Play all audios:
ABSTRACT Epidermal equivalents prepared with passaged keratinocytes are typically 10–20 μm thick, whereas intact human epidermis is up to 100 μm thick. Our established mathematical model of
epidermal homeostasis predicted that the undulatory pattern of the papillary layer beneath the epidermis is a key determinant of epidermal thickness. Here, we tested this prediction by
seeding human keratinocytes on polyester textiles with various fiber-structural patterns in culture dishes exposed to air, aiming to develop a more physiologically realistic epidermal model
using passaged keratinocytes. Textile substrate with fiber thickness and inter-fiber distance matching the computer predictions afforded a three-dimensional epidermal-equivalent model with
thick stratum corneum and intercellular lamellar lipid structure. The basal layer structure was similar to that of human papillary layer. Cells located around the textile fibers were
proliferating, as indicated by BrdU and YAP (Yes-associated protein) staining and expression of melanoma-associated chondroitin sulfate proteoglycan. Filaggrin, loricrin, claudin 1 and ZO-1
were all appropriately expressed. Silencing of transcriptional coactivator YAP with siRNA disturbed construction of the three-dimensional structure. Measurement of trans-epidermal water loss
(TEWL) indicated that the model has excellent barrier function. Our results support the idea that mathematical modeling of complex biological processes can have predictive ability and
practical value. SIMILAR CONTENT BEING VIEWED BY OTHERS A MECHANISTIC VIEW ON THE AGING HUMAN SKIN THROUGH EX VIVO LAYER-BY-LAYER ANALYSIS OF MECHANICS AND MICROSTRUCTURE OF FACIAL AND
MAMMARY DERMIS Article Open access 17 January 2022 UTILIZATION OF PATTERNED BIOPRINTING FOR HETEROGENEOUS AND PHYSIOLOGICALLY REPRESENTATIVE RECONSTRUCTED EPIDERMAL SKIN MODELS Article Open
access 18 March 2021 TRANSCRIPTOMIC ANALYSIS REVEALS DYNAMIC MOLECULAR CHANGES IN SKIN INDUCED BY MECHANICAL FORCES SECONDARY TO TISSUE EXPANSION Article Open access 29 September 2020
INTRODUCTION Experimental models of human epidermis are useful research tools for basic studies of skin biology and functional mechanisms, as well as for the development of transdermally
administrable medicines and safety testing of cosmetics and other products1. However, epidermis has a complex structure consisting of multiple layers2 and is not adequately mimicked by many
current models1. For example, epidermal models prepared with passaged keratinocytes are usually non-physiologically thin (10–20 μm), although thick epidermal equivalents (around 100 μm) have
been constructed with primary keratinocytes3. However, it is difficult to obtain primary keratinocytes with consistent properties in large amounts. We have established methodology for
simulating epidermal homeostasis4,5,6, employing a mathematical model composed of keratinocytes generated from stem cells distributed in the basal layer, and taking into account dynamic
cellular processes in the epidermis, such as migration and differentiation, as well as the interaction of intracellular Ca2+ dynamics with differentiation. Our previous numerical simulations
successfully reproduced a spatially and temporally stable epidermal structure with sufficient thickness and a flat stratum corneum/suprabasal layer interface6. Calculations suggested that
the epidermal structure and thickness are greatly influenced by the spatial distribution of stem cells and the structure of the basement membrane on which they are seeded. When we applied
sinusoidal modulation of the basement membrane shape and systematically changed the amplitude and wavelength, we found that formation of a thick and stable epidermal structure required
basement membrane undulations with large amplitude and short wavelength6. This result indicated that the undulatory pattern of the papillary layer, which lies at the top of the dermis
immediately below the epidermis, is critical for constructing an epidermal model with physiological thickness. However, one of the most important roles of the epidermis is its
water-impermeable barrier function, which requires a sufficiently thick stratum corneum. Since our previous model6 did not take into account flattening of corneocytes, it could not properly
simulate the thickness of stratum corneum. Here, we first established a new three-dimensional numerical simulation model that incorporates flattening of corneocytes to form a stratum corneum
sheet. Then, based on the computational predictions of the improved simulation model, we examined whether a full-thickness three-dimensional epidermal-equivalent model that included stratum
corneum and intercellular lamellar lipid structure, which are essential for skin barrier function, could be obtained by seeding passaged human keratinocytes on an undulating surface
consisting of polyester textile with an appropriate fiber pattern in culture dishes exposed to air. Our results represent a proof-of-principle of the value of this methodology for
establishing high-quality epidermal-equivalent models with passaged keratinocytes, and also indicate the importance of substrate structure for epidermal development. They also support the
idea that mathematical modeling of complex biological processes can have predictive ability and practical value. RESULTS STRUCTURE OF EPIDERMAL-EQUIVALENT MODELS GROWN ON PATTERNED TEXTILE
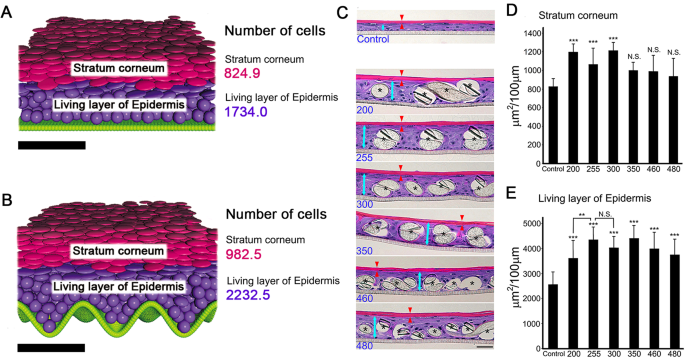
SUBSTRATES The results of the new three-dimensional computer simulation and the cultured epidermal models are illustrated in Fig. 1A–E. Compared with the result obtained using a flat
basement membrane (Fig. 1A), the stratum corneum and living layer were thicker on the sinusoidal basement membrane (Fig. 1B). The numbers of cells in the stratum corneum and the living layer
of the epidermis in the two cases are shown on the right. To validate the simulation results, we attempted to construct a thick epidermal model with passaged keratinocytes by using an
appropriately patterned substrate. For this purpose, we seeded keratinocytes on a series of textile samples with different textures in culture dishes. For comparison with the textile pattern
parameters (Table 1, Supplement Fig. 1), we also measured the corresponding values of human papillary layer, which exhibited a rete ridge height (corresponding to fiber thickness) of 51 μm
and an undulation interval (fiber interval) of 105 μm (n = 13, abdominal skin, average age of subjects 36.3 years). Representative microscopic hematoxylin and eosin (H&E)-stained images
of the grown epidermal models are shown in Fig. 1C. The results of quantification of the living layer of epidermis and stratum corneum are shown in Fig. 1D,E, respectively (no epidermal
sheet developed on samples #74, #86, #125, and #160). Textiles #200, #255 and #300 afforded significantly thicker stratum corneum than the control (no textile). The living layer of epidermis
on textile #255 was thicker than that on textile #200 (statistically significant) or #300 (not significant). Therefore, we focused on textile #255 for further study. INTEGRITY OF THE
EPIDERMAL-EQUIVALENT MODEL AND LOCATION OF PROLIFERATING CELLS Structural proteins filaggrin, loricrin, claudin 1 and ZO-1 play key roles in epidermis7,8 so to confirm that they were
appropriately expressed, we carried out immuno-histochemical studies of epidermal models grown on textile #255. As shown in Fig. 2, differentiation markers filaggrin (Fig. 2A) and loricrin
(Fig. 2B) and tight-junction markers ZO-1 (Fig. 2C) were all appropriately expressed at the upper layer of the epidermis. Claudin 1 was expressed in the cell membrane throughout the
epidermis (Fig. 2D). In addition, electron-microscopic images revealed thick stratum corneum (Fig. 2F, black asterisk) containing intercellular lipid bilayer structure (Fig. 2G, black
asterisk), suggesting that the model would show effective barrier function. Immunohistochemical studies of the model grown on textile #300 and the control are shown in supplement Fig. 2.
ZO-1 and claudin 1 were expressed in both cases, though expression of ZO-1 seemed weaker in the model grown on textile #300 than in that grown on textile #255. To confirm this, we measured
trans-epidermal water loss (TEWL) of control and epidermal models (Fig. 2H). Compared with the control, epidermal models grown on textile #255 showed significantly lower TEWL, indicating
that they have greater barrier function. In the present proof-of-concept study, we focused on TEWL to evaluate the skin equivalent model because it is widely used as a parameter of epidermal
water-impermeable barrier function, which is critical for the present purpose. Anti-BrdU staining revealed that proliferating cells were present only at the bottom in the control (Fig. 3A
no textile), whereas proliferating cells were also observed on fibers of the textile substrate in the experimental model (Fig. 3B, black arrows). We also examined expression of
melanoma-associated chondroitin sulfate proteoglycan (MCSP), which plays a role in stabilizing cell-substratum interactions9. Anti-MCSP immunostaining revealed positive cells on fibers (Fig.
3D, white arrows), supporting the idea that at least some cells on fibers had proliferative ability. Supplement Fig. 3 shows the results of double immunohistochemical staining with
anti-BrdU and K14, a basal-layer marker, of the model grown on textile #255 and the no-textile control. Co-expression of BrdU and K14 was observed on the top of the fibers in the model,
suggesting that proliferating cells may recognize the top of the fibers as a basal layer. ROLE OF YES-ASSOCIATED PROTEIN (YAP) IN THICK STRUCTURE DEVELOPMENT We next examined the role of the
key transcriptional regulator YAP in the present model by employing siRNA. In control epidermis, YAP was localized only at the basal layer (Fig. 3E), while in the textile-grown model, YAP
was expressed around fibers (Fig. 3F, red arrows). Representative images are shown in Fig. 3G–J. Application of YAP siRNA markedly disturbed the formation of three-dimensional structure, as
can be seen by comparison of H&E staining of control and YAP siRNA-applied textile-grown samples (Fig. 3G,H respectively). In control siRNA-treated epidermis, YAP was expressed around
fibers (Fig. 3I), while little expression was observed in YAP siRNA-treated samples (Fig. 3J). Application of YAP siRNA did not influence proliferation of keratinocytes in monolayer culture
(data not shown). YAP siRNA application also disturbed construction of three-dimensional structure in the control model (Supplement Fig. 4). The precise role of YAP in construction of the
thicker epidermal model remains to be established. DISCUSSION The _in vitro_ results confirmed our in-silico prediction that undulation of the basement membrane is critical for formation of
thick stratum and epidermal living layer. Indeed, the living layer of epidermis and the stratum corneum in the epidermal model were thickest when we used textile substrate having an
undulation pattern whose spatial scale was comparable to that predicted to be most effective by the computer simulation. Notably, this pattern is also similar to that of the human skin
papillary layer in 36-year-old healthy subjects. We also observed BrdU staining and expression of MCSP on cells located on textile fibers in our model, suggesting that cell contact with the
textile fibers might promote proliferation. The size and geometry of cells contribute to construction of tissue structure, because they influence intracellular biochemical patterns in
multi-cellular systems10. In addition, the spatial environment of bronchial epithelial cells influences proliferation rate11. Our results are thus consistent with the idea that the spatial
features of the basal layer of epidermis play a key role in epidermal structure development and homeostasis, and therefore that the substrate textile pattern is crucial for construction of a
thick epidermal model in our system. Further, since rete ridge height decreases with aging12 and epidermal permeability barrier recovery becomes slower with aging13, it will be interesting
to investigate whether aging-related changes of the undulation pattern of the basal layer are associated with impaired epidermal structure. Yes-associated protein (YAP) is a transcriptional
regulator associated with proliferation14. It is important in constructing three-dimensional body shape15 and in mechanotransduction16. In epidermis, YAP is involved in stem cell
proliferation17, terminal differentiation, epidermal permeability-barrier formation18 and wound healing19. Our findings indicate that YAP may also play a critical role in the development of
thick three-dimensional structure in our epidermal-equivalent model, though the mechanism involved remains to be determined. This work has confirmed the practical value of our mathematical
model for guiding the establishment of an epidermal equivalent model with a thick epidermal living layer and thick stratum corneum using passaged keratinocytes. Although other methods are
available to construct 3D epidermal equivalent models, as recently reviewed20, they have limitations. Various scaffold-based 3D models are available, but all of them require a hydrogel
scaffold, which our model does not. A scaffold-free 3D methodology has been described, but can only construct micro-scale models, so its usefulness is limited. Although we focused on TEWL to
evaluate our model, and did not carry out further evaluation of the barrier function, we believe our work provides a proof-of-concept of new methodology for the production of
physiologically realistic epidermal-equivalent models having a full-thickness epidermal layer that displays signs of differentiation, tight junctions and stratum corneum with intercellular
lipid bilayer structure, using passaged keratinocytes. Here, we used keratinocytes after three passages, which provided a 512-fold increase of cell number. This should make it possible to
construct low-cost epidermal-equivalent models while retaining high quality, although further optimization and validation studies will be necessary. These findings nicely illustrate the
utility of in-silico modeling of complex biological processes, since model parameters and conditions can be easily and inexpensively modified in computer simulations. MATERIALS AND METHODS
The mathematical model and computer simulation methodology are described in the supplement “Computer simulation”21. KERATINOCYTE CULTURE The basic methodology for constructing the skin
equivalent model was as described previously3. Normal human epidermal keratinocytes collected from neonate foreskin (NHEKs) from Kurabo (Osaka, Japan) were cultured in Epilife-KG2 (Kurabo)
containing 0.06 mM Ca2+ in 10 cm dishes and passaged three times. Then, 2.2 × 105 keratinocytes/500 μl CnT Prime medium (CELLnTEC, Berne, Switzerland) were plated on 12-well Millicells with
0.4 µm pore size PET hanging inserts (bore diameter 12 mm, Millipore, Billerica, MA). The inserts were precoated with CellStart (Invitrogen Life Technologies, Carlsbad, CA) in a 50X dilution
of DPBS. CnT Prime (CELLnTEC, Bern, Switzerland) (1 ml) was added to each well. At 72 hours after seeding (day 3) the medium was switched to CnT-PR-3D differentiation medium (CELLnTEC,
Bern, Switzerland) both inside and outside the inserts. Cultures were submerged in differentiation media for 16 hours and then lifted to the air-medium interface by removing excess medium
from the insert and reducing the volume of differentiation media on the outside to 500 µl. Cultures were fed daily with 500 µl of differentiation media for 9 days and then harvested. To
evaluate proliferative cells, BrdU was applied in the medium 24 hours before the harvest. POLYESTER TEXTILE SUBSTRATES Polyester textile (12-mm diameter; Clever, Toyohashi, Japan) with a
variety of fiber patterns was fixed with Rocktite 3554 (Henkel, Düsseldorf, Germany) and RTV118 (Momentive, New York, USA) to the bottom of the inserts, then pre-coated with CellStart, and
seeded with keratinocytes. Incubation was conducted as above. The characteristics of the textiles used are summarized in Table 1. EVALUATION OF FIBER PATTERNS IN TEXTILES AND NORMAL HUMAN
SKIN Fiber patterns of textiles and normal human skin were evaluated as illustrated in Supplement Fig. 1. Human tissues (non-sunexposed area from abdomen, n = 13; average age of subjects
36.3 years), obtained with informed consent following plastic surgery, were purchased from Biopredic International (Rennes, France) via KAC Co., Ltd. (Kyoto, Japan). This study was approved
by the ethics committee of Shiseido, and was conducted in accordance with the guideline of the National Institute of Health. YAP SIRNA APPLICATION One day before seeding cells onto 12-well
Millicells, the cells were grown to 80% confluency (approximately 2~3 × 106 cells), and transfected with 20 nM scramble control or YAP siRNA (GE Dharmacon, Lafayette, CO, USA) using the
transfection reagent RNA iMAX (Thermo Fisher Scientific, Waltham, MA USA) in OptiMem (Thermo Fisher Scientific) as described in the manual. Scramble control: ugguuuacaugucgacuaa,
ugguuuacauguuguguga, ugguuuacauguuuucuga and ugguuuacauguuuuccua. YAP: gcaccuaucacucucgaga, ugagaacaaugacgaccaa, ggucagagauacuucuuaa, ccaccaagcuagauaaaga (GE Dharmacon, Lafayette, USA).
Total RNA from human keratinocytes was isolated using and RNeasy mini kit (QIAGEN, Hilden, Germany) for quantitative real-time PCR (RT-PCR). Complementary DNA (cDNA) synthesis from 1 μg of
total RNA was performed using SuperScript VILO Master Mix (Invitrogen, Carlsbad, USA). The PCR reactions were performed using LightCycler 480 Probes Master (Roche, Basal, Switzerland), cDNA
and specific primer pairs: GAPDH: forward, gaaggtgaaggtcggagtc and reverse, gaagattggtgatgggatttc; YAP: forward, cccagatgaacgtcacagc and reverse, ttcccatccatcaggaagag, on an LightCycler 480
System II (Roche, Basel, Switzerland). Results were normalized with the GAPDH gene and showed that YAP siRNA decreased YAP expression by 87 ± 2%. HISTOLOGY For hematoxylin and eosin
(H&E) staining, anti-bromodeoxyuridine (BrdU) antibody or anti-YAP antibody staining, samples were fixed with 4% paraformaldehyde in PBS, embedded in paraffin, and sectioned at 3 μm. For
the evaluation of areas of stratum corneum and living layer of the epidermis, we constructed 4–6 samples sections per textile. We acquired 3 images per one section and took the average
value, and we used 3–5 sections from each condition. Image-J software was used to remove fiber cross-section areas from the images of the H&E-stained sections, and to evaluate the
remaining area of the living layer of the epidermis, and area of stratum corneum. For immunostaining of filaggrin, loricrin, and MCSP, samples were fixed in acetone at −20 °C for 30 min,
embedded in paraffin and sectioned at 3 μm for staining. For tight junction markers, 6 μm frozen sections were fixed in methanol at −20 °C for 30 min. Primary antibodies were rabbit
polyclonal anti-filaggrin (1/300, # sc-30229, Santa Cruz, Dallas, USA), rabbit polyclonal loricrin (1/500, # PRB-145P, Biolegend, San Diego, USA), mouse monoclonal-anti-NG2/MCSP (1/400, #
MAB2585, R&D Systems, Minneapolis, USA), rabbit polyclonal anti-claudin-1 antibody (1/500, #51–9000, Invitrogen, Carlsbad, USA) and mouse monoclonal anti-ZO-1/TJP1 antibody (1/500,
#33–9100, Invitrogen, Carlsbad, USA). Secondary antibodies were donkey anti-mouse Alexafluor 488, 594 and donkey anti-rabbit Alexafluor 594, all from Invitrogen (1/1000). For nuclear
staining, Hoechst 33258 (1/1000, Sigma-Aldrich, Taufkirchen, Germany) was used. For immunostaining of YAP, rabbit polyclonal YAP antibody (1/200, #4912, Cell Signaling, Danvers, USA) and DAB
substrate (Roche Diagnostics Inc, Manheim, Germany) were used. Samples were examined with a fluorescence microscope (BX51and DP80, Olympus, Tokyo, Japan) using cellSens software (Olympus,
Tokyo, Japan). After H&E staining, the areas of living layer and stratum corneum were calculated using ImageJ 1.47 v (NIH, Bethesda, MD, USA). ELECTRON-MICROSCOPIC OBSERVATION Epidermal
model samples for electron microscopy were minced (<0.5 mm3 pieces), fixed overnight in modified Karnovsky’s fixative, post-fixed in 2% aqueous osmium tetroxide or 0.2% ruthenium
tetroxide, dehydrated in graded ethanol solutions, and embedded in Epon-epoxy mixture. TRANSEPIDERMAL WATER LOSS Gravimetric transepidermal water loss (TEWL) was measured as described by
Hanley _et al_.21, Inserts with epidermal models were placed dermis-side down onto silicon rubber plates and the lateral edges were sealed with petrolatum, so that water loss occurred only
through the epidermal surface. Epidermis model sections were kept at ambient temperature (37 °C) and low humidity (<5%), and weighed for 2 hours. TEWL levels are reported as milligrams of
water lost per square millimeter per hour. Epidermal model sections from four different subjects were used. We used 6 control (on flat membrane) models and 6 models on textile #255. Each
sample was taken from an independent model. STATISTICS The results are expressed as the mean ± SD. Statistical significance of differences between two groups was determined by a two-tailed
Student’s t-test. In the case of more than 2 groups, statistical significance was determined by ANOVA with Tukey’s honestly significant difference (HSD), using KaleidaGraph (HULINKS, Tokyo,
Japan). P-values less than 0.05 were considered significant. REFERENCES * Schäfer-Korting, M. & Schreiber, S. Use of skin equivalents for dermal absorption and toxicity. Roberts, M. S.
and Walters, K. A. (eds) _Dermal Absorption and Toxicity Assessmen_t, Informa, USA pp141–159 (2008). * Elias, P. M. Defensive functions of the stratum corneum: Integrative aspects. Elias, P.
M. and Feingold, K. R. (eds) _Skin Barrier_, Taylor & Francis, USA, pp5–14 (2006). * Sun, R. _et al_. Lowered humidity produces human epidermal equivalents with enhanced barrier
properties. _Tissue Eng Part C Methods._ 21, 15–22 (2015). Article CAS Google Scholar * Denda, M. _et al_. Frontiers in epidermal barrier homeostasis - an approach to mathematical
modeling of epidermal calcium dynamics. _Exp Dermatol_ 23, 79–82 (2014). Article Google Scholar * Kobayashi, Y., Sawabu, Y., Kitahata, H., Denda, M. & Nagayama, M. Mathematical model
for calcium-assisted epidermal homeostasis. _J Theor Biol_ 397, 52–60 (2016). Article CAS Google Scholar * Kobayashi, Y. & Nagayama, M. Mathematical model of epidermal structure. R.
S. Anderssen _et al_. (eds), _Applications_ + _Practical Conceptualization_ + _Mathematics_ = _fruitful Innovation_, _Mathematics for Industry_ 11, Springer Japan, pp121–126 (2016). * Candi,
E., Schmidt, R. & Melino, G. The cornified envelope: a model of cell death in the skin. _Nat Rev Mol Cell Biol._ 6, 328–340 (2005). Article CAS Google Scholar * Brandner, J. M. _et
al_. Epidermal tight junctions in health and disease. _Tissue Barriers._ 3, e974451 (2015). Article CAS Google Scholar * Torkamani, N., Rufaut, N. W., Jones, L. & Sinclair, R.
Epidermal cells expressing putative cell markers in nonglabrous skin existing in direct proximity with the distal end of the arrector pili muscle. _Stem Cells Int._ 2016, 1286315 (2016).
Article CAS Google Scholar * Seirin, L. S. Lateral inhibition-induced pattern formation controlled by the size and geometry of the cell. _J Theor Biol._ 404, 51–65 (2016). Article
MathSciNet Google Scholar * Hagiwara, M. An _in vitro_-in silico interface platform for spatiotemporal analysis of pattern formation in collective epithelial cells. _Integr Biol (Camb)._
8, 861–868 (2016). Article CAS Google Scholar * Giangreco, A., Goldie, S. J., Failla, V., Saintigny, G. & Watt, F. M. Human skin aging is associated with reduced expression of the
stem cell markers beta1 integrin and MCSP. _J Invest Dermatol._ 130, 604–608 (2010). Article CAS Google Scholar * Ghadially, R., Brown, B. E., Sequeira-Martin, S. M., Feingold, K. R.
& Elias, P. M. The aged epidermal permeability barrier. Structural, functional, and lipid biochemical abnormalities in humans and a senescent murine model. _J Clin Invest._ 95, 2281–2290
(1995). Article CAS Google Scholar * Halder, G., Dupont, S. & Piccolo, S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. _Nat Rev Mol Cell Biol._ 13, 591–600
(2012). Article CAS Google Scholar * Porazinski, S. _et al_. YAP is essential for tissue tension to ensure vertebrate 3D body shape. _Nature._ 521, 217–221 (2015). Article ADS CAS
Google Scholar * Dupont, S. _et al_. Role of YAP/TAZ in mechanotransduction. _Nature._ 474, 179–183 (2011). Article CAS Google Scholar * Beverdam, A. _et al_. Yap controls
stem/progenitor cell proliferation in the mouse postnatal epidermis. _J Invest Dermatol._ 133, 1497–1505 (2013). Article CAS Google Scholar * Zhou, K. _et al_. Actin-related protein2/3
complex regulates tight junctions and terminal differentiation to promote epidermal barrier formation. _Proc Natl Acad Sci USA_ 110, E3820–3829 (2013). Article CAS Google Scholar * Lee,
M. J., Ran Byun, M., Furutani-Seiki, M., Hong, J. H. & Jung, H. S. YAP and TAZ regulate skin wound healing. _J Invest Dermatol._ 134, 518–525 (2014). Article CAS Google Scholar *
Randall, M. J., Jüngel, A., Rimann, M. & Wuertz-Kozak, K. Advances in the Biofabrication of 3D Skin _in vitro_: Healthy and Pathological Models. _Front Bioeng Biotechnol._ 6, 154 (2018).
Article Google Scholar * Hanley, K., Rassner, U., Elias, P. M., Williams, M. L. & Feingold, K. R. Epidermal barrier ontogenesis: maturation in serum-free media and acceleration by
glucocorticoids and thyroid hormone but not selected growth factors. _J Invest Dermatol._ 106, 404–411 (1996). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This work
was supported by JST CREST (Grant Number JPMJCR15D2). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Research Institute for Electronic Science, Hokkaido University, Sapporo, Japan Junichi
Kumamoto, Masaaki Uesaka & Masaharu Nagayama * Shiseido Global Innovation Center, Yokohama, Japan Shinobu Nakanishi, Mio Makita, Sumiko Denda & Mitsuhiro Denda * Graduate School of
Science, Hokkaido University, Sapporo, Japan Yusuke Yasugahira * Center for Simulation Sciences, Ochanomizu University, Tokyo, Japan Yasuaki Kobayashi Authors * Junichi Kumamoto View author
publications You can also search for this author inPubMed Google Scholar * Shinobu Nakanishi View author publications You can also search for this author inPubMed Google Scholar * Mio Makita
View author publications You can also search for this author inPubMed Google Scholar * Masaaki Uesaka View author publications You can also search for this author inPubMed Google Scholar *
Yusuke Yasugahira View author publications You can also search for this author inPubMed Google Scholar * Yasuaki Kobayashi View author publications You can also search for this author
inPubMed Google Scholar * Masaharu Nagayama View author publications You can also search for this author inPubMed Google Scholar * Sumiko Denda View author publications You can also search
for this author inPubMed Google Scholar * Mitsuhiro Denda View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.U., Y.Y., Y.K. and M.N.
established the mathematical model and carried out computer simulation. J.K. and M.D. planned _in vitro_ study and J.K., M.D., S.N. and M.M carried out the _in vitro_ study. S.D. provided
advice throughout these studies. All authors contributed to the refinement of all presented models and jointly wrote the article. All authors reviewed and approved the final version of the
paper. CORRESPONDING AUTHOR Correspondence to Mitsuhiro Denda. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE:
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENT COMPUTER SIMULATION AND
FIGURES RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and
reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes
were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If
material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain
permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Kumamoto, J., Nakanishi, S., Makita, M. _et al._ Mathematical-model-guided development of full-thickness epidermal equivalent. _Sci Rep_ 8, 17999 (2018).
https://doi.org/10.1038/s41598-018-36647-y Download citation * Received: 16 February 2018 * Accepted: 27 November 2018 * Published: 20 December 2018 * DOI:
https://doi.org/10.1038/s41598-018-36647-y SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative