Play all audios:
The faecal microbiota plays a critical role in host health, with alterations in the human faecal microbial composition associated with various conditions, particularly diarrhoeal diseases.
However, little is known about microbial changes during cryptosporidiosis, one of the most important diarrhoeal diseases caused by protozoa in cattle. In this study, alterations in the
faecal microbiota of neonatal calves as a result of Cryptosporidium parvum infection were investigated on a C. parvum-positive farm. Comparisons were made among groups of C. parvum-infected,
rotavirus-infected, and the pathogen-negative calves. A specific increase in the abundance of Fusobacterium was observed in the faecal microbiota of C. parvum-infected animals. Diarrhoea
severity increased in accordance with the abundance of C. parvum and Fusobacterium. Moreover, the specific increase of Fusobacterium appeared to be a universal feature of C. parvum
infection, since neonatal calves from geographically separated areas showed the same result. These observations indicated that the growth of Fusobacterium may be an important aggravating
factor of cryptosporidiosis.
The gut microbiota plays a critical role in the gut health of the host. It protects against enteropathogens, extracts nutrients and energy from food and contributes to normal immune
function. Disruption of the normal composition of the gut microbiota has been associated with obesity, malnutrition, inflammatory bowel disease, neurological disorders and several cancers1.
Alterations of the normal human gut microbiota have been documented, particularly in diarrhoeal diseases such as antibiotic-associated diarrhoea2, inflammatory bowel disease3, acute post
radiotherapy diarrhoea4 and irritable bowel syndrome5,6.
In a previous study of the faecal microbiota of neonatal calves, Firmicutes was the most abundant phylum, with a prevalence ranging from 63.84–81.90%, followed by Bacteroidetes
(8.36–23.93%), Proteobacteria (3.72–9.75%), Fusobacteria (0.76–5.67%) and Actinobacteria (1.02–2.35%)7. Changes in the digestive tract microbiome have also been identified in cattle
exhibiting diarrhoea8,9. While a shift in the faecal microbiota of cattle infected with Mycobacterium avium subsp. paratuberculosis (Johne’s disease) has been reported10, limited information
is available about the effects of other infectious diarrhoeal pathogens on the faecal microbiota of cattle.
Cryptosporidium parvum is a coccidian protozoan parasite that causes enteric infection and diarrhoeal disease in many mammals, including both immunocompetent and immunocompromised humans11.
The parasite is widely distributed and is a common cause of severe neonatal diarrhoea among calves, with cryptosporidiosis being one of the most important infectious diarrhoeal diseases
caused by protozoa for the cattle industry. While cryptosporidiosis is usually mild and self-limiting in immunocompetent humans, individuals with various immune disorders, including acquired
immune deficiency syndrome, often contract chronic, life-threating infections12. While nitazoxanide has been licensed for the treatment of Cryptosporidium-induced diarrhoea in humans13, its
efficacy in calves remains unclear14. Although one study reported beneficial effects of nitazoxanide in the treatment of cryptosporidiosis in experimentally-infected neonatal calves15,
another study revealed no prophylactic or therapeutic efficacy16. Therefore, clinical disease control in calves has been hampered by the lack of drugs and vaccines that are effective for
either treatment or prevention of cryptosporidiosis17,18.
The gut microbiota is thought to affect resistance to C. parvum infection because germfree adult immunocompetent mice showed high susceptibility to infection19. Moreover, a study using
severe combined immunodeficient (SCID) mice supported the hypothesis that resistance of adult mice to C. parvum infection does not require a specific immune response but can be mediated by
nonspecific mechanisms associated with the presence of intestinal microflora20. This hypothesis was based on results showing that C. parvum was not readily detected in flora-bearing adult
SCID mice, while germfree SCID mice were heavily infected following challenge with the parasite20. These observations indicate that some interaction occurs between C. parvum and the faecal
microbiota in infected hosts.
Administration of live Lactobacillus bacterial cell-free supernatants reduces the viability of C. parvum oocysts in vitro21,22. Furthermore, probiotics can limit C. parvum infection in
immunocompromised individuals in mouse models of the disease23,24, with similar results observed for human cases25. However, probiotic treatment of calves infected with C. parvum did not
result in a significant decrease in the incidence of diarrhoea or oocyst shedding compared with the controls26.
