Play all audios:
ABSTRACT Arbuscular mycorrhizal (AM) fungi play an important role in plant-fungi communities. It remains a central question of how the AM fungal community changes as plants grow. To
establish an understanding of AM fungal community dynamics associated with Chinese fir, Chinese fir with five different growth stages were studied and 60 root samples were collected at the
Jiangle National Forestry Farm, Fujian Province. A total of 76 AM fungal operational taxonomic units (OTUs) were identified by high-throughput sequencing on an Illumina Miseq platform. The
genera covered by OTUs were _Glomus_, _Archaeospora_, _Acaulospora_, _Gigaspora_ and _Diversispora. Glomus_ dominated the community in the whole stage. The number and composition of OTUs
varied along with the host plant growth. The number of OTUs showed an inverted V-shaped change with the host plant age, and the maximum occurred in 23-year. Overall, the basic species
diversity and richness in this study were stable. Non-metric multi-dimensional scaling (NMDS) analysis based on bray-curtis distance revealed that there were remarkable differentiations
between the 9-year and other stages. Besides, AM fungal community in 32-year had a significant difference with that of 23-year, while no significant difference with that of 45-year,
suggesting that 32-year may be a steady stage for AM fungi associated with Chinese fir. The cutting age in 32-year may be the most favorable for microbial community. The pH, total N, total
P, total K, available N, available P, available K, organic matter and Mg varied as the Chinese fir grows. According to Mantel test and redundancy analysis, available N, available P, K and Mg
could exert significant influence on AM fungal communities, and these variables explained 31% of variance in the composition of AM fungal communities. SIMILAR CONTENT BEING VIEWED BY OTHERS
BOTH ABUNDANT AND RARE FUNGI COLONIZING _FAGUS SYLVATICA_ ECTOMYCORRHIZAL ROOT-TIPS SHAPE ASSOCIATED BACTERIAL COMMUNITIES Article Open access 17 November 2022 ECTOMYCORRHIZAL FUNGAL
COMMUNITY VARIES ACROSS BROADLEAF SPECIES AND DEVELOPMENTAL STAGES Article Open access 26 February 2025 SPECIFICITY OF ASSEMBLAGE, NOT FUNGAL PARTNER SPECIES, EXPLAINS MYCORRHIZAL
PARTNERSHIPS OF MYCOHETEROTROPHIC _BURMANNIA_ PLANTS Article Open access 06 January 2021 INTRODUCTION Arbuscular mycorrhizal (AM) fungi, belonging to the phylum _Glomeromycotina_1, can form
mutualistic associations with more than 80% of land plant species2. Through AM associations, plants provide carbon for the fungi and AM fungi benefit the plants with improved access to soil
nutrients3,4, thus promoting plants’ growth and biomass production5. Furthermore, the fungi can improve soil physical structures and plants’ tolerance to abiotic stress, impact on nutrient
dynamics and carbon cycling, and contribute to the development of more sustainable ecosystems6,7,8. Given that AM associations play a crucial role in exchanging matters between the
aboveground and underground biotic communities9,10, understanding AM fungal community dynamics along the host plants’ growth status is essential for ecosystem management11. There has been
overwhelming researches that focus on how plant communities may structure AM fungal communities, and samples were required to be collected in a time series12. Most of the studies kept an eye
on short-lived hosts. For instance, AM fungal status and communities changed as the plants communities13. Besides the plant communities, environmental factors including spatial distance and
spatial variation may structure AM fungal communities. However, not all existing evidences support the successional changes of AM fungal communities. A study taken place in Calestienne
region indicated that AM fungal communities do not follow changes in the plant community14, and the strength of the correlation between plant and AM fungal communities do not change as
succession progressed15. There has also been some researches on long-lived plants influencing the AM fungal community. Liu _et al_. found that AM fungal communities did not vary across a
35-year chronosequence of _Caragana korshinskii_ plantations16. While others thought the early successional species accelerated AM fungal colonization four times than the late successional
species17. Researches on the relationship between woody plant and AM fungi also involves _Paraserianthes falcataria_18, breadfruit19 and willow20. However, compared with grassland and crops,
investigations of the impact of long-lived woody hosts on the AM fungal community structure are still limited, especially collecting woody plant roots as samples. Chinese fir (_Cunninghamia
lanceolata_ (Lamb.) Hook) is one of the most important timber plants in Southern China21. It is widely used for furniture making, bridge, boat and house building, general carpentry and
timber constructions. Chinese fir has been widely planted in subtropical China to meet increasing timber demands. The area was about 8.54 × 106 hectares, accounting for 21.35% of Chinese
plantations. According to the economic and ecological benefits, the rotation age of Chinese fir was usually 25~30 years. However, in order to meet the rising demand for wood products, the
rotation period of the Chinese fir plantations was shortened from 25–30 years to 20–25 years22. In some places, the plantations were harvested as young as 17 years23. Whether such a short
rotation period is conducive to the restoration of forest soil remains to be verified. Previous studies have mainly focused on the management of the aboveground plants in the forest, the
influence of soil nutrients and allelopathy on the growth of Chinese fir24,25,26. There have been limited researches on cutting age of Chinese fir, and the AM fungi community dynamic was
lack. The main aim of our study was to understand the dynamic of the AM fungal community associated in a chronosequence of Chinese fir, explore the driving environmental factors and provide
a foundation for cutting age of Chinese fir. To achieve that, 60 samples were obtained from Chinese fir stands with five different growth stages and some questions were raised: Do the AM
fungal communities change along a chronosequence of Chinese fir? If so, which stage (period) does the variation occurs? Which environmental factors are most closely related to the variation?
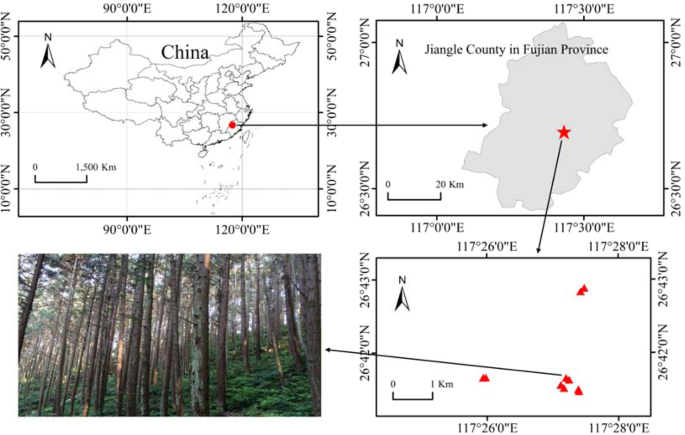
How long is the best rotation period? MATERIALS AND METHODS STUDY AREA AND SAMPLING The study was conducted at the Jiangle state-owned Forest Farm (E117°05′~117°40′, N26°26′~27°04′), Fujian
Province, China. This area is low hilly land with an average elevation of 258 m. The site is located in a typical subtropical climatic zone, with an annual mean temperature of 19.8 °C and
an annual mean precipitation of 1684 mm. The dominant trees are _Cunninghamia lanceolata_ Lamb, _Pinus massoniana_ L. and _Phyllostachys pubescens_ with a forest coverage rate of 84.5%. The
soil is red soil according to Chinese soil classification27. According to the distribution of Chinese fir in the forest farm, Chinese fir with five growth stages: 9-year, 17-year, 23-year,
32-year, 45-year were selected (Fig. 1). Two standard sites with 20 × 30 m2 were set up for each age group (the basic information of each standard site was shown in Table 1). Six trees were
selected as the research objects in each survey standard site, and 60 trees were got. The sources of all the sample trees were: Chinese fir seeds were collected from local seed orchards, and
planted in local nurseries. After growing for one year, the seedlings were transplanted to the mountains for afforestation. The soils in nurseries were local red loam, and the soils were
disinfected by potassium permanganate before sowing Chinese fir seeds. Sampling was conducted in April 2015. Fine roots and rhizosphere soil (20 cm in diameter and 20 cm in depth) were
collected in each sample tree from four directions (east, south, west and north), and then mixed for subsequent analysis. Finally, 60 root samples and 60 soil samples were obtained. The soil
samples were sieved with a 2-mm sieve, transported to the laboratory for further processing. ROOT PROCESSING, DEOXYRIBONUCLEIC ACID (DNA) EXTRACTION AND POLYMERASE CHAIN REACTION (PCR)
CONDITION Fresh fine roots (length = 1 cm) of individual tree were cleaned with distilled water and prepared for DNA extraction using a modified protocol28,29. Fine roots were ground with
sterilized quartz sand and an extra 650 uL cetyltrimethyl ammonium bromide(CTAB) (2% (w/v), 100 mM Tris-HCl, 1.4 M NaCl, 20 mM EDTA, pH = 8.0) was added and incubated in a 65 °C water bath
for 30 min with occasional shaking. 650 uL chloroform/isoamyl-alcohol solution (24:1) was added to each tube and shaken thoroughly to form an emulsion. The mixture was spun at 12,000 g for
15 min at 25 °C in a micro centrifuge and the supernatant phase decanted into a fresh 1.5 mL tube. Supernatant containing DNA was re-extracted with chloroform/isoamyl-alcohol solution (24:1)
at 4 °C until no interface was visible. Thirty uL 5 M KAC was added into the supernatant followed by 200 uL isopropanol and inverted gently to mix. The genomic DNA was precipitated at 9200
g for 2 min at 4 °C in a micro centrifuge. The DNA pellet was washed with 70% ethanol twice and dried using SpeedVac ® (AES 1010; Savant, Holbrook, NY, USA). The DNA pellet was then
re-suspended in 65 uL sterile deionized water and stored at -20 °C. Genomic DNA was amplified with nested polymerase chain reaction (PCR) for Illumina Miseq sequencing. GeoA230/AML231 were
used as the primers in the first amplification, while AMDGR32,33/NS3134 were chosen in the second amplification, and 12 np unique barcode was added at the 5′-end of NS31. The first PCR
mixture (25 uL) contained 12.5 uL 2 × Taq PCR MasterMix (Tiangen Biotech Co. Ltd, Beijing, China), each primer with 1 uL at 10 uM, 8.5 uL ddH2O, and 2 uL dilute DNA by a factor of 20, and
then followed the thermal cycling: an initial denaturation at 94 °C for 3 min, 30 cycles of denaturation at 94 °C for 30 s, annealing at 48 °C for 1 min, extension at 72 °C for 3 min, and a
final extension at 72 °C for 10 min. The product of the first amplification was diluted with sterilized deionized water by a factor of 100, and 2 uL of the resulting solution was used as a
template for the nested PCR (50 uL). The second amplification was performed as follows: 94 °C for 3 min; 30 cycles at 94 °C for 45 s, 45 °C for 45 s, 72 °C for 1 min and 72 °C for 10 min.
The PCR products were separated through a 1% agarose gel in 1X TAE. The correct bands were excised and purified with AxyPrep DNA Gel Extraction Kit (Axygen, Union City, CA, USA) and then
quantified with the Nanodrop 8000 (Thermo Scientific, Wilmington, DE, USA). All purified genomic DNA were mixed with an equal molar concentration and then subjected to the Illumina Miseq
platform for sequencing at the Environmental Genome Platform of the Chengdu Institute of Biology. BIOINFORMATICS ANALYSES The raw 18S rDNA paired-end reads were assembled by FLASH-1.2.835.
All reads were assembled to each sample based on the specific barcodes. Sequences without ambiguous nucleotides, longer than 300 bp and average quality score >30 were retained for further
analysis. The chimeras were checked and removed by “chimeras_check.py” command with usearch836. The non-chimeras sequences were clustered into operational taxonomic units (OTUs) with the
UPARSE at a 97% identity threshold37. The representative sequences were picked and assigned by blasting against the SILVA database38, and all non-“Glomeromycotina” sequences, as well as
fewer than five reads per OTU were removed to reduce the risk of artificially inflating richness due to sequencing error39. The Glomeromycotina sequences were resampled to eliminate the
effects of different read numbers. To further confirm the remaining OTUs belonging to AM fungi, a Neighbor joining tree was conducted in MEGA6 with the reference sequences from GenBank and
Maarj_AM_ using the Kimura 2-parameter model with 1000 replicates40. The references were chosen only if they met the followed criteria: the BLAST score >250, the query coverage >97%,
the similarity between OTU and reference >97%. All representative sequences of each OTU were submitted to GenBank (accession numbers: MK685901-MK685976). PHYSICAL AND CHEMICAL SOIL
PROPERTIES ANALYSES Soil pH was measured with a soil: water ratio of 1:2.5(w/v). Total nitrogen (N) and phosphorus (P) were extracted with H2SO4 + HClO4 and measured using a continuous flow
analyzer (AA3, SEAL, Germany). Total potassium (K) and Mg were extracted with HNO3 + HClO4 and measured with atomic absorption spectroscopy (AAS, TAS-900AFG, China). Alkaline hydrolysis
diffusion was used to determining alkaline-hydrolyzable nitrogen (N). Soil available phosphorus (P) was measured by the Mo-Sb anti-spectrophotometry method after being extracted with
HCl-NH4F41, while soil available potassium (K) was extracted with 1 M ammonium acetate42. Soil organic matter was measured by the wet-oxidation method43. STATISTICAL ANALYSES Shannon-wiener
index, Simpson index, Chao1, Observed_otus and PD_whole_tree were calculated to compare AM fungal community’s diversity and richness among different stages, and then One-way ANOVA were
performed for significant test with SPSS 18.0. Soil properties were also tested with SPSS 18.0. AM fungal community analyses were performed based on the abundance of OTUs in each sample. The
AM fungal community composition was subjected to non-metric multidimensional scaling (NMDS) with the Bray-Curtis dissimilarity measurement in the package Vegan in R, and then the “anosim”
function in the vegan package was carried out to test whether there were significant AM fungal community composition differences between the different stages. Redundancy analysis (RDA) was
performed to obtain the explanatory soil variables most related to the AM fungal community with vegan package in R44. Then the mantel and partial mantel test were used to test whether there
were significant relationships between the AM fungal communities and environmental factors45. RESULTS 18S RDNA SEQUENCING ANALYSIS A total of 1111 828 sequences were retained from raw reads.
A total of 1098 554 reads were clustered into 199 OTUs with UPARSE. Among these sequences, 1060 007 sequences (96.5% of total) which means 77 OTUs belonged to _Glomeromycotina_ (after being
assigned with the SILVA database). After removing sequences fewer than five from the data set, 1059 992 sequences were remained. Rarefaction curves were constructed showing the number of
observed OTUs (Fig. 2). When the number of sequences increased from 0 to 500, the number of observed OTUs increased sharply. Since then, as the number of sequences increased, the rate of
increase in the number of observed OTUs slowed down. And when the number of sequences reached 5000, the obserbed OTUs were nearly saturated. Therefore, 5000 sequences were retained for each
sample in the resampleing step. A total of 300 000 sequences were resampled to eliminate the effects of different sequences numbers. By comparing with the references sequences from GeneBank
and _MaarjAM_ through the Neighbor Joining tree, 76 OTUs were retained for further analysis (see the Supplementary Figure A1). The genera covered by OTUs were _Glomus_, _Archaeospora_,
_Acaulospora_, _Gigaspora_ and _Diversispora_. Among the 76 AM fungal OTUs, 57 OTUs belonged to _Glomus_, 11 OTUs belonged to _Archaeospora_, 5 OTUs belonged to _Acaulospora_, 1 OTUs
belonged to _Gigaspora_, and two OTUs belonged to _Diversispora_. From 9-year to 17-year, the number of _Glomus_ OTUs increased from 31 to 51 (an increase of 64.5%), and then began to
decline. When it reached 45-year, the number of _Glomus_ OTUs was 39. The number of _Archaeospora_ OTUs decreased in the whole stages. The maximum appeared in the 9-year (that was 10), and
the minimum appeared in the 32-year (that was 4). From 23-year to 45-year, there was no significant difference in the number of _Archaeospora_ OTUs (which was 5-4-5). The trend of
_Acaulospora_ OTUs was “V”-shaped. The number was biggest in the 9-year (that is 4), and smallest in the 17-year and 23-year (both were 2). Compared with other genera, the number of
_Gigaspora_ OTUs was always 1. _Diversispora_ only appeared in the 23-year, and the number of _Diversispora_ OTUs was 2. There was a maximum (61) of OTUs in the 23-year. 46 OTUs were from
the trees in the 9-year, 56 OTUs from the trees in 17-year, 61 OTUs from the trees in 23-year, 53 OTUs from the trees in 32-year, and 48 OTUs from the trees in 45-year. 32 OTUs were occupied
by the trees in all the growth stages, while 15 OTUs were unique (Fig. 3). AM FUNGAL COMMUNITY COMPOSITION There were four orders, five families and five genera in the samples. _Glomus_
dominated the community in the whole stage, the second was _Archaeospora_. While _Diversispora_ only existed in the 23-year stage. The proportion of _Glomus_ increased from 67% to 85% when
the host trees growed from 9-year to 32-year stage, while the _Archaeospora_ proportion decreased from 22% to 7% as the host plant grows. The _Acaulospora_ proportion decreased from 9-year
to 23-year, and turned to increase after 23-year. The minimum proportion of _Acaulospora_ was appeared in the 23-year with 3%. The proportion of _Gigaspora_ was always 2% in the whole stages
(Fig. 4). When we compared the composition of shared OTUs, the number of shared OTUs had a minimum in 9–32 year (that’s 34), and the maximum was 17–23 year (that’s 52) (Fig. 5). Among these
common OTUs, _Glomus_ accounted for the largest proportion (≥75%), followed by _Archaeospora_, _Acaulospora_ and _Gigaspora_. The proportion of shared OTUs in each pair varied from 51% to
80%. The number of shared OTUs between 9-year and other growth stages were less than 40, suggesting that the composition in 9-year had significant difference with other growth stages. The
number of shared OTUs between 17–23 year were over 50, suggesting that the difference was the smallest. The number of shared OTUs between 32-year and 9-year, 17-year, 23-year steadily
increased (from 34 to 46 and 49), while decreased in 45-year, indicating the composition difference increased from 9-year to 23-year, then decreased. The variety of the _Glomus_ composition
was similar to that of shared OTUs, indicating that the variety of _Glomus_ decided the whole variety of AMfungi. When it terms to the proportion of composition of each shared genus, the
biggest difference appeared in the 9–17 years, and the smallest difference appeared in the 9–45 years. With regard to unique OTUs, the number of OTUs only appeared in one growth stage was 15
(Fig. 6a), among those 2 OTUs belonged to _Diversispora_, 5 OTUs belonged to _Archaeospora_, 1 OTU belonged to _Acaulospora_, 7 OTUs belonged to _Glomus_. The number of OTUs appeared in two
growth stages was 7 (Fig. 6b), among those 6 OTUs belonged to _Glomus_, 1 OTU belonged to _Acaulospora_. The number of OTUs appeared in three growth stages was 13 (Fig. 6c), among those 2
OTUs belonged to _Acaulospora_, 2 OTUs belonged to _Archaeospora_, 9 OTUs belonged to _Glomus_. The number of OTUs appeared in four growth stages was 9 (Fig. 6d), all of those OTUs belonged
to _Glomus_. Non-metric multi-dimensional scaling (NMDS) analysis based on bray-curtis distance showed the differences in the AM fungal community composition between the different stages
(Fig. 7). The pattern was verified by the anosim results (Table A1). There were remarkable differentiations between the 9-year and other stages (_p_ < 0.01). There were also significant
differences between the 23-year/32-year, and 23-year/45-year (_p_ < 0.05). But there were no significant differences between 32-year and 45-year. AM fungal community’s diversity and
richness changes as the host plant grows (Table 2). The Shannon-wiener and Simpson indexes indicated that the most diverse AM fungal community was in the 9-year. Chao1 and Observed_otus
showed that the number of OTUs was largest in the 23-year. The PD_whole_tree was greatest in the 23-year. There were significant differences between 9-year and 45-year in the matter of
Shannon and Simpson indices. There also has significant difference between 23-year and 45-year of Observed_otus. The index of PD_whole_tree was maximum is 23-year indicating that the AM
fungal community composition was the most complex in 23-year, this is consistent with that there were the most genera in 23-year. RESPONSE OF AM FUNGAL COMMUNITY TO SOIL PHYSICOCHEMICAL
PROPERTIES pH, soil total nitrogen (N), total phosphorus (P), total potassium (K), available N, available P, available K, soil organic matter (SOM) and Mg were all significantly different
among the stages (Table 3). There were significant difference in pH among the 9-year, 17-year and 45-year, but no significant differences between the 23-year and 32-year. It is worth
mentioning that there were significant differences in total N and total P among different stages. As far as total K, available N and available P are concerned, the differences among the
pairs were significant except for the difference between 23-year and 32-year. The content of available K in 9-year was significantly different from that in other stages, and the difference
disappeared after 17-year. Soil organic matter (SOM) between the following pairs were significant: 9-year/17-year, 9-year/45-year, 17-year/23-year, 17-year/45-year. Mg was significantly
different in the first four stages, and the difference disappeared when it reached 32-year. Generally, there were significant differences in soil physical and chemical properties between
9-year to 17-year and 17-year to 23-year. Total N, total K, available N, available P, SOM and Mg were found to have significant influence on AMF communities based on the Mantel test (Table
4), and redundancy analysis (RDA) showed that these variables explained a total of 31% of the variance in the composition of AMF communities among stages (_F_ = 2.20, _P_ = 0.001, Fig. 8).
During the five stages, the AM fungal community composition in 9-year (red circle) was the most relevant to soil factors. When controlling other environmental factors, total K, available N,
available P and Mg were still significantly related with AM fungal community composition (_P_ < 0.05). DISCUSSION AM FUNGAL COMMUNITY CHANGED AS HOST PLANT GROWS Breadfruit has been
reported to have more AMF taxa in older trees than younger trees46. AMF species richness declined with _Populus-Salix_ successional stages47. This difference of fungal community response may
be due to different host plants. In our study, the number of AM fungal genera is most in the 23-year stage, and plant trees in 17-year had more OTUs than that of 9-year. This is in
accordance with the previous research in Hunan reported that 15–20 years old Chinese fir plants had a higher infection than 5–10–year–old plants48. It is interesting that AM fungal community
in 9-year was significantly different from the others, while the variation disappeared when it reached 32-year. This phenomenon could be due to adequate water and nutrient for tree growth
in the young period. However, as the plant trees grew faster with much fiercer competition than ever49, thus leading to the increased demand for effective absorption, and requiring more AM
fungi to participate in absorbing more nutrients and water. When it reached the 32-year, competition tended to be steady50. Our results were consistent with Gordon that plants from different
stages could tradeoff with nutrient gathering strategies through varying investment in AM fungi51. Moreover, it has been demonstrated that mycorrhizal community could evolve to suit the
plants’ current properties and local ecological conditions52,53. Although the biomass of fine root decreased, the speed of plant growth accelerated, and the AM fungal infection as well as
the number of OTUs increased, which also confirms that AM fungi can promote the plant to absorb nutrients. Due to the competition intensity, carbon content and fine roots are always changing
with the tree growth stages; therefore, we can consider that plant growth stage is an important predictor of fungal species community. Overall, this study showed that basic species richness
and diversity were stable. The survey of the ecological distribution of AM fungi and influencing factors revealed that both biological and abiotic factors such as soil type, vegetation,
geographical distance and climate could affect the AM fungal community composition. Soil type and climate determined the aeration and water content of soil, and the species diversity of AM
fungi decreased significantly with the increase of flooding degree54. Environmental, geographical and historical factors were determinant in shaping AM fungal community55,56. In the course
of evolution, the species richness and diversity of AM fungi changed with the evolution of host plants, and the host plants tended to choose AM fungi with high coexistence rate57. This study
was established on a regional (county) scale that shared similar environmental conditions including soil type, climate and altitude, indicating that the AM fungal community composition
varied slightly. Ecological processes structuring the fungal communities were inferred according to phylogenetic patterns and species abundance distributions58. Under conditions of similar
climate, precipitation, altitude and niche of host plants, the AM fungal communities clustered slightly suggesting that the central process structuring communities may be driven by the
growth stage of host plant. _Cunninghamia lanceolata_ generally has a rotation period of 25 years59,60. Considering timber yield only, the optimal rotation period can be 20 years or even 18
years61,62. However, studies on nitrogen deposition suggested that 30-year rotation period will be more conducive to the sustainable growth of forests63, some researches also recommended
cutting cycles of the stands should be increased from 20–25 years to 30 years of age64. Our results showed that the number of shared OTUs between 32-year and 9-year, 17-year, 23-year
steadily increased (from 34 to 46 and 49), while decreased in 45-year, indicating that 32-year may be a turning point in the growth of Chinese fir. The variation of _Glomus_ and
_Archaeospora_ tended to steady state, suggesting that the 32-year rotation period may be the most favorable for microbial community. EFFECTS OF SOIL PROPERTIES ON AM FUNGAL COMMUNITY AM
fungi have been shown to promote N nutrition and subsequently create mulches enriched in N65. Belowground N transfer can occur via plant-associated mycorrhizal fungi66, plant could control
over N transformations in and near the rhizosphere by releasing root exudates67,68. Enhanced root exudation has been demonstrated to accelerate rhizosphere N turnover, particularly under
N-limiting conditions69. Our research found that N was related with the AM fungal community, particularly in the 9-year. However, few studies have been done about relationships between root
exudate and nitrogen enrichment of Chinese fir. The research about how root exudates influence soil N accumulation of Chinese fir through AM fungi will be a hot topic in the future. Many
previous studies have claimed that P could affect AM fungal community composition. P not only lessened AM fungal community richness and Shannon diversity in a regional scale70, but also
affected AM fungal community diversity in a large environment71. Among the five stages, Chinese fir in the 9-year with a maximum AMF diversity index had minimum P content, which was 4.3
mg/kg. This phenomenon may be due to a scarcity of available P. Meanwhile, P availability might be influenced by pH, and a strongly acidic soil (pH <5) adverse to P transferring. As a
macronutrient, enzyme cofactor, and a component of chlorophyll molecule, Mg plays crucial roles in photosynthesis and stabilization of nucleotides and nucleic acids. This study demonstrated
that K and Mg were highlighted as important factors influencing the AM fungal community compositions. The result was consistent with the other studies. some studies emphasized out that Mg
and K were variables in the structure of the AM fungal community72. They also explored the relationship between the number of spores, the diversity of the AM fungal genera and some chemical
soil conditions, and found Mg and K were highly correlated with the density of spores73. Meanwhile, AM fungal species distribution was positively related to soil properties, especially soil
Mg contents74. More researches were concerned with the effects of AM fungal infection on Mg and K contents in plants. Compared with the control, the plant with additional AM fungi
inoculation exhibited significant differences in nutrient uptake75, plants treated with AM fungi possessed higher K and Mg contents in the roots76. However, it was also shown that AM fungi
inoculation had no positive influence on the contents of K and Mg77. CONCLUSIONS There were 76 OTUs were retained from the 60 root samples of Chinese fir with Illumina sequencing. The genera
covered by OTUs were _Glomus_, _Archaeospora_, _Acaulospora_, _Gigaspora_ and _Diversispora_, among those _Glomus_ occupied the majority(75%), followed by _Archaeospora_. The number and
composition of OTUs varied along with the host plant growth. The number of OTUs showed an inverted V-shaped change with the host plant age, and the maximum occurred in 23-year. When it comes
to shared OTUs, the number of shared OTUs between 32-year and 9-year, 17-year, 23-year, 45-year firstly increased then decreased, and the peak of that was in 32-year vs 23-year. There were
15 unique OTUs which only appeared in one growth stage. The proportion of each genus in 23-year was different with that of other stages. Non-metric multi-dimensional scaling (NMDS) analysis
based on bray-curtis distance showed that AM fungal community in 9-year was significantly differed with that of other stages. Besides, AM fungal community in 32-year had a significant
difference with that of 23-year, while no significant difference with that of 45-year, suggesting that 32-year may be a steady stage for AM fungi associated with Chinese fir. The cutting age
in 32-year may be the most favorable for microbial community. Available N, available P, K and Mg were significantly related with AM fungal community based on redundancy analysis. REFERENCES
* Spatafora, J. W., Chang, Y. & Benny, G. L. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. _Mycologia_ 108(5), 1028–1046 (2016). Article
CAS PubMed PubMed Central Google Scholar * Smith, S.E., Read, D. Mycorrhizal symbiosis (Third Edition). Academic Press, London (2008). Chapter Google Scholar * Bucher, M. Functional
biology of plant phosphate uptake at root and mycorrhizal interfaces. _New Phytol_ 173, 11–26 (2007). Article ADS CAS PubMed Google Scholar * Bainard, L. D., Bainard, J. D. & Hamel,
C. Spatial and temporal structuring of arbuscular mycorrhizal communities is differentially influenced by abiotic factors and host crop in a semi-arid prairie agroecosystem. _Fems Microbiol
Ecol_ 88, 333–344 (2014). Article CAS PubMed Google Scholar * Kim, Y., Gao, C. & Zheng, Y. Arbuscular mycorrhizal fungal community response to warming and nitrogen addition in a
semiarid steppe ecosystem. _Mycorrhiza_ 25, 267–276 (2015). Article CAS PubMed Google Scholar * Purin, S. & Rillig, M. C. The arbuscular mycorrhizal fungal protein glomalin:
Limitations, progress, and a new hypothesis for its function. _Pedobiologia_ 51, 123–130 (2007). Article CAS Google Scholar * Wilson, G. W. T., Rice, C. W. & Rilig, M. C. Soil
aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: results from long-term field experiments. _Ecol Lett_ 12, 452–461 (2009).
Article PubMed Google Scholar * Zhang, L. D., Zhang, J. L. & Christie, P. Effect of inoculation with the arbuscular mycorrhizal fungus Glomus intraradices on the root-knot nematode
Meloidogyne incognita in cucumber. _J Plant Nutr_ 32, 967–979 (2009). Article CAS Google Scholar * Antoninka, A., Reich, P. B. & Johnson, N. C. Seven years of carbon dioxide
enrichment, nitrogen fertilization and plant diversity influence arbuscular mycorrhizal fungi in a grassland ecosystem. _New Phytol_ 192, 200–214 (2011). Article PubMed Google Scholar *
Van Diepen, L. T. A., Lilleskov, E. A. & Pregitzer, K. S. Simulated nitrogen deposition affects community structure of arbuscular mycorrhizal fungi in northern hardwood forests. _Mol
Ecol_ 20, 799–811 (2011). Article CAS Google Scholar * Neuenkamp, L., Moora, M., Opik, M. The role of plant mycorrhizal type and status in modulating the relationship between plant and
arbuscular mycorrhizal fungal communities. New Phytol, https://doi.org/10.1111/nph.14995 (2018). Article CAS Google Scholar * Martínez-García, L. B., Richardson, S. J. & Tylianakis,
J. M. Host identity is a dominant driver of mycorrhizal fungal community composition during ecosystem development. _New Phytol_ 205(4), 1565–1576 (2015). Article PubMed CAS Google Scholar
* Krüger, C., Kohout, P. & Janouskova, M. Plant Communities Rather than Soil Properties Structure Arbuscular Mycorrhizal Fungal Communities along Primary Succession on a Mine Spoil.
_Front Microbiol_ 8, 719 (2017). Article PubMed PubMed Central Google Scholar * Honnay, O., Helsen, K. & Van Geel, M. Plant community reassembly on restored semi-natural grasslands
lags behind the assembly of the arbuscular mycorrhizal fungal communities. _Biol Conserv_ 212, 196–208 (2017). Article Google Scholar * de León, D. G., Moora, M. & Opik, M. Symbiont
dynamics during ecosystem succession: co-occurring plant and arbuscular mycorrhizal fungal communities. _Fems Microbiol Ecol_ 92(7), fiw097 (2016). Article CAS Google Scholar * Liu, Y.,
He, L. & An, L. Arbuscular mycorrhizal dynamics in a chronosequence of Caragana korshinskii plantations. _Fems Microbiol Ecol_ 67, 81–92 (2009). Article CAS PubMed Google Scholar *
Zangaro, W., Nisizaki, S. M. A. & Domingos, J. C. B. Mycorrhizal response and successional status in 80 woody species from South Brazil. _J Trop Ecol_ 19, 315–324 (2003). Article Google
Scholar * Wulandari, D., Saridi & Cheng, W. Arbuscular mycorrhizal fungal inoculation improves Albizia saman and Paraserianthes falcataria growth in post-opencast coal mine field in
East Kalimantan, Indonesia. _Forest Ecol Manag_ 379, 67–73 (2016). Article Google Scholar * Hart, M. M., Gorzelak, M. & Ragone, D. Arbuscular mycorrhizal fungal succession in a
long-lived perennial. _Botany_ 92, 313–320 (2014). Article Google Scholar * Corredor, A. H., Rees, K. V. & Vujanovic, V. Host genotype and health status influence on the composition of
the arbuscular mycorrhizal fungi in Salix bioenergy plantations. _Forest Ecol Manag_ 314, 112–119 (2014). Article Google Scholar * Yuan, Y., Yang, Y. & Chen, G. Fine root longevity of
a Cunninghamia lanceolata plantation estimated by minirhizotrons. _Journal of Subtropical Resources & Environment_ 4, 47–52 (2009). Google Scholar * Tian, D. L. _et al_. A long-term
evaluation of biomass production in first and second rotations of Chinese fir plantations at the same site. _Forestry_ 84, 411–418 (2011). Article Google Scholar * Bi, J. _et al_. Yield
decline in Chinese-fir plantations: a simulation investigation with implications for model complexity. _Can. J. For. Res_ 37, 1615–1630 (2007). Article Google Scholar * Chen, L. C. &
Wang, S. L. Allelopathic behaviour of Chinese fir from plantations of different ages. _Forestry_ 86(2), 225–230 (2013). Article Google Scholar * Fu, L., Sun, H. & Sharma, R. P.
Non-linear mixed-effects crown width models for individual trees of Chinese fir (Cunninghamia lanceolata) in south-central China. _Forest Ecol Manag_ 302, 210–220 (2013). Article Google
Scholar * Wang, Q. & Wang, S. Soil microbial properties and nutrients in pure and mixed Chinese fir plantations. _J Forestry Res_ 19, 131–135 (2008). Article CAS Google Scholar *
Chen, G., Yang, Z. & Gao, R. Carbon storage in a charonosequence of Chinese fir plantations in southern China. _Forest Ecol Manag_ 300, 68–76 (2013). Article Google Scholar * Guo, L.
D., Hyde, K. D. & Liew, E. C. Y. Identification of endophytic fungi from Livistona chinensis based on morphology and rDNA sequences. _New Phytol_ 47, 617–630 (2000). Article Google
Scholar * Maharachchikumbura, S. S. N., Guo, L. & Cai, L. A multi-locus backbone tree for Pestalotiopsis, with a polyphasic characterization of 14 new species. _Fungal Divers_ 56,
95–129 (2012). Article Google Scholar * Schwarzott, D. & Schüβler, A. A simple and reliable method for SSU rRNA gene DNA extraction, amplification, and cloning from single AM fungal
spores. _Mycorrhiza_ 10, 203–207 (2001). Article CAS Google Scholar * Lee, J., Lee, S. & Young, J. P. W. Improved PCR primers for the detection and identification of arbuscular
mycorrhizal fungi. _Fems Microbiol Ecol_ 65, 339–349 (2008). Article CAS PubMed Google Scholar * Lumini, E., Orgiazzi, A. & Borriello, R. Disclosing arbuscular mycorrhizal fungal
biodiversity in soil through a land-use gradient using a pyrosequencing approach. _Environ Microbiol_ 12, 2165–2179 (2009). PubMed Google Scholar * Sato, K., Suyama, Y. & Saito, M. A
new primer for discrimination of arbuscular mycorrhizal fungi with polymerase chain reaction-denature gradient gel electrophoresis. Japanese Society of Grassland. _Science_ 51, 179–181
(2005). CAS Google Scholar * Simon, L., Lalonde, M. & Bruns, T. D. Specific Amplification of 18S Fungal Ribosomal Genes from Vesicular-Arbuscular Endomycorrhizal Fungi Colonizing
Roots. _Appl Environ Microb_ 58, 291–295 (1992). CAS Google Scholar * Magoc, T. & Salzberg, S. L. FLASH: fast length adjustment of short reads to improve genome assemblies.
_Bioinformatics_ 27, 2957–2963 (2011). Article CAS PubMed PubMed Central Google Scholar * Edgar, R. C., Haas, B. J. & Clemente, J. C. UCHIME improves sensitivity and speed of
chimera detection. _Bioinformatics_ 27, 2194–2200 (2011). Article CAS PubMed PubMed Central Google Scholar * Edgar, R.C. UPARSE: highly accurate OUT sequences from microbial amplicon
reads. Nat Methods 10(10): 996-998 (2013). Article CAS PubMed Google Scholar * Opik, M., Zobel, M. & Cantero, J. J. Global sampling of plant roots expands the described molecular
diversity of arbuscular mycorrhizal fungi. _Mycorrhiza_ 23, 411–430 (2013). Article PubMed Google Scholar * Lindahl, B. D., Nilsson, R. H. & Tedersoo, L. Fungal community analysis by
high-throughput sequencing of amplified markers-a user’s guide. _New Phytol_ 199, 288–299 (2013). Article CAS PubMed PubMed Central Google Scholar * Tamura, K., Stecher, G. &
Peterson, D. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. _Molecular Biology and Evolution_ 30, 2725–2729 (2013). Article CAS PubMed PubMed Central Google Scholar *
Bray, R. H. & Kurtz, L. T. Determination of total, organic and available forms of phosphorus in soils. _Soil Sci_ 59, 39–46 (1945). Article ADS CAS Google Scholar * Bentonjonesjr.,
J. Soil testing in the United States. _Communications in Soil Sci & Plant Analysis_ 4, 16 (1973). Google Scholar * Walkley, A. A Critical examination of a rapid method for determining
organic carbon in soils-effect of variations in digestion conditions and of inorganic soil constituents. _Soil Sci_ 63, 251–264 (1947). Article ADS CAS Google Scholar * Bonfim, J. A.,
Vasconcellos, R. L. F. & Gumiere, T. Diversity of Arbuscular Mycorrhizal Fungi in a Brazilian Atlantic Forest Toposequence. _Microb Ecol_ 71, 164–177 (2016). Article PubMed Google
Scholar * Goslee, S. C. & Urban, D. L. The ecodist Package for Dissimilarity-based Analysis of Ecological Data. _J Stat Softw_ 22, 1–19 (2007). Article Google Scholar * Hart, M. M.,
Gorzelak, M. & Ragone, D. Arbuscular mycorrhizal fungal succession in a long-lived perennial. _Botany_ 92, 313–320 (2014). Article Google Scholar * Beauchamp, V. B., Stromberg, J. C.
& Stutz, J. C. Arbuscular mycorrhizal fungi associated with Populus–Salix stands in a semiarid riparian ecosystem. _New Phytol_ 170, 369–380 (2006). Article PubMed Google Scholar *
Li, L., Zhou, G. & Liu, J. The resource investigation and community structure characteristics of mycorrhizal fungi associated with Chinese fir. _African Journal of Biotechnology_ 10(30),
5719–5724 (2011). Google Scholar * Tian, D., Xiang, W., Yan, W. Effect of successive rotation on productivity and biomass of Chinese fir plantation at fast growing stage. Scientia Silvae
Sinicae 38: 14–18 (in Chinese) (2002). * Lin, C., Tao, H. & Jian, L. I. Intraspecific competition in a Cunninghamia lanceolata plantation with different age and diameter classes.
_Journal of Zhejiang A & F University_ 32, 353–360 (2015). Google Scholar * Gordon, W. S. B., J.R. Nutrient concentrations in fine roots. _Ecology_ 81, 275–280 (2000). Article Google
Scholar * Koziol, L. & Bever, J. D. Mycorrhizal response trades off with plant growth rate and increases with plant successional status. _Ecology_ 96, 1768–1774 (2015). Article PubMed
Google Scholar * Seifert, E. K., Bever, J. D. & Maron, J. L. Evidence for the evolution of reduced mycorrhizal dependence during plant invasion. _Ecology_ 90, 1055–1062 (2009).
Article PubMed Google Scholar * García, Ileana, Mendoza, Rodolfo & Pomar, MaríaC. Deficit and excess of soil water impact on plant growth of Lotus tenuis by affecting nutrient uptake
and arbuscular mycorrhizal symbiosis. _Plant and soil_ 304(1/2), 117–131 (2008). Article CAS Google Scholar * Chen, Y. L. _et al_. Nitrogen deposition and precipitation induced
phylogenetic clustering of arbuscular mycorrhizal fungal communities. _Soil biology & biochemistry_ 115, 233–242 (2017). Article CAS Google Scholar * Catarina Drumonde, Melo _et al_.
Communities of arbuscular mycorrhizal fungi under Picconia azorica in native forests of Azores. _Symbiosis._ 74, 43–54 (2018). Article Google Scholar * Yang, H. S. _et al_. Correlation
analysis between arbuscular mycorrhizal fungal community and host plant phylogeny. _Chinese journal of plant ecology_ 39(4), 383–387 (2015). (in Chinese). Article Google Scholar * Chai, Y.
X. _et al_. The effect of slope aspect on the phylogenetic structure of arbuscular mycorrhizal fungal communities in an alpine ecosystem. _Soil biology & biochemistry_ 126, 103–113
(2018). Article CAS Google Scholar * Zhang Jian _et al_. Stability of soil organic carbon changes in successive rotations of Chinese fir(Cunninghamia lanceolate(Lamb.) Hook) plantations.
_Journal of environmental sciences_ 21, 352–359 (2009). Article CAS Google Scholar * Zhang, X. Q. _et al_. Carbon stock changes in successive rotations of Chinese fir (Cunninghamia
lanceolate (Lamb) Hook) plantations. _Forest ecology and management_ 202, 131–147 (2004). Article Google Scholar * Influences of provenance and rotation age on heartwood rotio, stem
diameter and radial variation in tracheid dimension of Cunninghamia lanceolate. _Eur. J. Wood. Prod_ 76:669–677(2018). * Zhou, Wei & Gao, Lan The impact of carbon trade on the management
of short-rotation forest plantations. _Forest policy and economics_ 62, 30–35 (2016). Article Google Scholar * Zhao, M. F. _et al_. Effects of increased nitrogen deposition and rotation
length on long-term productivity of Cunninghamia lanceolate plantation in Southern China. _Plos One_ 8(2), e55376, https://doi.org/10.1371/journal.pone.0055376 (2013). Article ADS CAS
PubMed PubMed Central Google Scholar * Selvalakshmi Selvaraj _et al_. Influence of long-term successive rotations and stand age of Chinese fir (Cunninghamia lanceolate) plantations on
soil properties. _Geoderma_ 306, 127–134 (2017). Article ADS CAS Google Scholar * Mattoo, A. K. & Teasdale, J. R. Ecological and genetic systems underlying sustainable horticulture.
_Horticultural Reviews_ 37, 331–362 (2010). Google Scholar * Thilakarathna, M. S., McElroy, M. S. & Chapagain, T. Belowground nitrogen transfer from legumes to non-legumes under managed
herbaceous cropping systems – a review. _Agron Sustain Dev_ 36, 58 (2016). Article CAS Google Scholar * Bardgett, R. D., Mommer, L. & De Vries, F. T. Going underground: root traits
as drivers of ecosystem processes. _Trends in Ecology and Evolution_ 29, 692–699 (2014). Article PubMed Google Scholar * Finzi, A. C., Abramoff, R. Z. & Spiller, K. S. Rhizosphere
processes are quantitatively important components of terrestrial carbon and nutrient cycles. _Global Change Biol_ 21, 2082–2094 (2015). Article ADS Google Scholar * Meier, I. C., Finzi,
A. C. & Phillips, R. P. Root exudates increase N availability by stimulating microbial turnover of fast-cycling N pools. _Soil Biol Biochem_ 106, 119–128 (2017). Article CAS Google
Scholar * Sheng, M., Lalande, R. & Hamel, C. Effect of long-term tillage and mineral phosphorus fertilization on arbuscular mycorrhizal fungi in a humid continental zone of Eastern
Canada. _Regular Article_ 369, 599–613 (2013). CAS Google Scholar * Xiang, D., Verbruggen, E. & Hu, Y. Land use influences arbuscular mycorrhizal fungal communities in the
farming–pastoral ecotone of northern China. _New Phytol_ 204, 968–978 (2014). Article CAS PubMed Google Scholar * Irma Reyes-Jaramillo _et al_. The community of arbuscular mycorrhizal
fungi(Glomeromycota) associated with mescal agaves from Oaxaca and its relationship with some soil characteristics. _Revista Mexicana de biodiversidad_ 90, e902777 (2019). Google Scholar *
Giraldo, KarlaJaquelineRestrepo _et al_. Characterization of arbuscular mycorrhizal fungi of livestock soils in tropical lowlands and tropical highlands in the department of Antioquia,
Colombia. _IDESIA (Chile) Marzo_ 37, 35–44 (2019). Google Scholar * Martin Jemo _et al_. Mycorrhizal fungal community structure in tropical humid soil under fallow and cropping conditions.
_Scientific Reports_ 8, 17061, https://doi.org/10.1038/s41598-018-34736-6 (2018). Article ADS CAS PubMed PubMed Central Google Scholar * Wu, Q. P. _et al_. Additional AM fungi
inoculation increase Populus cathayana intersexual competetition. _Frontiers in plant science_ 9, 607, https://doi.org/10.3389/fpls.2018.00607 (2018). Article PubMed PubMed Central Google
Scholar * Bi, Yinli, Xiao, Li & Sun, Jinhua An arbuscular mycorrhizal fungus ameliorates plant growth and hormones after moderate root damage due to simulated coal mining subsidence: a
microcosm study. _Environmental science and pollution research_ 16, 11053–11061 (2019). Article CAS Google Scholar * Liu, Mohan _et al_. Rice straw biochar and phosphorus inputs have
more positive effects on the yield and nutrient uptake of Lolium multiflorum than arbuscular mycorrhizal fungi in acidic Cd-contaminated soils. _Chemosphere_ 235, 32–39 (2019). Article ADS
CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We wish to thank Ms. Jing Zhang for her help with data analysis. The authors are thankful to “The central university
special funding for basic scientific research business expenses (JD2010-2 and BLJD200907)” and the National Science and Technology Plan on rural areas (2012BAD22B05) for financial support.
Professor Ji kindly revised the English version of the manuscript. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * College of Forestry, Beijing Forestry University, Beijing, 100083, China Nini
Lu, Xuelei Xu, Ping Wang, Peng Zhang, Baoming Ji & Xinjie Wang * Key Laboratory for Silviculture and Conservation Joint-constructed by Province and Ministry of Education, Beijing
Forestry University, Beijing, 100083, China Nini Lu, Xuelei Xu, Ping Wang, Peng Zhang & Xinjie Wang * Experimental Forest Farm, Beijing Forestry University, Beijing, 100095, China Peng
Zhang Authors * Nini Lu View author publications You can also search for this author inPubMed Google Scholar * Xuelei Xu View author publications You can also search for this author inPubMed
Google Scholar * Ping Wang View author publications You can also search for this author inPubMed Google Scholar * Peng Zhang View author publications You can also search for this author
inPubMed Google Scholar * Baoming Ji View author publications You can also search for this author inPubMed Google Scholar * Xinjie Wang View author publications You can also search for this
author inPubMed Google Scholar CONTRIBUTIONS N.N.L., P.Z., B.M.J. and X.J.W. designed the experiment. N.N.L., X.L.X., P.Z. and P.W., carried out the experiment and performed the analyses.
N.N.L. and B.M.J. wrote the main manuscript text. N.N.L., P.W. and B.M.J. contributed to all the figures. CORRESPONDING AUTHOR Correspondence to Xinjie Wang. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION REVISED MANUSCRIPT RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons
license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Lu, N., Xu, X., Wang, P. _et al._ Succession in arbuscular mycorrhizal fungi can be
attributed to a chronosequence of _Cunninghamia lanceolata_. _Sci Rep_ 9, 18057 (2019). https://doi.org/10.1038/s41598-019-54452-z Download citation * Received: 18 June 2019 * Accepted: 14
November 2019 * Published: 02 December 2019 * DOI: https://doi.org/10.1038/s41598-019-54452-z SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content:
Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative