Play all audios:
ABSTRACT Hexagonal close-packed iron hydride, hcp FeH_x_, is absent from the conventional phase diagram of the Fe–H system, although hcp metallic Fe exists stably over extensive temperature
(_T_) and pressure (_P_) conditions, including those corresponding to the Earth’s inner core. _In situ_ X-ray and neutron diffraction measurements at temperatures ranging from 298 to 1073 K
and H pressures ranging from 4 to 7 GPa revealed that the hcp hydride was formed for FeH_x_ compositions when _x_ < 0.6. Hydrogen atoms occupied the octahedral interstitial sites of the
host metal lattice both partially and randomly. The hcp hydride exhibited a H-induced volume expansion of 2.48(5) Å3/H-atom, which was larger than that of the face-centered cubic (fcc)
hydride. The hcp hydride showed an increase in _x_ with _T_, whereas the fcc hydride showed a corresponding decrease. The present study provides guidance for further investigations of the
Fe–H system over an extensive _x–T–P_ region. SIMILAR CONTENT BEING VIEWED BY OTHERS MELTING PHASE RELATIONS IN FE–SI–H AT HIGH PRESSURE AND IMPLICATIONS FOR EARTH’S INNER CORE
CRYSTALLIZATION Article Open access 15 June 2022 INVERSION OF THE TEMPERATURE DEPENDENCE OF THERMAL CONDUCTIVITY OF HCP IRON UNDER HIGH PRESSURE Article Open access 09 October 2024
REDOX-STRUCTURE DEPENDENCE OF MOLTEN IRON OXIDES Article Open access 05 November 2020 INTRODUCTION Transition metals react with hydrogen to form hydrides, MH_x_, at hydrogen pressures of
several gigapascals (GPa; hereafter, the hydrogen pressure is referred to simply as “pressure”)1. Hydrogen molecules dissociate to hydrogen (H) atoms on the metal surface, where the H atoms
dissolve into the bulk to partially or fully occupy the interstitial sites of the metal lattice. The interstitial H atoms expand the volume of the metal lattice by 10–20% for a MH_x_
composition of _x_ = 11. The H composition _x_ varies as a function of the temperature (_T_) and pressure (_P_). Accordingly, the volume of hydride (_V_) varies via a hydrogen-induced volume
expansion. In addition to _V_, _x_ is an essential variable for describing the bulk state of a hydride. Hydrogen compositions for recovered specimens have been measured by neutron
diffraction or hot extraction of H2 gas at ambient pressure2. _In situ_ neutron diffraction was recently used to investigate the structure of iron deuteride under high _T–P_ conditions, and
the D composition was successfully determined3. Iron hydride (FeH_x_) has been intensively studied for half a century as a prototype of transition-metal hydrides2,3,4,5,6,7,8,9,10,11,12,13
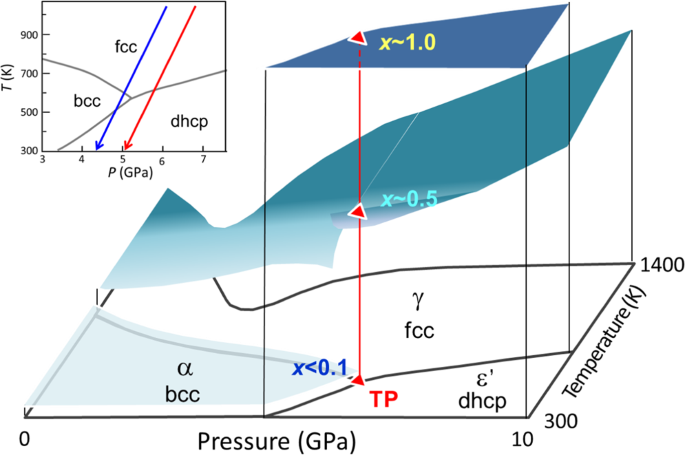
and an endmember of the constituents of the Earth’s core5,14,15,16,17,18,19,20,21,22,23,24. Figure 1, plotted from previously reported data2,3,4,5,6,7,8,9,10, shows a schematic of the
_x–T–P_ diagram of the Fe–H system at temperatures from 300 to 1400 K and pressures ranging from 0 to 10 GPa 2,3,4,5,6,7,8,9,10. Three phases exist: a low-pressure α phase with a
body-centered cubic (bcc) structure, wherein Fe atoms occupy the vertexes of the bcc lattice; a high-temperature γ phase with a face-centered cubic (fcc) structure and a high-pressure εʹ
phase with a double hexagonal close-packed (dhcp) structure. The bcc and fcc phases are a solid solution of hydrogen for _x_ <1.0, whereas the dhcp phase is a monohydride for _x_ = 1 over
almost the entire stable _T–P_ region. The triple point is located at approximately 520 K and 5 GPa2,4,9,10, where the bcc, dhcp, and fcc phases have approximate H compositions of 0.1, 1.0,
and 0.5, respectively5,7,8,9,10. The phase stability for iron hydride in equilibrium with fluid hydrogen has been investigated, where the composition _x_ was uniquely fixed to the highest
value at a given _T–P_ condition for each hydride phase. A different hydride should form for _x_ values below the equilibrium _x_ surface. The hcp hydride is absent in the conventional phase
diagram although the hcp phase of metallic iron is stable at extensive _T–P_ conditions up to those corresponding to the Earth’s inner core25. We performed structural investigations on the