At present, nothing is known about the faecal microbiota of neonatal calves infected with C. parvum. Interactions between the faecal microbiota and C. parvum must be analysed to understand
pathophysiological changes that occur during disease progression. To address this knowledge gap, we examined the faecal microbiota profiles of neonatal calves from a C. parvum-endemic farm.
Metagenomic sequencing analysis was conducted using the IonPGM, and the composition of the microbiota revealed by the high-throughput sequencing was re-examined and confirmed by quantitative
polymerase chain reaction (qPCR) analysis. Moreover, neonatal calves from different regions of Japan were examined to evaluate the universality of observed alterations in the faecal
microbiota caused by C. parvum infection.
Metagenomic analysis based on 16 S rRNA gene sequences was performed for a total of 120 faecal samples collected at six time points from 20 neonatal Holstein calves (Table S1). The calves
were aged between 0 and 15 days old and were located on a farm (farm #A) in Iwate Prefecture, Japan. All of the calves were female and were born in identical cattle sheds. Birth weights
ranged from 35–45 kg. All treatments after birth, including colostrum practices, were identical for all calves.
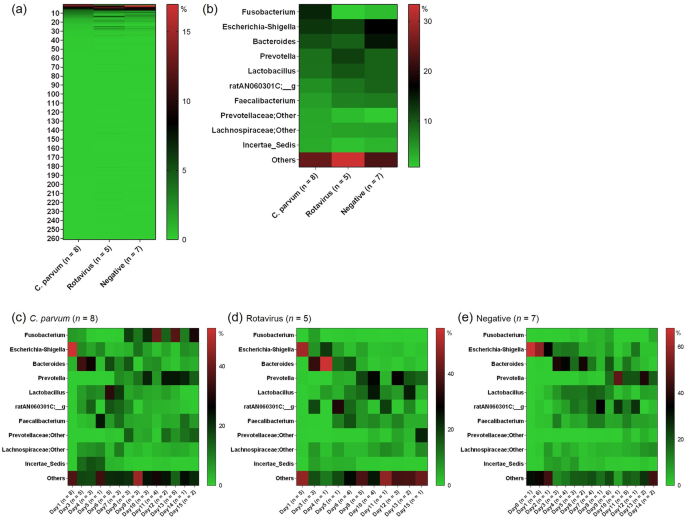
The distribution of the relative abundance of the different bacterial genera is shown in Fig. 1a for each of the three groups: C. parvum-only-infected (n = 8), rotavirus-only-infected (n =
5) and the pathogen-negative group (n = 7) determined by the commercial immunochromatographic test (ICT) strips (Bio-X Diagnostics SPRL, Jemelle, Belgium). None of the animals tested
positive for coronavirus infection.
Distribution of bacterial relative abundance among the three groups of faecal samples collected at farm #A: Cryptosporidium parvum-only-infected (n = 8), rotavirus-only-infected (n = 5) and
pathogen-negative (n = 7). An average value for the relative abundance across the six sampling points for each genus is shown using a gradient scale. (a) The 261 analysed genera (Table S2)
were plotted as rows in which the most abundant genus in the C. parvum-only infected group is shown at the top. The next most abundant genera are shown sequentially in order of abundance.
(b) The 10 most abundant genera (shown on the left-hand side) in the C. parvum-only-infected group in comparison with the corresponding abundances in the rotavirus-only-infected and
pathogen-negative groups. The top 10 genera were obtained from (a). A specific increase of Fusobacterium in the C. parvum-only-infected group was suggested. (c–e) Time-dependent change of
bacterial relative abundance among C. parvum-only-infected, rotavirus-only-infected and pathogen-negative groups. The column titles are the numbers of faecal samples collected in each age in
days (see Table S1).
The most abundant genus in C. parvum-only-infected calves was Fusobacterium (14.1%, average of all samples), followed by Escherichia-Shigella (12.8%). The relative ratio of Fusobacterium was
low in the rotavirus-only-infected (0.7%) and pathogen-negative (2.0%) groups, while the relative ratios of Escherichia-Shigella in the two groups (11.9% and 17.0%, respectively) were
similar to that observed in the C. parvum-infected group (Fig. 1b, Table S2).
The abundance of Fusobacterium appeared to be increased in the faecal microbiota of C. parvum-only-infected calves (Figs 1c and 2a). A significant increase was found in the abundance of the
bacteria between the 1st (0–1 day old) and the 6th (10–15 days old) sampling points (Fig. 2a, Wilcoxon test, P