grown to stationary phase at 37 °C with shaking at 200 RPM in cation-adjusted Muller-Hinton broth (CAMHB-Sigma-Aldrich.). This culture was then diluted to an OD600 of 0.05 and
grown to an OD of 0.1 (early exponential phase) prior to plating. 100 µL of this culture were added to a white Grenier LUMITRAC medium binding plate (Sigma-Aldrich, city, country) in
triplicate and incubated for 24 hours at 37 °C without shaking. After 24 hours, media were removed, and cells were gently washed 2X with fresh media. 100 µL of fresh CAMHB was added back to
the wells along with 100 µL of antibiotic, cationic peptide 6K-F17, or antibiotic plus peptide, for a final volume of 200 µL and mixed gently for 5 minutes on an orbital shaker. The plate
was then incubated for 16 hours at 37 °C. Following these procedures, 100 µL from each well was removed and placed into an empty well of a 96-well plate to monitor ATP in the planktonic or
detached fraction of the wells. 100 µL of the Bac-titer glo ATP cell viability solution (Promega, Madison, WI) was added to each well. Plates were gently mixed on an orbital shaker for 10
minutes prior to luminescence reading as per manufacturer’s suggested protocol. BIOFILM GROWTH IN CHAMBER SLIDES _P_. _aeruginosa_ was grown in chamber slides as previously described63.
Briefly, clinical isolates of _P_. _aeruginosa_ were grown overnight in 3 mL of lysogeny broth (Lennox formulation-LB) with shaking overnight. 40 μL of overnight culture was diluted into 4
mL of LB. This was diluted 1/10 and 220 µL was used to seed the wells of an 8-chambered cover-glass slide (Nunc Lab-tek II, VWR, Mississauga, Ontario, Canada). After 6 hours of attachment,
media were removed and replaced with fresh media. Biofilms were allowed to grow for a further 24–48 hours, replacing media every 12 hours until the conclusion of the experiment. Pre-formed
biofilms in chamber slides were also exposed to varying concentrations of cationic peptide, tobramycin (Sigma-Aldrich, Oakville, Ontario, Canada) or a combination of both as described.
24-hour biofilms were grown as described above prior to addition of various antibiotics for the indicated time periods. CONFOCAL MICROSCOPY Prior to confocal microscopy, biofilms were
stained using the Filmtracer Live/Dead biofilm viability kit (Life Technologies, Burlington, ON, Canada). Medium was gently removed from the chambers, and 200 μl total of the Live/Dead stain
was added to the chambers. After 45 min of incubation, the stain was removed, and fresh medium was placed in the wells. Confocal images were then acquired using a Quorum WaveFX spinning
disk confocal system (Quorum Technologies Inc., Guelph, Canada). All images were acquired using a 25X water objective (total magnification, X250) on a Zeiss AxioVert 200 M Microscope.
Spectral borealis lasers (green, 491 nm; red, 561 nm) were used for excitation. Emission filter sets of 515/40 and 624/40 were used to visualize the SYTO9 and propidium iodide stains,
respectively. Images in the z-stack were obtained at a distance of 0.8 μm to obtain the depth of the biofilm. For each experiment, each isolate tested was performed in duplicate technical
replicate (two chambers per condition) with three biological replicates (three separate experiments). Volocity software (PerkinElmer, Guelph, Canada) was used for acquisition and analysis of
images. SPUTUM PROCESSING Fresh sputum samples were collected with informed consent from CF patients at the Hospital for Sick Children (Toronto, Canada) according to the experimental
protocols and guidelines for research involving human subjects as approved by the Hospital for Sick Children [Research Ethics Board (REB) #100011132]. Samples were stored on ice, and PBS was
added to samples within 1 hour of collection at 3 times the volume of the sputum. Samples were vortexed for 5 min and 1 mL aliquots were placed into 1.5 mL microcentrifuge tubes and spun
down at 14,000 g for 10 min at 4 °C. Supernatant was removed gently and filtered sterilized through a 20 µm low-binding filter. The sample was plated on blood agar for 48 hours at 37° to
ensure no bacterial growth. Sputum samples were pooled from 5 patients. STATISTICAL ANALYSIS Continuous data were compared within groups, as performed using the Kruskal-Wallis test with a
Dunn’s multiple comparison post-test. For comparisons between tobramycin alone and tobramycin + peptide, means where compared with multiple t-tests with the Holm-Sidak method for multiple
comparisons. A _P-_value of < 0.05 was considered significant. All analyses were done using GraphPad Prism version 6.01. DATA AVAILABILITY STATEMENT All data generated or analyzed during
this study are included in this published article (and its Supplementary Information files). REFERENCES * Choo-Kang, L. R. & Zeitlin, P. L. Type I, II, III, IV, and V cystic fibrosis
transmembrane conductance regulator defects and opportunities for therapy. _Curr. Opin. Pulm. Med._ 6, 521–529 (2000). Article CAS Google Scholar * Ratjen, F. & Döring, G. Cystic
fibrosis. _Lancet_ 361, 681–689 (2003). Article CAS Google Scholar * Cohen, T. S. & Prince, A. Cystic fibrosis: a mucosal immunodeficiency syndrome. _Nat. Med._ 18, 509–519 (2012).
Article CAS Google Scholar * Cystic Fibrosis Canada. _Canadian Patient Data Registry Report_. (Toronto, Canada, 2017). * Bhagirath, A. Y. _et al_. Cystic fibrosis lung environment and
Pseudomonas aeruginosa infection. _BMC Pulm. Med._ 16, 174 (2016). Article Google Scholar * Govan, J. R. & Deretic, V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas
aeruginosa and Burkholderia cepacia. _Microbiol. Rev._ 60, 539–574 (1996). CAS PubMed PubMed Central Google Scholar * Lund-Palau, H. _et al_. Pseudomonas aeruginosa infection in cystic
fibrosis: pathophysiological mechanisms and therapeutic approaches. _Expert Rev. Respir. Med._ 10, 685–697 (2016). Article CAS Google Scholar * Maldonado, R. F., Sá-Correia, I. &
Valvano, M. A. Lipopolysaccharide modification in gram-negative bacteria during chronic infection. _FEMS Microbiol. Rev._ 40, 480–493 (2016). Article CAS Google Scholar * Rybtke, M.,
Hultqvist, L. D., Givskov, M. & Tolker-Nielsen, T. Pseudomonas aeruginosa Biofilm Infections: Community Structure, Antimicrobial Tolerance and Immune Response. _J. Mol. Biol._ 427,
3628–3645 (2015). Article CAS Google Scholar * Winstanley, C., O’Brien, S. & Brockhurst, M. A. Pseudomonas aeruginosa Evolutionary Adaptation and Diversification in Cystic Fibrosis
Chronic Lung Infections. _Trends Microbiol._ 24, 327–337 (2016). Article CAS Google Scholar * Davies, J. C. & Bilton, D. Bugs, biofilms, and resistance in cystic fibrosis. _Respir.
Care_ 54, 628–640 (2009). Article Google Scholar * Evans, T. J. Small colony variants of _Pseudomonas aeruginosa_ in chronic bacterial infection of the lung in cystic fibrosis. _Future
Microbiol._ 10, 231–239 (2015). Article CAS Google Scholar * Høiby, N., Ciofu, O. & Bjarnsholt, T. _Pseudomonas aeruginosa_ biofilms in cystic fibrosis. _Future Microbiol._ 5,
1663–1674 (2010). Article Google Scholar * Oliver, A., Mulet, X., López-Causapé, C. & Juan, C. The increasing threat of Pseudomonas aeruginosa high-risk clones. _Drug Resist. Updat._
21–22, 41–59 (2015). Article Google Scholar * Melvin, J. A., Montelaro, R. C. & Bomberger, J. M. Clinical potential of engineered cationic antimicrobial peptides against drug resistant
biofilms. _Expert Rev. Anti. Infect. Ther._ 14, 989–991 (2016). Article CAS Google Scholar * Bahar, A. & Ren, D. Antimicrobial Peptides. _Pharmaceuticals_ 6, 1543–1575 (2013).
Article Google Scholar * Hiemstra, P. S., Amatngalim, G. D., van der Does, A. M. & Taube, C. Antimicrobial Peptides and Innate Lung Defenses: Role in Infectious and Noninfectious Lung
Diseases and Therapeutic Applications. _Chest_ 149, 545–551 (2016). Article Google Scholar * Batoni, G., Maisetta, G. & Esin, S. Antimicrobial peptides and their interaction with
biofilms of medically relevant bacteria. _Biochim. Biophys. Acta - Biomembr._ 1858, 1044–1060 (2016). Article CAS Google Scholar * Hancock, R. E. & Scott, M. G. The role of
antimicrobial peptides in animal defenses. _Proc. Natl. Acad. Sci. USA_ 97, 8856–8861 (2000). Article ADS CAS Google Scholar * Matsuzaki, K., Sugishita, K. & Miyajima, K.
Interactions of an antimicrobial peptide, magainin 2, with lipopolysaccharide-containing liposomes as a model for outer membranes of gram-negative bacteria. _FEBS Lett._ 449, 221–224 (1999).
Article CAS Google Scholar * Zhang, L., Rozek, A. & Hancock, R. E. W. Interaction of Cationic Antimicrobial Peptides with Model Membranes. _J. Biol. Chem._ 276, 35714–35722 (2001).
Article CAS Google Scholar * Pimentel-Filho, N. _et al_. Bovicin HC5 and nisin reduce Staphylococcus aureus adhesion to polystyrene and change the hydrophobicity profile and Gibbs free
energy of adhesion. _Int. J. Food Microbiol._ 190, 1–8 (2014). Article CAS Google Scholar * Zhu, C. _et al_. Human β-defensin 3 inhibits antibiotic-resistant Staphylococcus biofilm
formation. _J. Surg. Res._ 183, 204–213 (2013). Article CAS Google Scholar * de la Fuente-Núñez, C. _et al_. Inhibition of Bacterial Biofilm Formation and Swarming Motility by a Small
Synthetic Cationic Peptide. _Antimicrob. Agents Chemother._ 56, 2696–2704 (2012). Article Google Scholar * Overhage, J. _et al_. Human Host Defense Peptide LL-37 Prevents Bacterial Biofilm
Formation. _Infect. Immun._ 76, 4176–4182 (2008). Article CAS Google Scholar * Mansour, S. C., Pena, O. M. & Hancock, R. E. W. Host defense peptides: front-line immunomodulators.
_Trends Immunol._ 35, 443–450 (2014). Article CAS Google Scholar * Deslouches, B. _et al_. _De novo_-derived cationic antimicrobial peptide activity in a murine model of Pseudomonas
aeruginosa bacteraemia. _J. Antimicrob. Chemother._ 60, 669–672 (2007). Article CAS Google Scholar * Deslouches, B. _et al_. Engineered cationic antimicrobial peptides to overcome
multidrug resistance by ESKAPE pathogens. _Antimicrob. Agents Chemother._ 59, 1329–1333 (2015). Article CAS Google Scholar * Lashua, L. P. _et al_. Engineered cationic antimicrobial
peptide (eCAP) prevents Pseudomonas aeruginosa biofilm growth on airway epithelial cells. _J. Antimicrob. Chemother._ 71, 2200–2207 (2016). Article Google Scholar * Glukhov, E., Burrows,
L. L. & Deber, C. M. Membrane interactions of designed cationic antimicrobial peptides: The two thresholds. _Biopolymers_ 89, 360–371 (2008). Article CAS Google Scholar * Yin, L. M.,
Edwards, M. A., Li, J., Yip, C. M. & Deber, C. M. Roles of hydrophobicity and charge distribution of cationic antimicrobial peptides in peptide-membrane interactions. _J. Biol. Chem._
287, 7738–7745 (2012). Article CAS Google Scholar * Döring, G., Flume, P., Heijerman, H. & Elborn, J. S. & Consensus Study Group. Treatment of lung infection in patients with
cystic fibrosis: Current and future strategies. _J. Cyst. Fibros._ 11, 461–479 (2012). Article Google Scholar * Zasloff, M. Antimicrobial peptides of multicellular organisms. _Nature_ 415,
389–395 (2002). Article ADS CAS Google Scholar * Stark, M., Liu, L. P. & Deber, C. M. Cationic hydrophobic peptides with antimicrobial activity. _Antimicrob Agents Chemother_ 46,
3585–3590 (2002). Article CAS Google Scholar * Glukhov, E., Stark, M., Burrows, L. L. & Deber, C. M. Basis for selectivity of cationic antimicrobial peptides for bacterial versus
mammalian membranes. _J. Biol. Chem._ 280, 33960–33967 (2005). Article CAS Google Scholar * Abou Alaiwa, M. H. _et al_. pH modulates the activity and synergism of the airway surface
liquid antimicrobials β-defensin-3 and LL-37. _Proc. Natl. Acad. Sci. USA_ 111, 18703–18708 (2014). Article ADS CAS Google Scholar * Doll, K., Jongsthaphongpun, K. L., Stumpp, N. S.,
Winkel, A. & Stiesch, M. Quantifying implant-associated biofilms: Comparison of microscopic, microbiologic and biochemical methods. _J. Microbiol. Methods_ 130, 61–68 (2016). Article
CAS Google Scholar * Junker, L. M. & Clardy, J. High-Throughput Screens for Small-Molecule Inhibitors of Pseudomonas aeruginosa Biofilm Development. _Antimicrob. Agents Chemother._ 51,
3582–3590 (2007). Article CAS Google Scholar * Sule, P. _et al_. A combination of assays reveals biomass differences in biofilms formed by _Escherichia coli_ mutants. _Lett. Appl.
Microbiol._ 49, 299–304 (2009). Article CAS Google Scholar * Konstan, M. W. _et al_. Safety, efficacy and convenience of tobramycin inhalation powder in cystic fibrosis patients: The
EAGER trial. _J. Cyst. Fibros._ 10, 54–61 (2011). Article CAS Google Scholar * Kennedy, S. _et al_. Activity of Tobramycin against Cystic Fibrosis Isolates of Burkholderia cepacia Complex
Grown as Biofilms. _Antimicrob. Agents Chemother._ 60, 348–355 (2016). Article CAS Google Scholar * Tom, S. K., Yau, Y. C. W., Beaudoin, T., LiPuma, J. J. & Waters, V. Effect of
High-Dose Antimicrobials on Biofilm Growth of Achromobacter Species Isolated from Cystic Fibrosis Patients. _Antimicrob. Agents Chemother._ 60, 650–652 (2016). Article CAS Google Scholar
* Batoni, G., Maisetta, G., Brancatisano, F. L., Esin, S. & Campa, M. Use of antimicrobial peptides against microbial biofilms: advantages and limits. _Curr. Med. Chem._ 18, 256–279
(2011). Article CAS Google Scholar * Saiman, L. _et al_. Cathelicidin peptides inhibit multiply antibiotic-resistant pathogens from patients with cystic fibrosis. _Antimicrob. Agents
Chemother._ 45, 2838–2844 (2001). Article CAS Google Scholar * Brogden, K. A. _et al_. The ovine cathelicidin SMAP29 kills ovine respiratory pathogens _in vitro_ and in an ovine model of
pulmonary infection. _Antimicrob. Agents Chemother._ 45, 331–334 (2001). Article CAS Google Scholar * Chmiel, J. F. _et al_. Antibiotic management of lung infections in cystic fibrosis.
I. The microbiome, methicillin-resistant Staphylococcus aureus, gram-negative bacteria, and multiple infections. _Ann. Am. Thorac. Soc._ 11, 1120–1129 (2014). Article Google Scholar *
Konstan, M. W. _et al_. Tobramycin inhalation powder for P. aeruginosa infection in cystic fibrosis: the EVOLVE trial. _Pediatr. Pulmonol._ 46, 230–238 (2011). Article Google Scholar *
Ramsey, B. W. _et al_. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. _N. Engl. J. Med._ 340, 23–30
(1999). Article CAS Google Scholar * Singh, P. K. _et al_. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. _Nature_ 407, 762–764 (2000).
Article ADS CAS Google Scholar * Poirel, L., Jayol, A. & Nordmann, P. Polymyxins: Antibacterial Activity, Susceptibility Testing, and Resistance Mechanisms Encoded by Plasmids or
Chromosomes. _Clin. Microbiol. Rev._ 30, 557–596 (2017). Article CAS Google Scholar * Miller, A. K. _et al_. PhoQ mutations promote lipid A modification and polymyxin resistance of
Pseudomonas aeruginosa found in colistin-treated cystic fibrosis patients. _Antimicrob. Agents Chemother._ 55, 5761–5769 (2011). Article CAS Google Scholar * Mangoni, M. L. & Shai, Y.
Temporins and their synergism against Gram-negative bacteria and in lipopolysaccharide detoxification. _Biochim. Biophys. Acta_ 1788, 1610–1619 (2009). Article CAS Google Scholar *
Moskowitz, S. M., Ernst, R. K. & Miller, S. I. PmrAB, a Two-Component Regulatory System of Pseudomonas aeruginosa That Modulates Resistance to Cationic Antimicrobial Peptides and
Addition of Aminoarabinose to Lipid A. _J. Bacteriol._ 186, 575–579 (2004). Article CAS Google Scholar * Stone, T. A. & Deber, C. M. Therapeutic design of peptide modulators of
protein-protein interactions in membranes. _Biochim. Biophys. Acta - Biomembr._ 1859, 577–585 (2017). Article CAS Google Scholar * Yin, L. M., Lee, S., Mak, J. S. W., Helmy, A. S. &
Deber, C. M. Differential binding of L- vs. D-isomers of cationic antimicrobial peptides to the biofilm exopolysaccharide alginate. _Protein Pept. Lett._ 20, 843–847 (2013). Article CAS
Google Scholar * Jacobs, M. A. _et al_. Comprehensive transposon mutant library of Pseudomonas aeruginosa. _Proc. Natl. Acad. Sci._ 100, 14339–14344 (2003). Article ADS CAS Google
Scholar * Clinical Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 22nd informational supplement M100-S22. CLSI (2018). * Liu, L. P. &
Deber, C. M. Anionic phospholipids modulate peptide insertion into membranes. _Biochemistry_ 36, 5476–5482 (1997). Article CAS Google Scholar * Bratosin, D., Mitrofan, L., Palii, C.,
Estaquier, J. & Montreuil, J. Novel fluorescence assay using calcein-AM for the determination of human erythrocyte viability and aging. _Cytometry. A_ 66, 78–84 (2005). Article Google
Scholar * Kunzelmann, K. _et al_. An immortalized cystic fibrosis tracheal epithelial cell line homozygous for the delta F508 CFTR mutation. _Am. J. Respir. Cell Mol. Biol._ 8, 522–529
(1993). Article CAS Google Scholar * Yau, Y. C. W. _et al_. Randomized controlled trial of biofilm antimicrobial susceptibility testing in cystic fibrosis patients. _J. Cyst. Fibros._ 14,
262–266 (2015). Article CAS Google Scholar * Stiefel, P. _et al_. Is biofilm removal properly assessed? Comparison of different quantification methods in a 96-well plate system. _Appl.
Microbiol. Biotechnol._ 100, 4135–4145 (2016). Article CAS Google Scholar * Beaudoin, T., Kennedy, S., Yau, Y. & Waters, V. Visualizing the Effects of Sputum on Biofilm Development
Using a Chambered Coverglass Model. _J. Vis. Exp._, https://doi.org/10.3791/54819 (2016). Article Google Scholar Download references ACKNOWLEDGEMENTS This work was supported, in part, by a
grant to C.M.D. from the Cystic Fibrosis Foundation (U.S.) (CFF Grant 1610). T.B. was funded by a Cystic Fibrosis Canada fellowship. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Division
of Translational Medicine, Research Institute, Hospital for Sick Children, Toronto, Canada Trevor Beaudoin, Christina Adams, Hartmut Grasemann & Valerie Waters * Division of Molecular
Medicine, Research Institute, Hospital for Sick Children, Toronto, Canada Tracy A. Stone, Miroslawa Glibowicka, Saumel Ahmadi, Christine E. Bear & Charles M. Deber * Department of
Biochemistry, University of Toronto, Toronto, Ontario, Canada Tracy A. Stone, Christine E. Bear & Charles M. Deber * Department of Physiology, University of Toronto, Toronto, Ontario,
Canada Saumel Ahmadi & Christine E. Bear * Division of Microbiology, Department of Pediatric Laboratory Medicine, Hospital for Sick Children, Toronto, Canada Yvonne Yau * Department of
Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada Yvonne Yau, Hartmut Grasemann & Valerie Waters * Division of Respiratory Medicine, Department of
Pediatrics, Hospital for Sick Children, Toronto, Canada Hartmut Grasemann * Division of Infectious Diseases, Department of Pediatrics, The Hospital for Sick Children, University of Toronto,
555 University Avenue, Toronto, M5G 1X8, Canada Valerie Waters Authors * Trevor Beaudoin View author publications You can also search for this author inPubMed Google Scholar * Tracy A. Stone
View author publications You can also search for this author inPubMed Google Scholar * Miroslawa Glibowicka View author publications You can also search for this author inPubMed Google
Scholar * Christina Adams View author publications You can also search for this author inPubMed Google Scholar * Yvonne Yau View author publications You can also search for this author
inPubMed Google Scholar * Saumel Ahmadi View author publications You can also search for this author inPubMed Google Scholar * Christine E. Bear View author publications You can also search
for this author inPubMed Google Scholar * Hartmut Grasemann View author publications You can also search for this author inPubMed Google Scholar * Valerie Waters View author publications You
can also search for this author inPubMed Google Scholar * Charles M. Deber View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS T.B., Y.Y., and
V.W. designed, performed, and analyzed biofilm experiments. T.S., M.G., S.A., and C.D. were involved in developing and testing the cationic peptide. C.A. was involved in confocal microscopy
and image analysis. H.G. and V.W. contributed tools to the experiments. T.B., T.S., Y.Y., C.B., H.G., V.W., and C.D. wrote and edited the manuscript. CORRESPONDING AUTHOR Correspondence to
Charles M. Deber. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with
regard to jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article
is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in
this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative
Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a
copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Beaudoin, T., Stone, T.A., Glibowicka, M. _et al._
Activity of a novel antimicrobial peptide against _Pseudomonas aeruginosa_ biofilms. _Sci Rep_ 8, 14728 (2018). https://doi.org/10.1038/s41598-018-33016-7 Download citation * Received: 12
June 2018 * Accepted: 17 September 2018 * Published: 03 October 2018 * DOI: https://doi.org/10.1038/s41598-018-33016-7 SHARE THIS ARTICLE Anyone you share the following link with will be
able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative KEYWORDS * Biovolume * Multidrug Resistance (MDR) * Cationic Antimicrobial Peptides (CAPs) * Colony Forming Units Per Ml (CFU/mL) * Cystic Fibrosis Bronchial Epithelial Cells
(CFBE)