Fe–H system to explore the formation of the hcp hydride at _x–T–P_ conditions below the equilibrium _x_ surface. _In situ_ X-ray diffraction measurements revealed that the hcp hydride
appeared at ~800 K while the fcc hydride was cooled from ~1000 K at ~7 GPa under conditions where there was no coexisting fluid H2 (red arrow in the inset of Fig. 1). The hcp hydride
subsequently decomposed into dhcp FeH and bcc Fe at ~430 K upon further cooling. The crystal structure including the site occupancy of deuterium (D) atoms was investigated via _in situ_
neutron diffraction measurements of the hcp deuteride, which was prepared by cooling the fcc deuteride (blue arrow in the inset). In this communication, we present _x_–_T–P_ conditions for
the formation of the hcp hydride and the variation of _x_ with the fcc–hcp–dhcp structural transitions. Hydrogen-induced volume expansion and the _x–T_ relation, which are essential
properties for characterizing metal hydrides, are derived from the structural data and compared with those of the fcc hydride. RESULTS X-RAY DIFFRACTION MEASUREMENTS The X-ray diffraction
profiles were collected while cooling fcc FeH_x_ with _x_ ≈ 0.6 from 1073 K to 298 K at an initial pressure of ~7 GPa. In this experiment, the amount of aluminum trihydride (AlH3) pellets
that was used as an internal H source was reduced to a H/Fe molar ratio of ~0.6 to prevent transformation from fcc FeH_x_ to the monohydride dhcp FeH with additional H absorption. The
temperature was continuously decreased at 10 K/min, and the pressure was reduced from 7.1 to 5.0 GPa because of the thermal contraction of the reaction cell. Time-resolved X-ray diffraction
profiles were collected with an exposure time of 20 s/profile using the energy dispersion method26. Figure 2(a) Shows the evolution of the diffraction profile with decreasing temperature.
The observed profiles are divided into approximately three regions for ease of explanation: fcc-dominant (Fig. 2(b)), hcp-dominant (Fig. 2(c)), and dhcp-dominant regions (Fig. 2(d)). When
the temperature decreased to ~800 K, only one peak appeared (~60 keV, _d_ ≈ 2.0 Å) in addition to those of the fcc hydride, as shown in Fig. 2(b). The peak intensity increased with further
decrease in the temperature to ~750 K, whereas the peak intensity of the fcc peaks remained unchanged. Other new peaks appeared at ~650 K. These peaks were assigned as hcp lattice
reflections, indicating a transformation from the fcc to the hcp structure. The most intense 101 peak (denoted by the arrow in Fig. 2(c)), which appeared on the low-energy side of the
preceding 101 peak, was shifted to a higher energy with decreasing temperature, thereby merging with the energy level of the 101 peak in Fig. 2(b). We assigned the preceding peak at
temperatures of 800–750 K as a hcp 101 peak from “precipitated hcp hydride” and the peaks appearing at ~650 K were assigned to “transformed hcp hydride.” The fcc–hcp structural
transformation proceeded until ~480 K (Fig. 2(c)). The fcc 111 peak was shifted to a lower energy because of volume expansion with H absorption; by contrast, the hcp 101 peak was shifted to
a higher energy because of volume contraction with H desorption. The hcp hydride eventually decomposed into dhcp FeH_x_ (_x_ ≈ 1.0) and bcc Fe (Fig. 2(d)) at ~430 K. The diffraction profiles
showed peaks from the fcc and hcp hydrides at temperatures below ~650 and ~430 K, respectively. A small amount of the fcc and hcp hydrides remained in a metastable state, probably because
of slow transformation kinetics at lower temperatures. The diffraction peaks continued to shift to lower or higher energies in the metastable temperature ranges, and the corresponding peak
positions were used to calculate the lattice constants or atomic volumes of Fe, as described in the following paragraphs. NEUTRON DIFFRACTION MEASUREMENTS We performed neutron diffraction
measurements with the same reaction cell as that used in the X-ray diffraction experiments, except that the AlH3 pellet was replaced with an AlD3 pellet3,27,28. An excess amount of AlD3 with
a Fe/D molar ratio of ~1.5 was charged into the cell to completely deuterize a bulk Fe specimen, 3.0 mm in diameter and 2.5 mm in thickness, within a short time. However, deuterization of
the Fe disc took 90 min even at temperatures as high as 1073 K. By contrast, only a few minutes were required for the hydrogenation of Fe flakes, which were mixed with the BN powder that was
used in the X-ray diffraction experiments. This result was expected because the surface areas for the Fe disc and flakes differed by orders of magnitude. After the formation of the fcc
deuteride at 1073 K and 6.0 GPa was confirmed by neutron diffraction, the temperature was rapidly decreased to 673 K to prevent the fcc deuteride from achieving an equilibrium D composition;
the hcp deuteride was thus prepared. The temperature was further decreased to 573 K and finally to 300 K. A neutron diffraction profile was collected at each temperature with a few hours of
integration time. The pressure decreased from 6.0 to 4.2 GPa upon cooling to 300 K. Figure 3 shows the neutron diffraction profiles that were recorded at 1073 K and 6.0 GPa (a), 673 K and
5.1 GPa (b), and 300 K and 4.2 GPa (c). The corresponding simulated and experimental profiles, as fitted by Rietveld refinement29, are shown. It should be noted that the Fe and D
compositions of the fcc deuteride are denoted by _x_ʹ in the panels (a) and (b). The site occupancy of the Fe atoms in the fcc lattice deviated slightly from unity to _x_ʹ < 1.0 because
of the formation of vacancies at the Fe sites9,10,30. The Rietveld refinement only provides the ratio of the site occupancies between Fe and D atoms; hence, the Fe composition is described
by _x_ʹ. The diffraction peaks at 673 K showed that the hcp structure was the dominant component. Rietveld refinement using a hcp model structure with D atoms randomly occupying the
interstitial sites yielded site occupations of 0.48(1) (hereafter, the numbers in parentheses denote the experimental error) and 0.0 for the octahedral and tetrahedral sites, respectively,
and a deuterium composition of _x_ = 0.48(1). A very similar diffraction profile was observed for the hcp deuteride at 573 K and 4.8 GPa, yielding _x_ = 0.48(1). In the 300-K profile, the
dominant diffraction peaks originated from dhcp FeD and bcc Fe; the hcp deuteride decomposed, as observed in the X-ray diffraction experiments. For dhcp FeD, we used a stacking fault model
that was presented in the early neutron diffraction study8. Because bcc Fe and dhcp Fe(H/D) are ferromagnetic31,32, each diffraction peak contains a magnetic scattering component in addition
to a nuclear one. The magnetic moment was optimized to 2.1 (1.1) in Bohr magnetons (μB) for bcc Fe. No magnetic contribution considered for dhcp FeD because the peak intensity was too low
for the magnetic structure to be refined. The structural parameters that were optimized by Rietveld refinement are summarized in Table 1. _V–T_ RELATION The atomic volume of Fe, _v_Fe, which
is calculated by dividing the unit cell volume of iron hydride by the number of Fe atoms contained in the cell, was obtained for each hydride from its X-ray and neutron diffraction data.
The atomic volume was plotted as a function of temperature in the 298–1073 K range in Fig. 4. The atomic volumes of the fcc and hcp Fe metals, which were calculated using their equations of
state33,34, are also plotted as references. For the precipitated hcp hydride, only the 101 peak was observed at temperatures in the 730–800 K range (Fig. 2(b)). The atomic volume for the
precipitated hcp hydride was estimated from the measured _d_ values of the 101 peak and the axial ratio of _c/a_ = 1.600 that was obtained for the transformed hcp hydride. The atomic volumes
were also calculated for the fcc and hcp hydrides that remained as metastable states below the transformation and decomposition temperatures, respectively. The _v_Fe–_T_ relations in Fig.
4a were used to derive the deuterium-induced volume _v_D = Δ_v_Fe/_x_1. Here, the excess amount of _v_D. that arises from the volume expansion of the metal lattice owing to the dissolution
of H/D atoms can be calculated using Δ_v_Fe = _v_Fe (hydride) − _v_Fe (reference metal). The value of _v_D for the hcp deuteride at 673 K and 5.1 GPa was found to be 2.51 (5) Å3/D atom using
Δ_v_Fe = 1.191 Å3, which was calculated from the _v_Fe (hcp FeD_x_) and the calculated _v_Fe (hcp Fe, which is plotted in Fig. 4b), and _x_ = 0.48(1). Using alternative data for Δ_v_Fe =
1.166 Å3 and _x_ = 0.48(1) at 573 K and 4.8 GPa, we obtained _v_D = 2.45(4) Å3/D atom. We took an averaged value of 2.48(5) Å3/D atom for the _v_D of hcp deuteride. For dhcp FeD, a 2.42 (4)
Å3/D atom was obtained using the structural data that are listed in Table 1, where the hcp Fe volume was used as the reference volume. The volume of ferromagnetic dhcp deuteride contains an
unknown contribution from magnetic volume expansion; hence, the calculated value is an upper limit on _v_D. The volume data for fcc FeD_x_ that were obtained by the neutron diffraction
should be regarded with caution because the lattice volume for this deuteride is substantially reduced owing to vacancy formation in the metal lattice9,10,30. Hence, we used the _v_D value
of 2.21(4) Å3/D atom, as has been previously reported for vacancy-free fcc FeD_x_3. The _v_D of the hcp hydride is the largest volume among those for iron hydrides. _X_–_T_ RELATION The
expanded volume, Δ_v_Fe, for the fcc and hcp hydrides that was measured over a temperature range of 298–1073 K by X-ray diffraction was converted to H compositions using a proportionality
relation, _x_ = Δ_v_Fe/_v_H, in which _v_H = _v_D is assumed. Figure 5 shows the _x_–_T_ relations of the fcc and hcp hydrides that were calculated using _v_H = 2.21 and 2.48 Å3/H atom,
respectively. The _x_ value of the fcc hydride decreased slightly from 0.68 at 1073 K to 0.66 at 600 K, before increasing towards a saturated value of 1.0; the fcc monohydride and the dhcp
monohydride formed at temperatures below ~430 K. The value of _x_ ≈ 0.5 was obtained for the precipitated hcp hydride in the 700 to 800 K range, whereas the transformed hcp hydride showed a
monotonic decrease in _x_ with decreasing temperature below 650 K. Despite the very similar values of _x_ ≈ 0.6 for the hcp and fcc hydrides at ~600 K, opposing trends were observed for the
variation in the H compositions with the temperature below 600 K. DISCUSSION Iron hydride/deuteride with an hcp metal lattice was formed by the transformation from the fcc hydride/deuteride.
In the early studies6,8, the hcp hydride/deuteride formed as an intermediate metastable state during the hydrogenation/deuterization of bcc Fe to dhcp Fe(H/D). The “transformed” hcp
deuteride has the same crystal structure as that of the “intermediate” hcp deuteride as shown by the structural parameters presented in Table I and Table 3 of ref.8. The D atoms occupy the
octahedral interstitial sites of the host metal lattice both partially and randomly; The present study provided a D composition of 0.48 for hcp deuteride at 673 K and 5.1 GPa, and at 573 K
and 4.8 GPa. This value was slightly higher than 0.42 reported for hcp deuteride prepared at 623 K and 9.2 GPa8. The tetrahedral site occupation, reported for fcc FeD_x_3, or the formation
of layered octahedral superstructures, reported for hcp TcH_x_ and MnD_x_ at _x_ = ~1/235 was not observed. The hcp iron hydride, in both its stable and metastable states, formed for _x_
< 0.6 at pressures from 4 to 6 GPa (Table 1 and Fig. 5). This hcp hydride lies under the equilibrium _x_-surface in the phase diagram that is shown in Fig. 1. Controlling of the H
composition plays a key role in the formation of hcp hydride. In the neutron diffraction measurements, the bulk fcc deuteride transformed to the hcp deuteride through the nonequilibrium
state formed due to the relatively low diffusion rate of D atoms at the measured temperatures. In the X-ray diffraction measurements, the powder fcc hydride transformed to the hcp hydride
under the condition of insufficient hydrogen supply. Both of the transformations occurred near the stable _T–P_ region of dhcp phase; the hcp hydride, instead of the dhcp monohydride, was
preferentially precipitated. These results suggest the formation of hcp hydride over a wide _T–P_ region under controlled H composition. The most recent theoretical calculations have shown
that the hcp hydride becomes more stable than the dhcp hydride when _x_ <~0.5 at extensively high _T–P_ conditions36. Further phase studies of the Fe–H system in extended _x–T–P_
conditions are required to clarify the structural stability of the hcp hydride in terms of the H composition. The crystal structures of iron hydride that appeared sequentially in the cooling
experiments are drawn in Fig. 6. The fcc, hcp, and dhcp structures can transform into each other by sliding metal planes and tuning the H composition. At the fcc–hcp transformation
temperature of ~650 K, the H composition of both hydrides is _x_ = 0.6; hence, the fcc structure can transform to the hcp structure by simply altering the stacking sequence of the metal
planes from ABCABC∙∙∙ to ABAB∙∙∙ along the body diagonal axis of the cubic lattice. For decomposition at ~430 K, the dhcp structure can form by altering the sequence from ABAB∙∙∙ to
ABABACAC∙∙∙ along the _c_ axis of the hcp lattice and filling all of the octahedral sites with H atoms. Although each of the metal lattices has one octahedral site per Fe atom available for
H-atom accommodation, the spatial arrangements of these lattices are quite different. The octahedra consisting of Fe atoms at the corners are connected by corner sharing in the fcc lattice
but by face-sharing in the hcp lattice (Fig. 6). The dhcp lattice consists of a mixture of two configurations, as seen in its sequence ABABACAC∙∙∙. The face-sharing configuration
substantially shortens the first-neighbor distance between the H atoms. For the coexisting state at 673 K and 5.06 GPa, the fcc lattice constant of _a_ = 3.6901(3) Å and the hcp lattice
constants of _a_ = 2.60047(10) Å and _c_ = 4.2280(4) Å were obtained (Table 1). These values provide first-neighbor distances of 2.609 Å and 2.114 Å for the fcc and hcp structures,
respectively. The latter distance of 2.114 Å is very close to the critical distance of 2.1 Å, below which dissolved H atoms in metals cannot approach each other owing to interatomic
repulsion forces37. Dissolved H atoms can preferentially occupy second-neighbor octahedral sites to avoid violating the 2.1-Å rule in the half-filled hcp lattice but not in the hcp
monohydride. The 2.1-Å rule is a possible factor in the stabilization of the hcp structure for _x_ < 0.6. The hcp and fcc solid solutions exhibited opposing variations in _x_ with the
temperature. The two-step variation of the fcc hydride was interpreted in terms of a miscibility gap; solid solutions with high and low H compositions can coexist below a critical _T–P_
point, at which the H solubility gap vanishes. For the fcc hydride, the miscibility gap was confirmed experimentally10,13 and the critical pressure was located at 4.0_–_4.5 GPa at a critical
composition of _x_ ≈ 0.413. The measured pressure range of 5.0_–_7.1 GPa was higher than the critical pressure; hence, the H composition of the fcc hydride increased along the
high-composition boundary of the miscibility gap with decreasing temperature below ~600 K, as shown in Fig. 5. For the hcp hydride, a miscibility gap has been theoretically predicted38, but
has not been experimentally confirmed. The observed monotonic decrease in the _x–T_ curve for the hcp hydride that is shown in Fig. 5 implies that the critical pressure was above ~6 GPa.
Consequently, the H composition decreased with decreasing temperature along the low-composition boundary of the miscibility gap. METHODS X-RAY DIFFRACTION The starting material was
reagent-grade pure iron flakes (purity: 99.9%) with a lateral particle size <100 μm and a thickness <20 μm. The flakes were mixed with BN powder (purity: 99% and grain size: >10 μm)
at a volume ratio of 2:3 and compacted into a disc that was 0.5 mm in diameter and 0.2 mm in height. The sample disc was loaded along with a compacted AlH3 disc, which served as an internal
H source, into a sleeve made of pyrolytic BN. This sleeve was placed into a NaCl capsule that was surrounded by a cylindrical graphite heater. The cell assembly was performed in the air.
High pressures and temperatures were generated using a cubic-type multi-anvil press. The internal H source decomposed into fluid H2 and Al metal upon heating above 800 K. The fluid H2
reacted with the Fe specimen to form FeH_x_ in the NaCl capsule. The temperature was monitored using Pt/Pt_–_13%Rh thermocouples with an uncertainty of less than 20 K. _In situ_ X-ray
diffraction measurements were conducted using synchrotron radiation at the BL14B1 beamline of SPring-8. Details of the high-pressure generation, the hydrogenation cell, and the _in situ_
synchrotron-radiation X-ray diffraction technique are described elsewhere26. NEUTRON DIFFRACTION The cell assembly for the high-pressure neutron diffraction measurements was essentially the
same as that used for X-ray diffraction. A compacted Fe disc, 3 mm in diameter and 2.5 mm in height, was prepared by pressing Fe flakes in a piston-cylinder-type mold. The Fe specimen was
placed at the center of a NaCl capsule (5.5 mm in diameter and 8 mm in height) and AlD3 (isotopic purity: 96 atom% D) pellets, which served as an internal D source, was placed above and
below the Fe specimen. The NaCl capsule was inserted into a cylindrical graphite heater and embedded in a pressure-transmitting medium made of MgO (17-mm edge cube). The cell assembly was
performed in the air. _In situ_ neutron diffraction measurements were conducted using the pulsed neutron source at the BL 11 (PLANET) beamline of J-PARC27. The collected diffraction profiles
were refined using Z-Rietveld software (version 0.9.42.2)29. In the refinement, H atoms that were included as an impurity at four atom% were assumed to randomly occupy the D atom sites. For
simplicity, the site occupancies of the H atoms and the H composition are notated as gD and x, respectively. The cell assembly and the high-pressure apparatus that was used for the neutron
diffraction experiments are described in detail elsewhere3. DATA AVAILABILITY All data supporting the findings of this study are available within the paper and Methods. The crystallographic
data are available from the corresponding authors upon request. REFERENCES * Fukai, Y. _The Metal–Hydrogen System_ 2nd edn (Springer-Verlag 2005). * Antonov, V. E. _et al_. High-pressure
hydrides of iron and its alloys. _J. Phys. Condens. Matter_ 14, 6427–6445 (2002). Article ADS CAS Google Scholar * Machida, A. _et al_. Site occupancy of interstitial deuterium atoms in
face-centred cubic iron. _Nat. Commun._ 5, 5063 (2014). Article ADS CAS Google Scholar * Antonov, V. E., Belash, I. T. & Ponyatovsky, E. G. T.-P. Phase diagram of the Fe-H system at
temperatures to 450 C and pressures to 6.7 GPa. _Scr. Metal._ 16, 203–208 (1982). Article CAS Google Scholar * Badding, J. V., Hemley, R. J. & Mao, H. K. High-pressure chemistry of
hydrogen in metals: _In situ_ study of iron hydride. _Science_ 253, 421–424 (1991). Article ADS CAS Google Scholar * Yamakata, M., Yagi, T., Utsumi, W. & Fukai, Y. _In situ_ X-ray
observation of iron hydride under high pressure and high temperature. _Proc. Japan Acad._ 68B, 172–176 (1992). Article Google Scholar * Fukai, Y., Yamakata, M. & Yagi, T. Some
high-pressure experiments on the Fe–H system. _Z. Phys. Chem._ 179, 119–123 (1993). Article CAS Google Scholar * Antonov, V. E. _et al_. Neutron diffraction investigation of the dhcp and
hcp iron hydrides and deuterides. _J. Alloys Compd._ 264, 214–222 (1998). Article CAS Google Scholar * Fukai, Y., Mori, K. & Shinomiya, H. The phase diagram and superabundant vacancy
formation in Fe–H alloys under high hydrogen pressures. _J. Alloys Compd._ 348, 105–109 (2003). Article CAS Google Scholar * Hiroi, T., Fukai, Y. & Mori, K. The phase diagram and
superabundant vacancy formation in Fe–H alloys revisited. _J. Alloys Compd._ 404–406, 252–255 (2005). Article Google Scholar * Pépin, C. M., Dewaele, A., Geneste, G., Loubeyre, P. &
Mezouar, M. New Iron Hydrides under High Pressure. _Phys. Rev. Lett._ 113, 265504 (2014). Article ADS Google Scholar * Pépin, C. M., Geneste, G., Dewaele, A., Mezouar, M. & Loubeyre,
P. Synthesis of FeH5: A layered structure with atomic hydrogen slabs. _Science_ 357, 382–385 (2017). Article ADS Google Scholar * Saitoh, H., Machida, A., Sugimoto, H., Yagi, T. &
Aoki, K. P-V-T relation of the Fe-H system under hydrogen pressure of several gigapascals. _J. Alloys Compd._ 706, 520–525 (2017). Article CAS Google Scholar * Stevenson, D. J. Hydrogen
in the Earth’s core. _Nature_ 268, 130–131 (1977). Article ADS CAS Google Scholar * Stevenson, D. J. Models of the Earth’s core. _Science_ 214, 611–619 (1981). Article ADS CAS Google
Scholar * Fukai, Y. & Akimoto, S. Hydrogen in the Earth’s core: experimental approach. _Proc. Japan Acad._ 59B, 158–162 (1983). Article Google Scholar * Fukai, Y. The iron–water
reaction and the evolution of the Earth. _Nature_ 308, 174–175 (1984). Article ADS CAS Google Scholar * Jephcoat, A. & Olson, P. In the inner Core of the Earth pure iron? _Nature_
325, 332–335 (1987). Article ADS CAS Google Scholar * Stixrude, L., Wasserman, E. & Cohen, R. E. Composition and temperature of Earth’s inner core. _J. Geophys. Res._ 102,
24729–24739 (1997). Article ADS CAS Google Scholar * Anderson, O. L. & Isaak, D. G. Another look at the core density deficit of Earth’s outer core. _Phys. Earth Planet Inter._ 131,
19–27 (2002). Article ADS CAS Google Scholar * Hirao, N., Kondo, T., Ohtani, E., Takemura, K. & Kikegawa, T. Compression of iron hydride to 80 GPa and hydrogen in the Earth’s inner
core. _Geophy. Res. Lett._ 31, L06616 (2004). Article ADS Google Scholar * Sakamaki, K. _et al_. Melting phase relation of FeH_x_ up to 20 GPa: Implication for the temperature of the
Earth’s core. _Phys. Earth Planet. Inter._ 174, 192–201 (2009). Article ADS CAS Google Scholar * Narygina, O. _et al_. X-ray diffraction and Mӧssbauer spectroscopy study of fcc iron
hydride FeH at high pressures and implications for the composition of the Earth’s core. _Earth Planet. Sci. Lett._ 307, 409–414 (2011). Article ADS CAS Google Scholar * Iizuka-Oku, R.
_et al_. Hydrogenation of iron in the early stage of Earth’s evolution. _Nat Commun._ 8, 14096 (2017). Article ADS CAS Google Scholar * Tateno, S., Hirose, K., Ohishi, Y. & Tatsumi,
Y. The Structure of Iron in Earth’s Inner Core. _Science_ 330, 359–361 (2010). Article ADS CAS Google Scholar * Saitoh, H., Machida, A. & Aoki, K. Synchrotron X-ray diffraction
techniques for _in situ_ measurement of hydride formation under several gigapascals of hydrogen pressure. _Chin. Sci. Bull._ 59, 5290–5301 (2014). Article CAS Google Scholar *
Sano-Furukawa, A. _et al_. Six-axis multi-anvil press for high-pressure, high-temperature neutron diffraction experiments. _Rev. Sci. Instrum._ 85, 113905 (2014). Article CAS Google
Scholar * Hattori, T. _et al_. Design and performance of high-pressure PLANET beamline at pulsed neutron source at J-PARC. _Nucl. Instrum. Methods Phys. Res. Sect. A_ 780, 55–67 (2015).
Article ADS CAS Google Scholar * Oishi, R. _et al_. Rietveld analysis software for J-PARC. _Nucl_. _Instrum_. _Methods_. _Phys. Res. Sect. A_ 600, 94–96 (2009). CAS Google Scholar *
Fukai, Y. & Sugimoto, H. Formation mechanism of defect metal hydrides containing superabundant vacancies. _J. Phys.: Condens. Matter_ 19, 436201 (2007). ADS Google Scholar * Antonov,
V. E., Belash, I. T., Ponyatovskii, E. G., Thiessen, V. G. & Shiryaev, V. I. Magnetization of iron hydride. _Phys. Stat. Sol. (a)_ 65, K43–48 (1981). Article ADS CAS Google Scholar *
Wordel, R. _et al_. Mössbauer Study of Iron Hydride Produced Under High Pressure. _Z. Phys. Chem. N.F._ 145, 121–127 (1985). Article CAS Google Scholar * Uchida, T., Wang, Y., Rivers, M.
L. & Sutton, S. R. Stability field and thermal equation of state of ε-iron determined by synchrotron X-ray diffraction in a multianvil apparatus. _J. Geophys. Res._ 106, 21799–21810
(2001). Article ADS CAS Google Scholar * Tsujino, N. _et al_. Equation of state of γ-Fe: reference density for planetary cores. _Earth Planet. Sci. Lett._ 375, 244–253 (2013). Article
ADS CAS Google Scholar * Shilstein, S. _et al_. The Crystal Structure of High-Pressure Hydrides. _Z. Phys. Chem. N.F._ 146, 129–135 (1985). Article CAS Google Scholar * Gomi, H., Feil,
Y. & Yoshino, T. The Effects of Ferromagnetism and Interstitial Hydrogen on the Physical Properties of hcp and dhcp FeHx: Implications for the Density and Magnetism of a
Hydrogen-bearing Core. _Lunar and Planetary Science_ XLVIII, 1775 (2017). Google Scholar * Westlake, D. G. Stoichiometries and interstitial site occupation in the hydrides of zrni and other
isostructural intermetallic compounds. _J. Less-Common Mel._ 75, 177–185 (1980). Article CAS Google Scholar * Sugimoto, H. & Fukai, Y. Solubility of hydrogen in metals under high
hydrogen pressures: Thermodynamical calculations. _Acta Metall. Mater._ 40, 2327–2336 (1992). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS Neutron diffraction
experiments were performed under proposal no. 2014B0017 at J-PARC. Synchrotron X-ray diffraction experiments were performed under proposal numbers 2015A3602 and 2016B3651 at SPring-8. This
work was performed under the Inter-university Cooperative Research Program of the Institute for Materials Research, Tohoku University, proposal numbers 16K0075 and 17K0018, and supported
partially by the Grants-in-aid for Scientific Research, grant numbers 24241032, 25220911 and 18H05224 of Japan Society for the Promotion of Science. We thank T. Yagi and H. Kagi for their
polite discussions. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Quantum Beam Science Research Directorate, National Institutes for Quantum and Radiological Science and Technology, 1-1-1,
Kouto, Sayo-cho, Sayo-gun, Hyogo, 679-5148, Japan Akihiko Machida & Hiroyuki Saitoh * J-PARC Center, Japan Atomic Energy Agency, Tokai, Naka, Ibaraki, 319-1195, Japan Takanori Hattori
& Asami Sano-Furukawa * Neutron Science and Technology Center, Comprehensive Research Organization for Science and Society, Shirakata, Tokai, Naka, Ibaraki, 319-1106, Japan Ken-ichi
Funakoshi * Institute for Materials Research, Tohoku University, 2-1-1 Katahira, Aoba-ku, Sendai, 980-8577, Japan Toyoto Sato & Shin-ichi Orimo * WPI-Advanced Institute for Materials
Research (AIMR), Tohoku University, 2-1-1, Katahira, Aoba-ku, Sendai, 980-8577, Japan Shin-ichi Orimo * Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo,
113-0033, Japan Katsutoshi Aoki Authors * Akihiko Machida View author publications You can also search for this author inPubMed Google Scholar * Hiroyuki Saitoh View author publications You
can also search for this author inPubMed Google Scholar * Takanori Hattori View author publications You can also search for this author inPubMed Google Scholar * Asami Sano-Furukawa View
author publications You can also search for this author inPubMed Google Scholar * Ken-ichi Funakoshi View author publications You can also search for this author inPubMed Google Scholar *
Toyoto Sato View author publications You can also search for this author inPubMed Google Scholar * Shin-ichi Orimo View author publications You can also search for this author inPubMed
Google Scholar * Katsutoshi Aoki View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS A.M., H.S., T.H., A.S.-F. and K.F. performed the
high-pressure neutron diffraction experiments. H.S. performed the high-pressure X-ray diffraction experiments. T.S. and S.O. prepared AlD3 and AlH3. A.M. and K.A. analyzed the neutron and
X-ray diffraction data. A.M. and K.A. wrote the manuscript. K.A. directed this study. CORRESPONDING AUTHORS Correspondence to Akihiko Machida or Katsutoshi Aoki. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published
maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,
adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Machida, A., Saitoh, H., Hattori, T. _et al._ Hexagonal Close-packed Iron Hydride behind the Conventional Phase Diagram. _Sci Rep_ 9, 12290 (2019).
https://doi.org/10.1038/s41598-019-48817-7 Download citation * Received: 21 February 2019 * Accepted: 12 August 2019 * Published: 23 August 2019 * DOI:
https://doi.org/10.1038/s41598-019-48817-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative