Play all audios:
ABSTRACT Starch makes up 70% of the wheat grain, and is an important source of calories for humans, however, the overconsumption of wheat starch may contribute to nutrition-associated health
problems. The challenge is to develop resistant starch including high amylose wheat varieties with health benefits. Adapting advance genomic approaches in EMS-induced mutant lines differing
in amylose content, basic leucine zipper (bZIP) regulatory factors that may play role in controlling amylose biosynthesis were identified in wheat. bZIP transcription factors are key
regulators of starch biosynthesis genes in rice and maize, but their role in regulating these genes in wheat is poorly understood. A genome-wide survey identified 370 wheat bZIPs, clustered
in 11 groups, showing variations in amino acids composition and predicted physicochemical properties. Three approaches namely, whole transcriptome sequencing, qRT-PCR, and correlation
analysis in contrasting high and low amylose mutants and their parent line identified 24 candidate bZIP (positive and negative regulators), suggesting bZIPs role in high amylose
biosynthesis. bZIPs positive role in high amylose biosynthesis is not known. _In silico_ interactome studies of candidate wheat bZIP homologs in _Arabidopsis_ and rice identified their
putative functional role. The identified bZIPs are involved in stress-related pathways, flower and seed development, and starch biosynthesis. An in-depth analysis of molecular mechanism of
novel candidate bZIPs may help in raising and improving high amylose wheat varieties. SIMILAR CONTENT BEING VIEWED BY OTHERS GENOME-WIDE ANALYSIS OF RING-TYPE E3 LIGASE FAMILY IDENTIFIES
POTENTIAL CANDIDATES REGULATING HIGH AMYLOSE STARCH BIOSYNTHESIS IN WHEAT (_TRITICUM AESTIVUM_ L.) Article Open access 01 June 2021 RNA-SEQ TRANSCRIPTOME PROFILING OF IMMATURE GRAIN WHEAT IS
A TECHNIQUE FOR UNDERSTANDING COMPARATIVE MODELING OF BAKING QUALITY Article Open access 13 May 2024 EQTL MAPPING OF THE 12S GLOBULIN CRUCIFERIN GENE _PGCRURSE5_ AS A NOVEL CANDIDATE
ASSOCIATED WITH STARCH CONTENT IN POTATO TUBERS Article Open access 13 October 2020 INTRODUCTION Cereal grains largely contain starch1,2, and are important sources of calories for humans.
Starch is highly digestible, about 99% is digested in human gut and converted into glucose, raising the glycemic index. Its overconsumption causes nutrition-associated health problems3,4,5.
There is a surge to develop cereal crops with resistant starch and food grains rich in dietary fibre6,7. Resistant starch is categorized as ‘good dietary fibre’3. Due to its slow digestion
in gut, high amylose starch is categorized as resistant or healthy starch that results in low glycemic index. Amylose is further converted into short chain fatty acids (SCF) by bacteria in
large intestine4,5. SCFs are known prebiotics with proven health benefits4,5. Functional genomics approaches are used to identify regulatory factors controlling amylose biosynthesis that may
be manipulated to increase its content in grains8,9,10. In this study, genome-wide analysis of contrasting mutant lines for amylose content led to the identification of candidate bZIPs that
correlate with the expression of two key genes of amylose biosynthesis pathway i.e., Granule-bound starch synthase I (GBSSI) and starch branching enzyme II (SBEII). Briefly, starch is a
semi-crystalline structure composed of two fractions, amylose and amylopectin11,12. Amylose is largely linear chain of glucose moiety whereas amylopection is highly branched. Amylose is a
non-digestible or slow-digestible fraction of starch and thus considered as resistant starch. In cereal, amylose makes ~25% of total starch. In this study, two contrasting mutant lines
differing in amylose content were used for genome-wide analysis. High amylose mutant line ‘TAC 75’ with 65% amylose content and low amylose mutant line ‘TAC 6’ with 7% amylose content along
with the parent line ‘C 306’ with ~26% amylose content were investigated. The key enzymes involved in starch biosynthesis are ADP-glucose pyrophosphorylase, starch synthases (soluble and
granule-bound), and starch branching and debranching enzymes11,12,13. GBSSI is largely responsible for amylose biosynthesis14,15, whereas SBEII is responsible for amylopectin
biosynthesis16,17. The differential expression of GBSSI and SBEII have been correlated with high amylose biosynthesis18,19. Unlike in other plants, their regulation by bZIPs is largely
unknown in wheat. bZIP transcription factor family members are well-known for their roles in growth and development20,21. bZIPs are also involved in the regulation of starch biosynthesis in
the endosperm that determine starch quality and quantity22,23,24,25. OsbZIP58 in rice23, ZmbZIP91 in maize26 and bZIP58 in wheat18 are reported to regulate starch biosynthesis. However,
their role in amylose biosynthesis in wheat is not well-understood. Genome-wide analysis identified many bZIP family members (genes) in various plant species like maize27, cucumber28, rice29
and _Arabidopsis_30. For example, there are 94 bZIPs identified in _Oryza sativa subsp_. _indica_, 140 in _O_. _sativa subsp_. _japonica_, 216 in _Zea mays_, 127 in _Arabidopsis thaliana_,
and 187 in wheat31. Due to its large genome size and lack of complete genome information, the structural and functional characterization of bZIPs is lacking in wheat. Publically available
genome sequence of wheat can be used for the genome-wide analysis of bZIPs and their roles in high amylose biosynthesis. The sequence based structured domain information of bZIPs can be used
to identify their putative functional role by _in silico_ analysis using the published validated functional roles, phylogenetic group information, and interactome analysis in the other
plant species such as _Arabidopsis_. In the present study, genome-wide analysis and phylogenetic analysis of bZIPs were undertaken in wheat. The 284 Gb transcriptome sequence data was
generated from the two contrasting mutant lines, ‘TAC 75’ and ‘TAC 6’ and their parent variety, ‘C 306’. Transcriptome analysis, qRT-PCR data, and correlation analysis identified candidate
wheat bZIPs (TabZIPs) regulating high amylose biosynthesis. The putative functional role of the candidate TabZIPs in amylose biosynthesis were predicted by using phylogenetic group
information and protein interacting networks of _Arabidopsis_ databases. RESULTS IDENTIFICATION AND CHARACTERIZATION OF WHEAT BZIPS GENOME SURVEY OF BZIP TRANSCRIPTION FACTORS IN WHEAT Using
whole genome sequence databases, wheat bZIPs TFs were identified through sequence similarity match with maize, rice, barley and _Arabidopsis_. A total of 370 wheat bZIPs (TabZIPs) were
identified by a Hidden Markov Model (HMM) profile ‘PF00170’ search against the whole wheat proteome ensembl database by HMMER3.0 and BLASTp search using plant bZIP sequences. Subsequently,
after validating the integrity of the bZIP domain using NCBI-CDD and InterproScan, a total of 370 wheat bZIP proteins encoded by 238 bZIP genes were identified. Each wheat bZIP protein was
assigned a unique identifier from TabZIP1 to TabZIP370. The gene isoforms and their proteins were assigned the same gene/protein number with decimal point. The information regarding TabZIP
transcription factors is listed in Supplementary Table S1. PHYLOGENETIC ANALYSIS OF TABZIPS The sequence homology relationship of 370 TabZIP proteins with that of _Arabidopsis_, maize, rice,
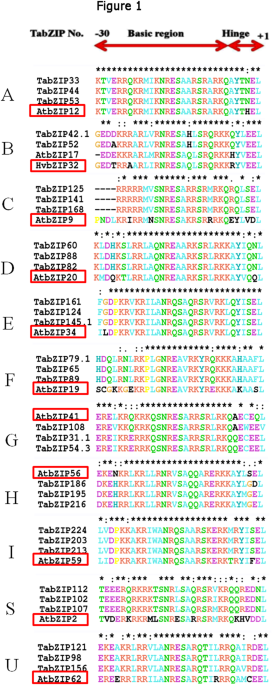
and barley was analysed by multiple sequence alignment. The analysis revealed a low level of variation (Fig. 1, Supplementary Fig. S1). The phylogenetic analysis grouped the 370 TabZIP
proteins in 11 clades (Fig. 2), and named as groups A to I and S to U, in accordance with those reported in _Arabidopsis_30. Two groups, A and D, were the largest, each with 83 TabZIPs
followed by group C (48 TabZIPs), group I (44 TabZIPs), group G (32 TabZIPs), group S (19 TabZIPs), group B, E and F (each with 14 TabZIPs), group H (9 TabZIPs), while group U is the
smallest with only 5 TabZIPs. TABZIP PHYSICOCHEMICAL PROPERTIES AND CONSERVED MOTIFS ANALYSIS The 370 predicted TabZIPs varied in their amino acid composition ranging from 129 (TabZIP48.1
and TabZIP94) to 920 (TabZIP106.1) residues with a molecular mass from 14 kDa (TabZIP94) to 103 kDa (TabZIP106.1). Their theoretical isoelectric point (pI) varied between 4.65 (TabZIP198.1
and TabZIP219.1) to 11.38 (TabZIP133). The grand average of hydropathicity (GRAVY) of each amino acid residue in the 370 TabZIP proteins was very low, which indicates better interaction
between TabZIP proteins and water molecules. The information regarding the physiochemical properties of TabZIPs is provided in Supplementary Table S2. In TabZIPs, ten motifs were identified
by the MEME database that gave insight into their function and divergence (Table 1, Fig. 3, Supplementary Fig. S2, and Supplementary Table S3). Motif 1 was present in all the TabZIP
proteins, while motif 7 was present in all the groups except group D. Group D comprised all motifs except motif 7 and 8. Motif 8 was shared by groups A, B, C, F, G and I. The results showed
that the TabZIPs share similar sequences and clustered in the same group. ANALYSIS OF CIS-REGULATING ELEMENTS OF STARCH PATHWAY GENES AND PREDICTION OF DNA BINDING DOMAIN (DBD) OF TABZIPS To
understand the mechanism of transcriptional regulation of wheat starch amylose and amylopectin biosynthesis pathway genes, their promoter regions were analysed for the cis-regulating
element using the available online wheat genome sequences datasets. It has been previously reported that in plants bZIPs prefer to bind ACGT core sequence like G-box (CACGTG), C-box
(GACGTC), and A-box (TACGTA) motifs. Promoter analysis showed the presence of A and G boxes which are the putative sites for bZIP DNA binding domains. To analyse the cis-regulating region,
up to 1.0 kb sequence upstream to open reading frames of starch pathways genes (GBSSI, GBSSII, SSI, SSII, SSIII, SSIV, SBEI, SBEIIa and SBEIIb) were identified. Five (GBSSI), seven (GBSSII),
four (SSI), four (SSII), six (SSIII), one (SSIV), thirteen (SBEI), six (SBEIIa), and one (SBEIIb) ACGT core sequence were found in the up-stream sequences of these genes. Three A boxes were
identified in SSII and one A box was identified in GBSSII and SSIV (Supplementary Fig. S3). IDENTIFICATION OF CANDIDATE TABZIPS REGULATING AMYLOSE BIOSYNTHESIS IN WHEAT Three approaches,
namely genome-wide transcriptome sequencing, candidate genes based qRT-PCR, and a statistical correlation analysis of GBSSI and SBEII in the contrasting mutant lines for amylose content,
were applied for the identification of candidate TabZIPs for high amylose biosynthesis. SEQUENCE CHARACTERIZATION AND FUNCTIONAL ANALYSIS OF GBSSI, SBEIIA, AND SBEIIB IN MUTANTS The
sequences of GBSSI, SBEIIa, and SBEIIb, which were retrieved from transcriptome sequence data, identified both synonymous and non-synonymous mutations in their coding regions (Supplementary
Fig. S4). The mutations were also detected in their homoeologous loci. Three, one, and two non-synonymous mutation frequency were identified in the catalytic domain of GBSSI-2BL, GBSSI-7AS,
and GBSSI-7DS, respectively. In SBEII isoforms (SBEIIa and SBEIIb), mutation frequency was high and the sequencing errors cannot be ruled out. Both SBEII isoforms were located at three
homoeologous chromosomes i.e. 2AL, 2BL, and 2DL. The frequency of mutations in SBEIIa and SBEIIb were relatively higher than GBSSI (Supplementary Fig. S4). The comparative gene expression
analysis of GBSSI, SBEIIa and SBEIIb starch metabolic genes at three development stages (21, 28 and 35 days after anthesis, DAA) revealed variation in their expression levels. GBSSI showed
high expression during seed development in the high amylose mutant line compared to the low amylose mutant line (Supplementary Fig. S5). During seed development, SBEII isoforms (SBEIIa and
SBEIIb) showed very low expression levels in the high amylose mutant line in comparison to that of low amylose mutant line. The higher expression level of GBSSI and lower expression of both
isoforms of SBEIIa and SBEIIb in the high amylose mutant line were correlated with increased amylose content during seed development (Supplementary Fig. S5). TRANSCRIPTOME SEQUENCING OF
MUTANT LINES FPKMs (Fragments Per Kilobase of transcript per Million mapped reads) value is a normalization method for gene expression study. FPKMs of the 370 TabZIP genes were retrieved
from the transcriptomic sequence data. The sequence data was generated on two biological replicates of the developing seeds (28 days after anthesis) belonging to the two mutant lines, ‘TAC
75’ with 65% amylose (high amylose mutant line) and ‘TAC 6’ with 7% amylose (low amylose mutant line), and the parent wheat variety, ‘C 306’ with 26% amylose. The genes having FPKM values of
at least 0.02 were considered to be expressed and were used for differential gene expression analysis (Supplementary Table S4A, S4B). Considering an FPKM value of 0.02 as the cutoff point,
81 out of 370 (~22%) TabZIPs did not show expression in any three genotypes i.e. 289 (78%) TabZIPs showed expression in at least one genotype at a given time. Individual genotype data showed
that 236 (63.8%) TabZIPs showed expression in the high amylose mutant, 241 (63.6%) in the low amylose mutant, and 226 (61.1%) in their parent variety. In pairwise comparison, 177 TabZIPs
showed expression in all the three genotypes as shown in the Venn diagram (Fig. 4A). The graph also revealed that 12 TabZIPs were only expressed in the high amylose mutant and therefore
unique to the mutant line. The twelve TabZIPs were TabZIP15, TabZIP50.1, TabZIP54.5, TabZIP56.3, TabZIP91.1, TabZIP91.2, TabZIP120.2, TabZIP128, TabZIP167.2, TabZIP173.2, TabZIP184.2, and
TabZIP220.2. The FPKM values of these 12 TabZIPs were below 0.5, indicating low expression. The pairwise differential gene expression analysis of TabZIPs was done among three genotypes (two
mutant lines and one parent variety), using log 2-fold of mean FPKM data (two biological replicates). It involved three pairs, ‘TAC 75’ vs ‘TAC 6’, ‘TAC 75’ vs ‘C 306’, and ‘TAC 6’ vs ‘C
306’ (Fig. 4B). Out of the three, only two pairs (‘TAC 75’ vs ‘TAC 6’ and ‘TAC 75’ vs ‘C 306’) were used to identify candidate TabZIPs that may be involved in high amylose biosynthesis by
comparing bZIPs expression in the above said genotypes. In comparison, the TabZIP that have FPKM value of zero in other genotype was not taken into consideration as reasons for absence
cannot be determined. In this study a relatively stringent criterion of 5-fold differential expression was considered to identify putative candidate TabZIP for high amylose biosynthesis. A
total of 147 TabZIPs showed at least 2-fold differential expression among the three pairs, ‘TAC 75’ vs ‘TAC 6’, ‘TAC 75’ vs ‘C 306’, and ‘TAC 6’ vs ‘C 306’ (Fig. 4B). In the pair, ‘TAC 75’
vs ‘TAC 6’, a total of 89 (39 + 18 + 9 + 23) TabZIPs showed differential expression (at least 2-fold) in the high amylose mutant line (‘TAC 75’) in comparison to the low amylose mutant line
(‘TAC 6’) (Fig. 4B, Supplementary Table S5). In this pair, 18 (out of 89) TabZIPs were unique to the pair ‘TAC 75’ vs ‘TAC 6’ (Fig. 4B). Out of 18 unique TabZIPs, two TabZIPs (TabZIP101.1
and TabZIP238.2) showed at least a 5-fold higher and three TabZIPs (TabZIP229.1, TabZIP229.3, and TabZIP238.1) showed at least a 5-fold lower in the high amylose mutant line in comparison to
the low amylose mutant line. Out of 18 candidate TabZIPs, five TabZIPs (TabZIP101.1, TabZIP238.2, TabZIP229.1, TabZIP229.3, and TabZIP238.1) may be candidate genes, regulating high amylose
biosynthesis. In the pair, ‘TAC 75’ vs ‘C 306’, a total of 91 (9 + 8 + 39 + 35) TabZIPs showed at least 2-fold differential expression in the high amylose mutant line in comparison to the
parent variety. In this pair, 8 TabZIPs were unique to the pair ‘TAC 75’ vs ‘C 306’ (Fig. 4B). Out of 8 unique TabZIP, only one TabZIP i.e. TabZIP145.3 showed at least 5-fold lower
expression in the high amylose mutant line in comparison to the parent variety. Therefore, TabZIP145.3 may regulate high amylose biosynthesis. Between the two pairs involving high amylose
mutant line (TAC 75’ vs ‘TAC 6’ and ‘TAC 75’ vs ‘C 306’), 9 TabZIPs were common in both the pairs (Fig. 4B). Out of 9, 7 TabZIPs (TabZIP237.1, TabZIP110, TabZIP157.1, TabZIP188.5,
TabZIP194.3, TabZIP117.1, and TabZIP137) showed >5-fold lower expression in the high amylose mutant line in both the pairs. These 7 TabZIPs may also regulate high amylose biosynthesis.
Among the three pairs (‘TAC 75’ vs ‘TAC 6’, ‘TAC 75’ vs ‘C 306’, and ‘TAC 6’ vs ‘C 306’), 39 TabZIPs were common (Fig. 4B). Out of 39, three TabZIPs (TabZIP117.2, TabZIP167.2, and
TabZIP184.2) showed at least a 5-fold higher expression in the high amylose line than the low amylose mutant line (‘TAC 6’) and the parent variety (‘C 306’) in the two pairs (‘TAC 75’ vs
‘TAC 6’, and ‘TAC 75’ vs ‘C 306’). These three TabZIPs showed at least 5-fold lower expression in the low amylose mutant line (‘TAC 6’) compared to the parent variety ‘C 306’ (Supplementary
Table S5). Similarly, three TabZIPs (TabZIP54.1, TabZIP59.2, and TabZIP77.1) showed at least a 5-fold lower expression in the high amylose line than the low amylose mutant line (‘TAC 6’) and
the parent variety (‘C 306’) in the two paired genotypes (‘TAC 75’ vs ‘TAC 6’, and ‘TAC 75’ vs ‘C 306’). These three TabZIPs showed at least 5-fold higher expression in the low amylose
mutant line (‘TAC 6’) than the parent variety (Supplementary Table S5). Therefore, these six TabZIPs (TabZIP117.2, TabZIP167.2, TabZIP184.2, TabZIP54.1, TabZIP59.2, and TabZIP77.1) are
putative candidate genes for high amylose biosynthesis. After three pair-wise comparisons, a total of 19 (5 + 1 + 7 + 6) candidate TabZIPs (5 positive regulators: TabZIP101.1, TabZIP117.2,
TabZIP167.2, TabZIP184.2, and TabZIP238.2 and 14 negative regulators: TabZIP54.1, TabZIP59.2, TabZIP77.1, TabZIP110, TabZIP117.1, TabZIP137, TabZIP145.3, TabZIP157.1, TabZIP188.5,
TabZIP194.3, TabZIP229.1, TabZIP229.3, TabZIP237.1, and TabZIP238.1) were identified for their suggested role in high amylose biosynthesis. In summary, differential expression (at least
5-fold) analysis of the transcriptome data of the two contrasting mutants and parent varieties identified 19 candidate TabZIPs for high amylose biosynthesis. These TabZIPs may play a pivotal
role in the high amylose biosynthesis regulation. The stringent criterion used in this study led to the identification of only few candidate TabZIPs. QRT-PCR-BASED CANDIDATE GENE EXPRESSION
ANALYSIS IN MUTANT LINES The detailed information on primers designed for qRT-PCR is provided in Supplementary Table S6. Pairwise differential expression analysis of quantitative expression
data of the randomly selected 52 (out of 370) TabZIPs was done at three seed developmental stages (21, 28, and 35 DAA) and is given in Supplementary Table S7. The pairwise differential gene
expression analysis was done among three genotypes (two mutant lines, ‘TAC 75’ & ‘TAC 6’ and one parent variety, ‘C 306’), using log 2-fold of mean expression data. It involved three
pairs, ‘TAC 75’ vs ‘TAC 6’, ‘TAC 75’ vs ‘C 306’, and ‘TAC 6’ vs ‘C 306’ (Supplementary Table S7). Out of the three pairs, only two pairs (‘TAC 75’ vs ‘TAC 6’ and ‘TAC 75’ vs ‘C 306’) are of
interest and were analysed further to identify candidate TabZIPs for high amylose biosynthesis by comparing expression of TabZIPs in the high amylose mutant line (‘TAC 75’) in comparison to
the low amylose line (‘TAC 6’) and the parent variety (‘C 306’). Differential expression analysis of the 52 TabZIPs revealed largely negative expression in the high amylose mutant line in
comparison to the low amylose mutant line (‘TAC 75’ vs ‘TAC 6’) and the parent variety (‘TAC 75’ vs ‘C 306’) (Supplementary Table S7). The majority of the TabZIPs showed a similar pattern
during three different seed development stages i.e. 21, 28 and 35 DAA. Differential gene expression analysis revealed five bZIPs (TabZIP69.1, TabZIP111, TabZIP121, TabZIP151, and TabZIP236)
that showed low expression in the high amylose line in comparison to the low amylose mutant line (‘TAC 75’ vs ‘TAC 6’) as well as to the parent variety (‘TAC 75’ vs ‘C 306’) (Fig. 4C,D) at
the three seed development stages. These five bZIPs may be involved in negative regulation of high amylose biosynthesis. However, two bZIPs, TabZIP50.2 and TabZIP54, showed at least 4-fold
positive expression in the high amylose line in comparison to the low amylose mutant line and the parent variety during late stage of seed development i.e. 28 and 35 DAA, the stages at which
amylose biosynthesis is very high18. Further analysis of differential gene expression data between the high amylose (‘TAC 75’) and low amylose (‘TAC 6’) mutant line showed development stage
specific expression, for example, at the 21 DAA seed development stage, 46 TabZIPs showed >2-fold negative expression and 2 TabZIPs showed >2-fold positive expression in the high
amylose mutant line. At 28 DAA seed development stage, 46 TabZIPs showed >2-fold negative expression and 5 TabZIPs showed >2-fold positive expression in the high amylose line. At 35
DAA seed development stage, 26 TabZIPs showed >2-fold positive expression and 47 TabZIPs showed >2-fold negative expression. Three biological replications, each with three technical
replicates, by and large provided similar results. Therefore, the above analysis identified five bZIPs, TabZIP69.1, TabZIP111, TabZIP121, TabZIP151, and TabZIP236 that showed lower
expression in the high amylose line in comparison to the low amylose mutant line and the parent variety (Fig. 4C,D) during the three seed development stages and may be the candidate genes
for high amylose biosynthesis. These five TabZIPs are distinct from the 19 TabZIPs identified in the transcriptome sequence data. Therefore, a total 24 candidate TabZIPs regulating high
amylose biosynthesis are identified in the two transcriptome studies. CORRELATION ANALYSIS OF TABZIPS WITH KEY ENZYMES FOR AMYLOSE BIOSYNTHESIS Two enzymes, ‘GBSSI’ and ‘SBEIIb’, are key
genes mainly responsible for amylose and amylopectin biosynthesis, respectively. The over-expression of GBSSI or down-expression of SBEIIb is functionally validated to high amylose
biosynthesis and vice-versa for amylopectin biosynthesis32. Therefore, it is important to analyze statistical correlation of expression data of TabZIPs with that of GBSSI and SBEIIb to
identify genes responsible for the regulation of the starch biosynthesis pathway. The pairwise statistical correlation analysis of the normalized expression data of the 52 TabZIPs with that
of GBSSI and SBEIIb identified 31 TabZIPs showing positive correlation with GBSSI, while 34 of 52 TabZIPs showed positive correlation with SBEIIb (Fig. 5). Among them, 14 TabZIPs showed
positive correlation with both enzymes, and therefore, possibly regulate the expression of both GBSSI and SBEIIb. However, no negative correlation was observed for SBEIIb. The correlation
analysis identified three TabZIPs (TabZIP 151, TabZIP121, TabZIP69.1) showing moderate negative to moderate positive correlation with GBSSI and SBEIIb, respectively (Fig. 5). The three are
candidate genes for high amylose biosynthesis. These three TabZIPs are also present in the identified 24 candidate TabZIPs. In summary, three approaches (transcriptome studies, qRT-PCR
analysis, and correlation data) identified 24 candidate TabZIPs for high amylose biosynthesis. _IN SILICO_ PREDICTION OF FUNCTIONAL ROLE OF CANDIDATE TABZIPS REGULATING HIGH AMYLOSE
BIOSYNTHESIS The functional role of the 24 candidate TabZIPs were predicted by two methods: 1) engaging phylogenetic group information and 2) protein interacting network analysis using
_Arabidopsis_ databases. FUNCTIONAL PREDICTIONS USING PHYLOGENETIC GROUP INFORMATION The functional role of the 24 candidate TabZIPs was determined using the functional information of
_Arabidopsis_ bZIP groups30. Their phylogenetic group information (Supplementary Table S1, Supplementary Fig. S2) revealed that the majority of the TabZIPs (10 TabZIPs) belonged to Group D
(Table 2). Other TabZIPs belonged to Group C (3 TabZIPs), Group I (3 TabZIP), Groups A (2 TabZIPs), Group G (2 TabZIPs), and one TabZIP each in Groups E, H, and U. However, one TabZIP is not
assigned to any group. The 10 TabZIPs of Group D were TabZIP59.2, TabZIP77.1, TabZIP117.1, TabZIP117.2, TabZIP167.2, TabZIP184.2, TabZIP229.1, TabZIP229.3, and TabZIP238.2. The majority of
Group D members are involved in plant development and defense30. Group I TabZIPs were TabZIP111, TabZIP137, and TabZIP157.1. Group I members are involved in vascular development33,34. The
Group C TabZIPs were TabZIP151, TabZIP188.5, and TabZIP194.3, and this group shows homology to Opaque 2, which regulates starch and carbohydrate biosynthesis in maize23. Group A TabZIPs were
TabZIP110 and TabZIP236 and its member are regulators of ABA-mediated signaling pathways and abiotic stress responsive genes. Group G TabZIPs were TabZIP101.1 and TabZIP237.1. Members of
this group are reported to be involved in regulation of light-mediated cell elongation. Member of Group E (TabZIP145.3) is reported to be involved in pollen development35. Member of Group H
(TabZIP69.1) is reported to be involved in systemic acquired resistance36. Member of Group U (TabZIP121) are involved in cellular transport and lipid metabolism37. PUTATIVE FUNCTIONAL
PREDICTION USING PROTEIN-PROTEIN INTERACTION NETWORK ANALYSIS Due to availability of limited genomic information and protein databases in wheat, the functional role of possible 24 candidate
bZIPs regulating high amylose biosynthesis were predicted _in silico_, using their _Arabidopsis_ homologs. The study of gene family interaction (protein-protein interaction) is important for
prediction of function in biological processes. In this the study, 13 potential protein interaction networks (N1 to N13) were identified for 24 TabZIPs (Table 2, Fig. 6). The master
regulators of the 13 networks were GBF1, AREB3, BZ02H3, BZIP9, PAN, TGA10, BZIP34, BZIP16, TGA6, AT1G06070, VIP1, AT1G19490, and TGA9. The four master regulators, TGA6, TGA9, TGA10, and PAN
belonged to TGACG (TGA) motif-binding bZIP sub-family. These master regulators are regulating 9 (out of 24) candidate TabZIPs for high amylose biosynthesis. The previous studies reported
their high degree of functional redundancy, mainly in plant disease resistance and stress responses. bZIPs like PAN, TGA9 and TGA10 are also involved in flower development. The master
regulator, AREB3 regulates 4 TabZIPs (TabZIP110, TabZIP117.1, TabZIP117.2, and TabZIP236) and belonged to ABA-response elements (ABREs) binding proteins (AREB). AREBs are involved in
ABA-responsive abiotic stress, mainly drought and high salinity stresses, and are involved in ABA-dependent signal transduction pathway. The functional roles of other master regulators are
given in Table 2. DISCUSSION The genome-wide analysis of wheat genome sequence data identified many TabZIPs, which are more than that reported in other major plant species, for example 75 in
_Arabidopsis_30, 89 in rice29, 92 in sorghum38, 170 in maize27, 121 in banana39, 77 in cassava40, and 96 in _Brachypodium_41. The previous report identified 182 bZIP proteins in wheat31. In
this study, out of 370, 184 new TabZIPs were identified (Supplementary Tables S1 and S2) and were confirmed by motif and domain analysis. The presence of a large number of TabZIPs in wheat
is expected due to its large genome size. bZIP numbers may further increase when the complete reference genome will be available. Multiple sequence alignment and phylogenetic analysis
clustered the wheat bZIP into 11 groups (Figs 1–3 and Supplementary Fig. S1), which is largely in agreement with the previous phylogenetic classification of plant bZIPs. Earlier, major plant
bZIPs were grouped in 10–11 groups, for example, 10 groups are reported in _Arabidopsis_30 and cassava40, and 11 groups in maize27, rice29, and banana39. Earlier bZIPs of wheat and its two
wild relatives and other plant species were grouped into 14 groups31. In this study, among the eleven phylogenetic groups identified, Group A and D are large cluster groups, each containing
83 TabZIPs. It is reported that bZIPs in Group A play an important role in abscisic acid (ABA) signaling and abiotic stresses. The abiotic stresses and ABA help to induce transcriptional and
post-translational regulation42,43. Several bZIPs were identified and shown to be ABA-responsive and improve multiple abiotic stress tolerance42,43,44. For example, OsbZIP23, a bZIP from
rice, plays a major role in ABA dependent drought and salinity tolerance43. Group D members are involved in plant development and defence30. For example, TGA (TGACG sequence binding protein)
family in _Arabidopsis_ interacts with the non-expresser of PR1 (NPR1), which is a key component in the salicylic acid defense signaling pathway45,46,47,48,49. The tobacco TGA1 and
_Arabidopsis_ TGA2 proteins are responsive to salicylic acid and bind to xenobiotic responsive promoters47,48,49. The functional information on Group B 14 TabZIPs is very limited. The
members of this group have trans-membrane domain and specific domain at C-terminus which are important for ER stress response39. Group C contains 48 TabZIPs and the members of this group
include _Opaque2_, which plays an important role in modulating seed-specific gene expression30. _Opaque2_ regulates seed storage protein expression by interacting with the PBF protein.
Groups E and F each contain 14 TabZIPs, but their functional information is not available. Group G comprises 32 TabZIPs and it is named so due to its members being G-box binding factors30.
The G-box binding factor genes from _Arabidopsis_ are connected to the regulation of light-responsive promoters, and are involved in biotic and abiotic stresses39,50. Group H contains nine
TabZIPs. The name of this group refers to the HY5 gene which is a bZIP transcription factor in _Arabidopsis_ that binds to a G-box, and regulates the stimulus-induced development30. The
control of HY5 activity by light is also well documented in dark-grown _Arabidopsis_30,40. Group I contains 44 TabZIPs. Various studies on Group I genes have been reported from different
plant species (Rice RF2a and tomato VSF-1), which indicate their role in vascular development51,52. Group S in wheat is the third smallest group in wheat with 19 TabZIPs, whereas in
_Arabidopsis_ it is the largest30. In _Arabidopsis_ the A_TBZIP11/ATB2_ gene is regulated by light, and play role in carbohydrate-consuming53 (i.e. sink) and in the vascular system30. The
_ATB2_ is involved in post-transcriptional repression by sucrose and carbohydrate balancing53. While S Group, in monocot and dicot species get activated during stress54. Group U in wheat is
the smallest with five TabZIPs, which are very similar to the members of other plant species, such as maize and rice30. Members of this group have hydrophobic isoleucine instead of conserved
arginine 10 (Supplementary Fig. S1). This substitution in amino acid affects the DNA binding specificity of bZIP, which is documented in earlier studies55. This classification was further
supported by conserved motif analysis. Conserved motif analysis indicated that almost all the TabZIPs in wheat contained typical bZIP domains. Additionally, each subfamily had some common
motifs and some subfamilies also contained the special motifs. These features in conserved bZIP motifs were also observed in maize, rice and _Arabidopsis_. Generally, most TabZIP genes in
the same subfamilies showed similar gene structure and conserved motifs, which support their close evolutionary relationship and the classification of subfamilies (Table 1, Fig. 3, and
Supplementary Table S3). As wheat is among the most significant crops, the biological processes like seed development and maturation are vital for starch quality. The bZIP family has been
reported to have role in the seed development processes of many plant species, however, the role of bZIPs in amylose or amylopectin biosynthesis regulation is undetermined in wheat. TabZIPs
act as positive as well as negative regulators of genes, which actively take part in physiological processes mainly via DNA binding. In the current study, cis-regulatory sequences analysis
of the starch biosynthesis pathway indicate the presence of high density of G and A boxes in GBSS I, and SBE II genes (Supplementary Fig. S3), indicating a high probability of bZIPs binding
at their promoter regions. Therefore, some may have active role in regulation of amylose and amylopectin biosynthesis. Before comparative gene expression analysis and next generation
sequencing (NGS), the mutation lines were characterized by sequencing two key genes (GBSSI and SBEII) responsible for amylose and amylopectin biosynthesis, both synonymous and non-synonymous
mutations were detected in their isoforms. The frequency of mutations in SBEIIa and SBE IIb were relatively higher than GBSSI (Supplementary Fig. S4). The comparative gene expression
analysis of GBSSI, SBEIIa, and SBEIIb starch metabolic genes at three development stages (21, 28 and 35 DAA) in mutant lines validated high and low amylose mutants as reported earlier in
wheat and other crops10,12,17,18,56. Differential gene expression analysis using whole transcriptome NGS data (two biological replicates) and qRT-PCR data using randomly selected 52 TabZIPs
of the two contrasting mutants and parent varieties identified a total of 24 TabZIPs, which are potential candidate genes for high amylose biosynthesis. These TabZIPs may play role in high
amylose biosynthesis by either positive or negative regulation. bZIP transcription factors are key regulators of starch biosynthesis genes in rice and maize, but their role in regulating
these genes in wheat is poorly understood. It is reported earlier in rice where bZIP transcription factor OsbZIP5823 was regulator of six starch metabolic pathway genes including GBSSI and
SBEII in starch biosynthesis23. The identified candidate TabZIPs were categorized into 8 bZIP groups, namely A, C, D, E, G, H, I and U and one TabZIP (TabZIP54,1) was not assigned to any
groups (Table 2). The putative functional role of the 24 candidate TabZIPs were predicted by using phylogenetic group information as well as protein interacting network analysis using
_Arabidopsis_ databases. Their putative functional role is described in the Results section. The putative functional role can be grouped into four categories- stress (abiotic and biotic),
flower and seed development, starch biosynthesis, and seed storage protein regulations. Interactome analysis identified 13 protein interaction networks (N1 to N13, Fig. 6, Table 2). The
master regulators and partners of networks showed that many TabZIPs play important role in biological processes. The four master regulators, TGA6, TGA9, TGA10, and PAN identified in this
study belonged to TGACG (TGA) motif-binding bZIP sub-family. These master regulators are represented by 9 (out of 24) candidate TabZIPs for high amylose biosynthesis. Group D TabZIPs
(TabZIP59.2, 77.1, 167.2, 184.2) show homology with TGA9 and TGA10 (networks N1 and N4). TGA9 and TGA10 interact with floral glutaredoxins ROXY1 and ROXY2 and are required for anther
development57. They also interact with other TGAs (TGA4) and PAN58. The master regulator of other Group D members (TabZIP 229.1, TabZIP229.3, TabZIP238.1 and TabZIP238.2) in network N4 is
PAN (PERIANTHIA). PAN is involved in the determination of floral organ number and also in a post-translational modification by GRXC7/ROXY159. It also binds with BOP1 and BOP2 which are
involved in growth asymmetry, an important aspect of patterning in leaves and flowers60. Group I members are involved in vascular development30,51,52. TabZIP111 shows homology with VIP1 in
network N8 and TabZIP137 and TabZIP157.1 to _Arabidopsis_ bZIP (AT1G06070) in network N7 in _Arabidopsis_. They play role in vascular development in tobacco and when over-expressed in
_Arabidopsis_, caused growth retardation under a mannitol-stressed condition61. Group G members (TabZIP101.1 and 237.1) show homology with GBF and bZIP16 in network N13 (Table 2). GBF2 and
AtbZIP16, G-box binding proteins, are involved in the regulation of light or hormone induced stresses62,63. Group C members (TabZIP151, 188.5 and 194.3) show homology with BZ02H3 in network
N6 and AtbZIP9 in network N5. BZ02H3 regulates seed storage protein expression24. Group C TabZIPs also shows homology with _Opaque2_, which is more closely related to monocot species and
regulate seed storage protein production by interacting with the PBF protein in _Arabidopsis_’s embryo. Rice OsbZIP58 and OsbZIP20 regulate starch and carbohydrate biosynthesis23. The master
regulator of Group A members (TabZIP110 and TabZIP236 in network N2) is AREB (ABA-response elements binding proteins) which is induced by ABA and osmotic stresses64. AREB is activated by
SnRKs and is involved in the PP2C-SnRK-AREB pathway and is an important component of the ABA signalling pathway. The Interactome analysis provides broad sight to understand the regulation
and interacting partners’ of these candidate TabZIPs and suggest their putative functions. These wheat bZIPs will be used for validation in molecular breeding programme on large germplasm
set through e-QTL analysis and using functional genomics tools. CONCLUSION High amylose starch is considered to be a good dietary fibre, rich healthy starch, as it is not easily or slowly
digested in our guts and is finally transformed into small chain fatty acids (SCFAs) (prebiotics) by bacteria in the large intestine. There is a global demand to develop cereal crops with
high levels of resistant starch or dietary fibre rich food grains. Starch is composed of two fractions, amylose and amylopectin, which are synthesised by starch metabolic pathway genes. We
identified 370 TabZIP genes from wheat and unravelled their basic classification and evolutionary relationships using evolutionary and conserved protein motif analyses. This will provide
ample knowledge for functional characterization of bZIP genes. 24 candidate TabZIPs were identified for high amylose biosynthesis using whole transcriptome data of wheat contrasting mutants
for amylose content. Their putative functional roles were determined using protein-protein network analysis. The bZIPs are being used in our lab in molecular breeding for the improvement of
amylose content in wheat. All this information will lay a platform for future research on the functional characterization of potential TabZIPs and regulatory mechanism of high amylose
biosynthesis in cereal crops. This study therefore is advancing our understanding of the molecular basis of genetic enhancements of amylose content in wheat. MATERIALS AND METHODS PLANT
MATERIALS AND TRANSCRIPTOME SEQUENCE DATA Two contrasting mutant lines, ‘TAC 75’ (amylose content ~65%) and ‘TAC 6’ (amylose content ~7%) in M6 generation were used for the identification of
putative bZIPs for high amylose biosynthesis. The lines were developed after the EMS treatment of the parent bread wheat (_Triticum aestivum_ L.) variety ‘C 306’ (amylose content ~26%)56.
The 284 Gb transcriptome sequence data was generated from the two biological replicates of two contrasting mutant lines, ‘TAC 75’ and ‘TAC 6’ and their parent variety, ‘C 306’ (unpublished).
The whole transcriptome data will be made available by requesting the corresponding author and it is available at NABI’s intranet. For transcriptome sequencing, RNAs were extracted from
developing seeds at 28 days after anthesis. _IN SILICO_ IDENTIFICATION, PHYLOGENETIC ANALYSIS, AND PHYSIOCHEMICAL PROPERTIES OF BZIPS IN BREAD WHEAT The identification of the genome wide
distribution of bZIPs was performed in two steps. In the first step, the hidden Markov model profiles65 of the bZIP domain, _viz_. PF0017066, were used as queries to search the bZIP proteins
in the wheat proteome Ensembl database (http://plants.ensembl.org/index.html) using HMMER3.065. In the second step, a local BLASTp search was performed to identify the predicted wheat bZIPs
by HMMER3.0 with already known bZIPs from _Arabidopsis_30, maize27, rice29 and barley67. These potential wheat bZIPs were further examined for the existence and integrity of the bZIP domain
by using NCBI-CDD68 and InterproScan69. The bZIP protein sequences of wheat, rice, maize, barley, and _Arabidopsis_ were aligned by using ClustalW70 with gap opening and gap extension
penalties of 10 and 0.1, respectively. The neighbour-Joining (NJ) method was used to infer the evolutionary history of all bZIP protein sequences. The associated taxa clustered together in
the bootstrap test of 1000 replicas. The phylogenetic tree was constructed using MEGA software, version 671. The visualization and annotation of the constructed phylogenetic tree was carried
out by using EvolView72. The physiochemical properties of protein sequences such as molecular weight, isoelectric point, theoretical pI and GRAVY (Grand average of Hydropathicity) values of
TabZIPs were calculated using ExPASy Protparm73. MEME (version 4.11.2) was used for the prediction of conserved motifs. The limits specified for minimum width, maximum width, and maximum
numbers of motifs were 8, 50 and 10, respectively. The motifs were numbered according to their order displayed by MEME Suite. _IN SILICO_ CIS-REGULATING ELEMENTS MAP ANALYSIS OF STARCH
BIOSYNTHESIS PATHWAY GENES Cis-regulating elements of starch metabolic pathway genes were analysed to explore the DNA binding domains of bZIPs. The genomic sequences of starch metabolic
pathway genes (GBSSSI, GBSSII, SSI, SSII, SSIII, SSIV, SBEI, SBEIIa and SBEIIb) were retrieved from the International Genome Sequencing Consortium database (https://www.wheatgenome.org/)
(IWGSC). They were processed through Regulatory Sequence Analysis Tools (RSAT: http://rsat.ulb.ac.be/rsat/)74 to determine binding sites using their up to 1000 bp upstream sequences. _IN
SILICO_ DIFFERENTIAL GENE EXPRESSION ANALYSIS The transcriptome sequence data of the mutant lines and parent (unpublished) was used for the identification of wheat bZIPs (TabZIPs). The
identified bZIPs were further confirmed based on gene ontology and Pfam domain analysis. FPKMs (Fragments per kilobase of transcript per million mapped reads) values of TabZIPs were used for
gene expression study. FPKMs of the 370 TabZIP genes were retrieved from the transcriptomic sequence data on two biological replicates of the developing seeds (28 days after anthesis)
belonging to the two mutant lines and the parent wheat variety. In this study the TabZIP genes having FPKM values of at least 0.02 were considered to be expressed and used for differential
gene expression analysis. GENE EXPRESSION ANALYSIS BY QRT-PCR DURING SEED DEVELOPMENT The primers of 52 TabZIPs along with two key genes (GBSSI and SBEII) of starch metabolic pathway that
are largely responsible for amylose and amylopectin biosynthesis were designed using Primer Express Software Tool version 3.0 (Thermo Fisher Scientific, USA). The tagged spikes were
harvested at three seed developmental stages i.e. 21, 28, and 35 DAA for RNA extraction by Trizol method and cDNA preparation. The relative expression levels of the target bZIPs were
calculated by ΔΔCt method (Schmittgen and Livak _et al_., 2001). Wheat ADP-Ribosylation Factor, ARF (AB050957.1) was used as an internal control gene for normalization of gene expression
data. STATISTICAL CORRELATION ANALYSIS Pearson’s correlation analysis was performed between the normalized expression data (qRT-PCR) of 52 TabZIPs with that of SBEIIb and GBSSI (all values
taken in this study are normalized Ct values with housekeeping ARF gene). INTERACTOME ANALYSIS The putative function was determined for the candidate TabZIPs identified for high amylose
biosynthesis through interactome analysis i.e. protein interacting network analysis. Wheat bZIPs homolog’s were determined in _Arabidopsis_ and then their protein-protein interaction (PPI)
networks were identified in _Arabidopsis thaliana_ databases (https://string-db.org/) using default parameters. REFERENCES * Pfister, B. & Samuel, C. Z. Formation of starch in plant
cells. _Cellular and Molecular Life Sciences._ 73(14), 2781–2807 (2016). Article CAS Google Scholar * Zeeman, S. C., Jens, K. & Smith, A. M. Starch: its metabolism, evolution, and
biotechnological modification in plants. _Annual review of plant biology._ 61, 209–234 (2010). Article CAS Google Scholar * Birt, D. F. _et al_. Resistant starch: promise for improving
human health. _Advances in Nutrition: An International Review Journal._ 4(6), 587–601 (2013). Article CAS Google Scholar * Topping, D. L. & Peter, M. Clifton. Short–chain fatty acids
and human colonic function: roles of resistant starch and nonstarch polysaccharides. _Physiological reviews._ 81(3), 1031–1064 (2001). Article CAS Google Scholar * Jenkins, D. J. A. _et
al_. Glycemic index: overview of implications in health and disease. _The American journal of clinical nutrition._ 76(1), 266S–273S (2002). Article MathSciNet CAS Google Scholar * Eskin,
N. A. Michael, and Fereidoon Shahidi. _Biochemistry of foods_. Academic Press, (2012). * Messer, E. Food Systems and Dietary Perspective: Are Genetically Modified Organisms the Best Way to
Ensure Nutritionally Adequate Food? _Indiana Journal of Global Legal Studies_. 65–90 (2001). * Fitzgerald, M. A., Susan, R. M. C. & Robert, D. Hall. Not just a grain of rice: the quest
for quality. _Trends in plant science._ 14(3), 133–139 (2009). Article CAS Google Scholar * Tang, G., Galili, G. & Zhuang, X. RNAi and microRNA: breakthrough technologies for the
improvement of plant nutritional value and metabolic engineering. _Metabolomics._ 3(3), 357–369 (2007). Article CAS Google Scholar * Tetlow, I. J. Understanding storage starch
biosynthesis in plants: a means to quality improvement. _Botany._ 84(8), 1167–1185 (2006). CAS Google Scholar * Tester, R. F., Karkalas, J. & Qi, X. Starch composition, fine structure
and architecture. _Journal of Cereal Science._ 39(2), 151–165 (2004). Article CAS Google Scholar * Jenkins, P. J. & Donald, A. M. The influence of amylose on starch granule structure.
_International Journal of Biological Macromolecules._ 17(6), 315–321 (1995). Article CAS Google Scholar * Jobling, S. Improving starch for food and industrial applications. _Current
opinion in plant biology._ 7(2), 210–218 (2004). Article CAS Google Scholar * Tatsuro, H. & Terao, T. A comprehensive expression analysis of the starch synthase gene family in rice
(_Oryza sativa_ L.). _Planta._ 220(1), 9–16 (2004). Article Google Scholar * Pandey, M. K. _et al_. Different isoforms of starch–synthesizing enzymes controlling amylose and amylopectin
content in rice _(Oryza sativa_ L.). _Biotechnology advances._ 30(6), 1697–1706 (2012). Article CAS Google Scholar * Smith, A. M. The biosynthesis of starch granules. _Biomacromolecules._
2(2), 335–341 (2001). Article CAS Google Scholar * Carciofi, M. _et al_. Concerted suppression of all starch branching enzyme genes in barley produces amylose–only starch granules. _BMC
plant biology._ 12(1), 223 (2012). Article CAS Google Scholar * Singh, A. _et al_. Expression patterns of genes involved in starch biosynthesis during seed development in bread wheat
(_Triticum aestivum_). _Molecular breeding._ 35(9), 184 (2015). Article ADS Google Scholar * Yamakawa, H. _et al_. Comprehensive expression profiling of rice grain filling–related genes
under high temperature using DNA microarray. _Plant physiology._ 144, 258–277 (2007). Article CAS Google Scholar * Landschulz, W. H., Peter, F. J. & Steven, L. M. K. The leucine
zipper: a hypothetical structure common to a new class of DNA binding proteins. _Science._ 240(4860), 1759–1765 (1988). Article ADS CAS Google Scholar * Ellenberger, T. E. _et al_. The
GCN4 basic region leucine zipper binds DNA as a dimer of uninterrupted α helices: crystal structure of the protein–DNA complex. _Cell_ 71, 1223–1237 (1992). Article CAS Google Scholar *
She, K. –C. _et al_. A novel factor FLOURY ENDOSPERM2 is involved in regulation of rice grain size and starch quality. _The Plant Cell._ 22(10), 3280–3294 (2010). Article CAS Google
Scholar * Wang, J. –C. _et al_. OsbZIP58, a basic leucine zipper transcription factor, regulates starch biosynthesis in rice endosperm. _Journal of experimental botany._ 64(11), 3453–3466
(2013). Article CAS Google Scholar * Maddaloni, M. _et al_. The transcriptional activatorOpaque–2 controls the expression of a cytosolic form of pyruvate orthophosphate dikinase–1 in
maize endosperms. _Molecular and General Genetics._ 250(5), 647–654 (1996). CAS PubMed Google Scholar * Fu, F.-F. & Xue, H.-W. Coexpression analysis identifies Rice Starch Regulator1,
a rice AP2/EREBP family transcription factor, as a novel rice starch biosynthesis regulator. _Plant Physiology._ 154(2), 927–938 (2010). Article CAS Google Scholar * Chen, J. _et al_.
ZmbZIP91 regulates expression of starch synthesis-related genes by binding to ACTCAT elements in their promoters. _Journal of Experimental Botany_ 67(5), 1327–1338 (2016). Article CAS
Google Scholar * Wei, K. _et al_. Genome–wide analysis of bZIP–encoding genes in maize. _DNA research._ 19(6), 463–476 (2012). Article CAS Google Scholar * Baloglu, M. C. _et al_.
Genome–wide analysis of the bZIP transcription factors in cucumber. _PLoS One_ 9(4), e96014 (2014). Article ADS Google Scholar * Nijhawan, A. _et al_. Genomic survey and gene expression
analysis of the basic leucine zipper transcription factor family in rice. _Plant physiology_ 146(2), 333–350 (2008). Article CAS Google Scholar * Jakoby, M. _et al_. bZIP transcription
factors in Arabidopsis. _Trends in plant science_ 7(3), 106–11116 (2002). Article CAS Google Scholar * Li, X. _et al_. Genome–wide identification and evolutionary analyses of bZIP
transcription factors in wheat and its relatives and expression profiles of anther development related TabZIP genes. _BMC genomics_. 16 (2015). * Szklarczyk, D. _et al_. STRINGv10:
protein–protein interaction networks, integrated over the tree of life. _Nucleic Acids Res._ 43(D1), D447–52 (2015). Article CAS Google Scholar * Liu, C., Wu, Y. & Wang, X. bZIP
transcription factor OsbZIP52/RISBZ5: a potential negative regulator of cold and drought stress response in rice. _Planta._ 235(6), 1157–1169 (2012). Article CAS Google Scholar * Nakase,
M., Aoki, N., Matsuda, T. & Adachi, T. Characterization of a novel rice bZIP protein which binds to the α-globulin promoter. _Plant molecular biology._ 33(3), 513–522 (1997). Article
CAS Google Scholar * Gibalova, A. Rena kD, Matczuk K, Duplakova N, Chab D, Twell D, Honys D. AtbZIP34 is required for Arabidopsis pollen wall patterning and the control of several
metabolic pathways in developing pollen. _Plant Mol Biol_ 70, 581–601 (2009). Article CAS Google Scholar * Zhang, Y. _et al_. Knockout analysis of Arabidopsis transcription factors TGA2,
TGA5, and TGA6 reveals their redundant and essential roles in systemic acquired resistance. _The Plant Cell._ 15(11), 2647–2653 (2003). Article CAS Google Scholar * Gibalová, A. _et al_.
Characterization of pollen-expressed bZIP protein interactions and the role of ATbZIP18 in the male gametophyte. _Plant reproduction._ 30(1), 1–17 (2017). Article Google Scholar * Wang, J.
_et al_. Genome wide expansion and expression divergence of the basic leucine zipper transcription factors in higher plants with an emphasis on Sorghum. _Journal of integrative plant
biology._ 53(3), 212–231 (2011). Article CAS Google Scholar * Hu, W. _et al_. Genome–wide analyses of the bZIP family reveal their involvement in the development, ripening and abiotic
stress response in banana. _Scientific reports_. 6 (2016). * Hu, W. _et al_. Genome–wide characterization and analysis of bZIP transcription factor gene family related to abiotic stress in
cassava. _Scientific reports._ 6, 22783 (2016). Article ADS CAS Google Scholar * Xiang, L. & Chu, Z. Genome–wide evolutionary characterization and analysis of bZIP transcription
factors and their expression profiles in response to multiple abiotic stresses in. _Brachypodiumdistachyon. BMC genomics._ 16(1), 227 (2015). Article Google Scholar * Johnson, R. R.,
Wagner, R. L., Verhey, S. D. & Walker–Simmons, M. K. The abscisic acid–responsive kinase PKABA1 interacts with a seed–specific abscisic acid response element–binding factor, TaABF, and
phosphorylates TaABF peptide sequences. _Plant Physiology._ 130(2), 837–846 (2002). Article Google Scholar * Xu, D. B. _et al_. ABI–like transcription factor gene TaABL1 from wheat
improves multiple abiotic stress tolerances in transgenic plants. _Functional & integrative genomics._ 14(4), 717–730 (2014). Article CAS Google Scholar * Nakamura, S., Komatsuda, T.
& Miura, H. Mapping diploid wheat homologues of _Arabidopsis_ seed ABA signaling genes and QTLs for seed dormancy. _Theoretical and Applied Genetics._ 114(7), 1129–1139 (2007). Article
CAS Google Scholar * Xiang, Y., Tang, N., Du, H., Ye, H. & Xiong, L. Characterization of OsbZIP23 as a key player of the basic leucine zipper transcription factor family for conferring
abscisic acid sensitivity and salinity and drought tolerance in rice. _Plant physiology._ 148(4), 1938–1952 (2008). Article CAS Google Scholar * Alves, M. S. _et al_. Plant bZIP
transcription factors responsive to pathogens: a review. _International journal of molecular sciences._ 14(4), 7815–7828 (2013). Article Google Scholar * Singh, K. B., Foley, R. C. &
Oñate–Sánchez, L. Transcription factors in plant defense and stress responses. _Current opinion in plant biology._ 5(5), 430–436 (2002). Article CAS Google Scholar * Després, C., DeLong,
C., Glaze, S., Liu, E. & Fobert, P. R. The Arabidopsis NPR1/NIM1 protein enhances the DNA binding activity of a subgroup of the TGA family of bZIP transcription factors. _The Plant
Cell._ 12(2), 279–290 (2000). Article Google Scholar * Zhou, J. M. _et al_. NPR1 differentially interacts with members of the TGA/OBF family of transcription factors that bind an element
of the PR–1 gene required for induction by salicylic acid. _Molecular Plant–Microbe Interactions._ 13(2), 191–202 (2000). Article MathSciNet CAS Google Scholar * Zhang, L. _et al_. A
novel wheat bZIP transcription factor, TabZIP60, confers multiple abiotic stress tolerances in transgenic Arabidopsis. _Physiologiaplantarum._ 153(4), 538–554 (2015). CAS Google Scholar *
Ringli, C. & Keller, B. Specific interaction of the tomato bZIP transcription factor VSF–1 with a non–palindromic DNA sequence that controls vascular gene expression. _Plant molecular
biology._ 37(6), 977–988 (1998). Article CAS Google Scholar * Yin, Y., Zhu, Q., Dai, S., Lamb, C. & Beachy, R. N. RF2a, a bZIP transcriptional activator of the phloem specific rice
tungro bacilliform virus promoter, functions in vascular development. _The EMBO Journal._ 16(17), 5247–5259 (1997). Article CAS Google Scholar * Rook, F. _et al_. Sucrose‐specific
signalling represses translation of the Arabidopsis ATB2 bZIP transcription factor gene. _The Plant Journal_ 15(2), 253–263 (1998). Article CAS Google Scholar * Chen, H. _et al_. Basic
leucine zipper transcription factor OsbZIP16 positively regulates drought resistance in rice. _Plant science._ 193, 8–17 (2012). Article Google Scholar * Wei, K. A. I. F. A. _et al_.
Genome-wide analysis of bZIP-encoding genes in maize. _DNA research_ 19(6), 463–476 (2012). Article CAS Google Scholar * Mishra, A. _et al_. Development of EMS–induced mutation population
for amylose and resistant starch variation in bread wheat (_Triticum aestivum_) and identification of candidate genes responsible for amylose variation. _BMC plant biology_ 16(1), 217
(2016). Article MathSciNet Google Scholar * Murmu, J. _et al_. Arabidopsis bZIP transcription factors TGA9 and TGA10 interact with floral glutaredoxins ROXY1 and ROXY2 and are redundantly
required for anther development. _Plant Physiology_. pp-110 (2010). * Deppmann, C. D., Rebecca, S. & Elizabeth, J. T. Cross-species annotation of basic leucine zipper factor
interactions: Insight into the evolution of closed interaction networks. _Molecular biology and evolution._ 23(8), 1480–1492 (2006). Article CAS Google Scholar * Li, S. _et al_. Nuclear
activity of ROXY1, a glutaredoxin interacting with TGA factors, is required for petal development in Arabidopsis thaliana. _The Plant Cell_ 21(2), 429–441 (2009). Article CAS Google
Scholar * Hepworth, S. R. _et al_. BLADE-ON-PETIOLE–dependent signaling controls leaf and floral patterning in Arabidopsis. _The Plant Cell._ 17(5), 1434–1448 (2005). Article CAS Google
Scholar * Tsugama, D., Shenkui, L. & Tetsuo, T. Analysis of functions of VIP1 and its close homologs in osmosensory responses of Arabidopsis thaliana. _PLoS One_ 9(8) (2014). Article
ADS Google Scholar * Menkens, A. E. & Anthony, R. C. Isolation and characterization of a fourth Arabidopsis thaliana G-box-binding factor, which has similarities to Fos oncoprotein.
_Proceedings of the National Academy of Sciences._ 91(7), 2522–2526 (1994). Article ADS CAS Google Scholar * Shaikhali, J. GIP1 protein is a novel cofactor that regulates DNA-binding
affinity of redox-regulated members of bZIP transcription factors involved in the early stages of Arabidopsis development. _Protoplasma._ 252(3), 867–883 (2015). Article CAS Google Scholar
* Kim, S. _et al_. ARIA, an Arabidopsis arm repeat protein interacting with a transcriptional regulator of abscisic acid-responsive gene expression, is a novel abscisic acid signaling
component. _Plant physiology_ 136(3), 3639–3648 (2004). Article MathSciNet CAS Google Scholar * Finn, R. D., Clements, J. & Eddy, S. R. HMMER web server: interactive sequence
similarity searching. _Nucleic acids research_ 367 (2011). * Finn, R. D. _et al_. The Pfam protein families database: towards a more sustainable future. _Nucleic acids research._ 44(D1),
D279–D285 (2016). Article CAS Google Scholar * Pourabed, E., Golmohamadi, F. G., Monfared, P. S., Razavi, S. M. & Shobbar, Z. S. Basic leucine zipper family in barley: genome–wide
characterization of members and expression analysis. _Molecular biotechnology._ 57, 12–26 (2015). Article CAS Google Scholar * Marchler–Bauer, A. _et al_. NCBI’s conserved domain
database. _Nucleic Acids Res._ 43, D222–226 (2015). Article Google Scholar * Jones, P. _et al_. InterProScan 5: genome–scale protein function classification. _Bioinformatics._ 30,
1236–1240 (2014). Article CAS Google Scholar * Thompson J. D., Gibson T. & Higgins D. G. Multiple sequence alignment using ClustalW and ClustalX. _Current protocols in
bioinformatics_, 2–3 (2002). * Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. _Molecular biology and
evolution._ 30(12), 2725–2729 (2013). Article CAS Google Scholar * Zhang, H., Gao, S., Lercher, M. J., Hu, S. & Chen, W. H. EvolView, an online tool for visualizing, annotating and
managing phylogenetic trees. _Nucleic acids research._ 40(W1), W569–W572 (2012). Article CAS Google Scholar * Gasteiger, E. _et al_. Protein identification and analysis tools on the
ExPASy server (pp. 571–607). Humana Press (2005). * Thomas–Chollier, M. _et al_. RSAT: regulatory sequence analysis tools. _Nucleic acids research. 36, suppl_ 2, W119–W127 (2008). Article
Google Scholar Download references ACKNOWLEDGEMENTS We would like to thank the Executive Director of the National Agri-Food Biotechnology Institute (NABI), Mohali, India for funds and
support. The research work presented in the manuscript was funded by Department of Biotechnology (DBT), Ministry of Science & Technology, Government of India. PK is thankful to the ICMR,
India, for the grant of Junior Research Fellowship (JRF) and Senior Research Fellowship (SRF) towards his Ph.D. The authors are thankful to Gregory Hoover of Clemson University for
manuscript editing. The authors are also thankful to DeLCON for online library facility. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * National Agri-Food Biotechnology Institute (NABI),
Sector-81, SAS Nagar, Mohali, 140306, Punjab, India Pankaj Kumar, Ankita Mishra, Himanshu Sharma, Mohammed Saba Rahim, Monica Sharma, Afsana Parveen, Prateek Jain, Vikas Rishi & Joy Roy
* Department of Biotechnology, Panjab University, Chandigarh, 160014, India Pankaj Kumar, Ankita Mishra, Afsana Parveen & Prateek Jain * Centre for Computational Biology and
Bioinformatics, School of Life Sciences, Central University of Himachal Pradesh, Kangra, 176206, Himachal Pradesh, India Dixit Sharma & Shailender Kumar Verma * Department of Plant
Sciences, School of Basic and Applied Sciences, Central University of Punjab, Bathinda, 151001, India Mohammed Saba Rahim Authors * Pankaj Kumar View author publications You can also search
for this author inPubMed Google Scholar * Ankita Mishra View author publications You can also search for this author inPubMed Google Scholar * Himanshu Sharma View author publications You
can also search for this author inPubMed Google Scholar * Dixit Sharma View author publications You can also search for this author inPubMed Google Scholar * Mohammed Saba Rahim View author
publications You can also search for this author inPubMed Google Scholar * Monica Sharma View author publications You can also search for this author inPubMed Google Scholar * Afsana Parveen
View author publications You can also search for this author inPubMed Google Scholar * Prateek Jain View author publications You can also search for this author inPubMed Google Scholar *
Shailender Kumar Verma View author publications You can also search for this author inPubMed Google Scholar * Vikas Rishi View author publications You can also search for this author
inPubMed Google Scholar * Joy Roy View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS J.R. conceived and designed the experiments. P.K.
conducted experiment works, data analysis, and manuscript writing. A.M. had developed mutant lines. H.S., M.S.R., M.S., A.P., P.J. helped in experimental works. D.S. helped in the
identification, phylogenetic and conserved motif analysis. V.R. and S.K.V. helped in data analysis and edited the manuscript. All authors read and approved the final manuscript.
CORRESPONDING AUTHOR Correspondence to Joy Roy. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature
remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION TABLE S4 RIGHTS AND
PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any
medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The
images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not
included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly
from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kumar, P., Mishra,
A., Sharma, H. _et al._ Pivotal role of bZIPs in amylose biosynthesis by genome survey and transcriptome analysis in wheat (_Triticum aestivum_ L.) mutants. _Sci Rep_ 8, 17240 (2018).
https://doi.org/10.1038/s41598-018-35366-8 Download citation * Received: 20 December 2017 * Accepted: 31 October 2018 * Published: 22 November 2018 * DOI:
https://doi.org/10.1038/s41598-018-35366-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * Amylose Biosynthesis * Wheat bZIP * High Amylose *
Amylose Mutants * Amylose Content