Play all audios:
ABSTRACT Serotonin (5-hydroxytryptamine: 5-HT) is a biogenic monoamine that mediates immune responses and modulates nerve signal in insects. _Se-5HTR_, a specific receptor of serotonin, has
been identified in the beet armyworm, _Spodoptera exigua_. It is classified into subtype 7 among known 5HTRs. _Se-5HTR_ was expressed in all developmental stages of _S_. _exigua_. It was
expressed in all tested tissues of larval stage. Its expression was up-regulated in hemocytes and fat body in response to immune challenge. RNA interference (RNAi) of _Se-5HTR_ exhibited
significant immunosuppression by preventing cellular immune responses such as phagocytosis and nodulation. Treatment with an inhibitor (SB-269970) specific to 5HTR subtype 7 resulted in
significant immunosuppression. Furthermore, knockout mutant of _Se-5HTR_ by CRISPR-Cas9 led to significant reduction of phagocytotic activity of _S_. _exigua_ hemocytes. Such
immunosuppression was also induced by bacterial secondary metabolites derived from _Xenorhabdus_ and _Photorhabdus_. To determine specific bacterial metabolites inhibiting Se-5HTR, this
study screened 37 bacterial secondary metabolites with respect to cellular immune responses associated with Se-5HTR and selected 10 potent inhibitors. These 10 selected compounds
competitively inhibited cellular immune responses against 5-HT and shared phenylethylamide (PEA) chemical skeleton. Subsequently, 46 PEA derivatives were screened and resulting potent
chemicals were used to design a compound to be highly inhibitory against Se-5HTR. The designed compound was chemically synthesized. It showed high immunosuppressive activities along with
specific and competitive inhibition activity for Se-5HTR. This study reports the first 5HT receptor from _S_. _exigua_ and provides its specific inhibitor designed from bacterial metabolites
and their derivatives. SIMILAR CONTENT BEING VIEWED BY OTHERS STRUCTURE-ACTIVITY RELATIONSHIPS OF SEROTONERGIC 5-MEO-DMT DERIVATIVES: INSIGHTS INTO PSYCHOACTIVE AND THERMOREGULATORY
PROPERTIES Article Open access 14 March 2024 MARINE DITERPENOID TARGETS STING PALMITOYLATION IN MAMMALIAN CELLS Article Open access 18 July 2023 STRUCTURAL PHARMACOLOGY AND THERAPEUTIC
POTENTIAL OF 5-METHOXYTRYPTAMINES Article 08 May 2024 INTRODUCTION Serotonin or 5-hydroxytryptamine (5-HT) is a biogenic monoamine found across most phyla of life1. This indolamine compound
is biosynthesized from tryptophan by successive catalytic activities of tryptophan hydroxylase and aromatic-L-amino acid decarboxylase2,3,4 primarily in nervous systems5,6. In human and
other vertebrates, serotonin is a well-known neurotransmitter involved in mood, appetite, sleep, anxiety, cognition, and psychosis7,8,9. Outside the nervous system, serotonin plays important
roles as growth factor and regulator of hemostasis and blood clotting10,11. In plants, serotonin is basically involved in stress signaling12. Serotonin plays crucial role in physiological
and behavioral processes in insects and other invertebrates13,14. In _Drosophila melanogaster_, there is evidence that serotonin is required for courtship and mating15, circadian
rhythm16,17, sleep18, locomotion13,19, aggression20, insulin signaling and growth21, and phagocytosis22. Serotonin is also involved in olfactory processing23, feeding behavior19, heart
rate24, and responses to light25 in _D_. _melanogaster_ larvae. Serotonin modulates physiological processes by binding to specific receptors. Seven main subtypes of serotonin receptors have
been classified in vertebrates26. Except 5-HT3 receptor, the other six classes (5-HT1, 5-HT2, 5-HT4, 5-HT5, 5-HT6, and 5-HT7 receptors) belong to G protein-coupled receptor family27. Among
these receptors, 5-HT1 and 5-HT5 receptors can inhibit cAMP synthesis by preferentially coupling to a trimeric G protein Gi/o28. 5-HT2 receptor uses Gq/11 to induce breakdown of inositol
phosphates, resulting in an increase in cytosolic Ca2+ level28. Besides, 5-HT4, 5-HT6, and 5-HT7 receptors coupled to Gs can stimulate cAMP production28. However, 5-HT3 receptor is a
ligand-gated cation channel that mediates neuronal depolarization29. Insects have at least three subtypes of 5-HT receptors. Five different 5-HT receptors as orthologous to mammalian 5-HT1A,
5-HT1B, 5-HT2A, 5-HT2B, and 5-HT7 have been identified in _D_. _melanogaster_14. In addition, partial sequences of two 5-HT1, two 5-HT2, and one 5-HT7 receptors have been identified in the
field cricket _Gryllus bimaculatus_30. Two 5-HT1 receptors and two 5-HT1 splice variants have been described in _Tribolium castaneum_ and _Papilio xuthus_, respectively31,32. As from _D_.
_melanogaster_ and _G_. _bimaculatus_, two 5-HT2 receptors have been described from _Apis mellifera_ while only one 5-HT2 receptor has been reported in other insects such as _Periplaneta
americana_ and _Locusta migratoria_333,34. In a lepidopteran species, _Pieris rapae_, four different 5-HT receptors including a novel subtype 8 have been reported35,36. 5-HT modulates
various physiological processes via expression of different receptor types in various tissues. 5-HT receptors are expressed highly in brain and ventral nerve cord of insects32. 5-HT1
receptor in honey bee brain is involved in visual information processing37. Expression pattern of 5-HT7 receptor in honey bee nervous system suggests its possible roles in information
processing, learning, and memory38. 5-HT receptors might play roles in neuroendocrine secretion and gut motility in cockroaches29. In salivary gland of several insects, 5-HT7 receptor has
been reported to be involved in salivary secretion mediated by cAMP level elevation33,39,40. Moreover, 5-HT7 receptor mediates visceral muscle contraction in the gastrointestinal tract of
several insects including _A_. _aegypti_ and _T_. _castaneum_41,42. 5-HT not only has neurophysiological roles, but also mediates cellular immune responses in insects by enhancing
phagocytosis and nodulation43. Two different 5-HT receptors (1B and 2B) are expressed in hemocytes of _P_. _rapae_, of which 5-HT receptor 1B mediates cellular immune response22. In another
lepidopteran insect, _Spodoptera exigua_, 5-HT mediates increase of total circulatory hemocyte number by stimulating sessile hemocytes and mediating cellular immune responses such as
phagocytosis and nodule formation44,45. However, 5-HT receptor in _S_. _exigua_ has not been reported yet. Two entomopathogenic bacteria, _Xenorhabdus_ and _Photorhabdus_, can inhibit insect
immune responses to protect themselves and their symbiotic nematodes46. To accomplish host immunosuppression, these bacteria can synthesize and secrete secondary metabolites to inhibit
immune signals and effectors47. Among these bacterial metabolites, tryptamine and phenylethylamide derivatives have been identified with suggested function of interrupting 5-HT signaling48.
The objective of the present study was to determine bacterial secondary compound(s) that could inhibit 5-HT signaling. To this end, we identified 5-HT receptor that could mediate insect
immunity in _S_. _exigua_. To determine a potent inhibitor of this receptor, we screened bacterial secondary metabolites and their potent derivatives. RESULTS BIOINFORMATICS ANALYSES REVEAL
THAT _S_. _EXIGUA_ CONTAINS A 5-HT RECEPTOR From a short read archive database (GenBank accession number: SRR1050532) of _S_. _exigua_, a highly matched contig (accession number:
GARL01017386.1) containing 3,308 bp nucleotide sequence with an open reading frame (ORF) from 996th to 2,690th bp was identified. Prediction of amino acid sequence by BlastP analysis
revealed that its ORF sequence shared identities with other insect 5-HT receptors: 99% with _Spodoptera litura_ 5HTR (XP_022827337.1), 98% with _Helicoverpa armigera_ 5HTR (XP_021195909.1),
95% with _Trichoplusia ni_ 5HTR (XP_026729862.1), and 85% with _Manduca sexta_ 5HTR (AGL46976.1). This novel 5-HT receptor from _S_. _exigua_ (Se-5HTR) encoded a sequence of 564 amino acids
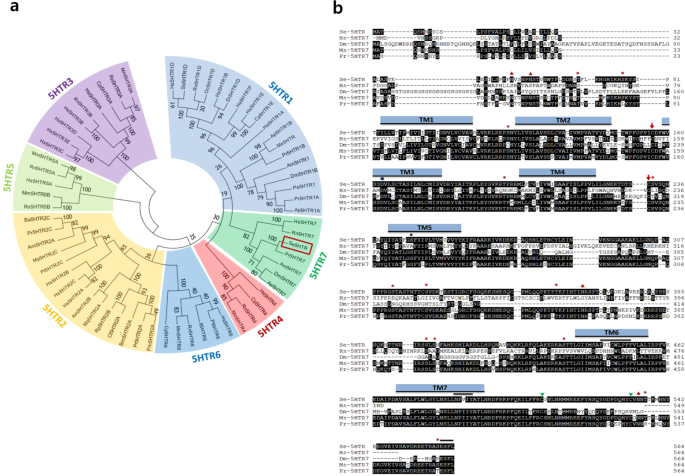
having a predicted molecular weight of about 63.23 kDa. Phylogenetic analysis of its protein sequence indicated that Se-5HTR was clustered with other 5-HT7 receptors (Fig. 1a). Predicted
amino acid sequence of Se-5HTR contained a signal peptide of 35 residues and attained GPCR character with seven transmembrane domains. Consensus N-linked glycosylation sites are located in
the N-terminus (Asn48 and Asn53), the third intracellular loop (Asn362), and C-terminus (Asn534). Several consensus sites for phosphorylation by protein kinase A and/or protein kinase C were
predicted throughout the length of the sequence. A disulfide bond between two Cys residues at extracellular matrix (‘ECM’, Supplementary Fig. 1) is also predicted. _Se-5HTR_ sequence
attains highly conserved amino acid residues (Fig. 1b) found uniquely in the biogenic monoamine receptor family49,50,51. Negatively charged Asp residue in TM3 (Asp163) might interact with
positively charged amino group of the ligand. A hydrogen bond between the hydroxyl group of serine residue in TM5 (Ser247) and the hydroxyl group of 5-HT was predicted. In TM6, the consensus
sequence unique to aminergic receptors (Phe444-X-X-X-Trp448-X-Pro450-X-Phe452) is conserved. Like other GPCRs, a possible motif (Asn485-Pro486-X-X-Tyr489) that might participate in
agonist-mediated sequestration and re-sensitization of Se-5HTR is conserved in TM7. The C-terminus of the receptor contains two potential post-translational palmitoylation cysteine residues
(Cys508 and Cys-532) and PDZ (post synaptic density protein, _D__rosophila_ disc large tumor suppressor, and zonula occludens-1 protein)-domain binding motif (Glu561-Ser562-Phe563-Leu564).
SE-5HTR IS EXPRESSED IN ALL DEVELOPMENTAL STAGES AND LARVAL TISSUES Expression of _Se-5HTR_ in different developmental stages of _S_. _exigua_ was assessed by RT-PCR. Results showed its
expression from egg to adult stages (Fig. 2a). RT-qPCR revealed variation in its expression among developmental stages, with L5 larvae and adults showing the highest expression levels. In L5
larvae, all tissues analyzed by RT-PCR showed its expression (Fig. 2b). RT-qPCR revealed that the midgut exhibited the highest expression level of _Se-5HTR_. Hemocytes and brain also showed
relatively high levels of its expression. Basal expression levels of _Se-5HTR_ were highly up-regulated in response to immune challenge (Fig. 2c). _Se-5HTR_ expression was increased
∼125-fold after challenge with fungus, _Beauveria bassiana_ compared to control (unchallenged). It was increased 60∼90-fold after bacterial challenge compared to naïve larvae. SPECIFIC
INHIBITORS AGAINST 5-HTR SUPPRESS HEMOCYTE BEHAVIORS OF _S_. _EXIGUA_ A commercial inhibitor (SB-269970) specific to 5-HT7 was used to assess its effect on hemocyte behaviors of _S_.
_exigua_ (Fig. 3). Total hemocyte count (THC) of L5 larvae was ~1.2 × 107 cells/mL (Fig. 3a). THC was significantly (_P_ < 0.05) increased in response to 5-HT or bacterial challenge.
SB-269970 prevented the increase of THC in response to bacterial challenge. Its IC50 value was estimated to be 1.925 μM. These results suggest that Se-5HTR can mediate hemocyte mobilization
in response to 5-HT upon bacterial challenge. To determine the modulation effect of Se-5HTR on hemocyte-spreading behavior, competitive inhibition between SB-269970 and 5-HT against Se-5HTR
was assessed (Fig. 3b). Hemocytes were spread on slide glass with growth of F-actin. However, SB-269970 significantly (_P_ < 0.05) suppressed such hemocyte-spreading behavior. The
inhibitory effect of the inhibitor was rescued by addition of 5-HT. A relatively low dose (SB-269970: 5-HT = 10:1) of 5-HT did not rescue the inhibitory effect. However, a relatively high
dose (1:10) of 5-HT significantly (_P_ < 0.05) rescued such inhibitory effect, indicating a specific competition between inhibitor and ligand against Se-5HTR. RNA INTERFERENCE (RNAI) OF
SE-5HTR To address the immunomodulatory effect of Se-5HTR on hemocytes, its gene expression was knocked-down by RNAi (Fig. 4). RNAi was performed using dsRNA specific to _Se-5HTR_.
dsRNA-injected larvae exhibited significant (_P_ < 0.05) reduction of _Se-5HTR_ expression. RT-qPCR analysis showed that about 90% of _Se-5HTR_ mRNA was suppressed by dsRNA treatment at
24 h PI (Fig. 4a). At 24 h PI of dsRNA, _Se-5HTR_ expression was analyzed in four different tissue samples. Compared to control tissues, dsRNA-treated larvae exhibited significant (_P_ <
0.05) reduction of _Se-5HTR_ expression in all tissues including hemocytes. RNAI OF SE-5HTR SUPPRESSES HEMOCYTE BEHAVIORS OF _S_. _EXIGUA_ The effect of RNAi specific to Se-5HTR on hemocyte
mobilization in response to bacterial challenge was analyzed (Fig. 4b). Upon bacterial challenge, THC increased by more than two folds. However, RNAi treatment significantly (_P_ < 0.05)
suppressed such increase of THC. The RNAi treatment also significantly (_P_ < 0.05) influenced hemocyte-spreading behavior (Fig. 4c). On glass slide, hemocytes exhibited spreading
behavior in 40 min by cytoskeletal rearrangement through F-actin growth (see phalloidin staining). However, hemocytes collected from larvae treated with RNAi specific to Se-5HTR lost such
behavior. MODULATION OF CELLULAR IMMUNE RESPONSES BY SE-5HTR The influence of Se-5HTR on modulating hemocyte-spreading behavior suggested that it might mediate cellular immune responses
against bacterial infection. Phagocytosis against FITC-labeled _Escherichia coli_ was observed in hemocytes from control larvae. Labeled bacteria were observed within hemocytes of control
larvae (Fig. 5a). However, hemocytes of larvae treated with dsRNA specific to Se-5HTR significantly (_P_ < 0.05) lost such phagocytosis, similar to that found for hemocytes of larvae
treated with SB-269970. Phagocytosis was decreased around 57% or 78% after treatment with dsRNA or inhibitor, respectively. In response to bacterial challenge, _S_. _exigua_ formed ∼78
hemocytic nodules per larva (Fig. 5b). However, RNAi specific to Se-5HTR or inhibitor treatment significantly (_P_ < 0.05) reduced such cellular immune response. CRISPR-CAS9 OF _SE-5HTR_
AND ITS INFLUENCE ON PHAGOCYTOSIS To support the function of Se-5HTR in mediating cellular immunity, a CRISPR-Cas9-mediated knockout of _Se5HTR_ was performed (Fig. 6). In _in vitro_ test,
the designed sgRNA was able to specifically bind to target DNA (638 bp, Fig. 6a) and mediated DNA cleavage by Cas9 (Fig. 6b). The sgRNA and Cas9 enzyme complex was injected to newly laid
eggs. Control (Cas9 only) injection (n = 300) showed 66.3% of hatching ration while treatment (sgRNA + Cas9) injection (n = 800) showed 12.1%. Among hatched larvae, 94.4% larvae developed to
pupae in control compared to 15.4% in treatment. At L5 larvae, the mutant larvae were assessed in their genomic DNA sequences at the target region and showed deletions compared to control
larvae (Fig. 6c). The deletion mutations led to missense and partial deletion in amino acid sequence (‘M2’) or early termination in other mutants. Under this knockout condition, mutant
larvae were assessed in cellular immune response using phagocytosis (Fig. 6d). Control larvae enhanced the phagocytotic activity with addition of 5-HT. However, knockout mutants were
significantly impaired in the immune response, in which the enhancement of the phagocytotic activity in response to 5-HT was lost. INFLUENCE OF BACTERIAL SECONDARY METABOLITES ON IMMUNE
RESPONSES MEDIATED BY SE-5HTR RNAi or specific inhibitor assays indicated that 5-HTR could mediate both cellular immune responses of phagocytosis and nodule formation in response to
bacterial challenge. Bacterial metabolites of two entomopathogens, _Xenorhabdus nematophila_ (Xn) and _Photorhabdus temperata temperata_ (Ptt), were extracted from their culture broth using
different organic solvents and their inhibitory activities against cellular immune responses were then assessed (Fig. 7). Organic solvent extracts of Xn- or Ptt-cultured broth exhibited
significant (_P_ < 0.05) inhibitory activities against phagocytosis (Fig. 7a) and nodulation (Fig. 7b), although there were variations in their inhibitory activities among extracts. To
identify bacterial secondary compounds that could inhibit Se-5HTR, 37 compounds (HB 4 – HB 602) derived from _Xenorhabdus_ and _Photorhabdus_47 were screened for their inhibitory activities
against hemocyte nodule formation and phagocytosis (Fig. 8). More than three 75% (28 out of 37 compounds) of these test compounds exhibited significant (_P_ < 0.05) inhibition against
phagocytosis (Fig. 8a). In nodulation assay, all test compounds exhibited significant (_P_ < 0.05) inhibition (Fig. 8b). Since Se-5HTR could mediate both cellular immune responses,
bacterial compounds that highly inhibited both phagocytosis (<60%) and nodulation (<30 nodules per larva) were selected. As a result, 10 potent chemicals (HB 4, HB 5, HB 23, HB 30, HB
44, HB 45, HB 50, HB 223, HB 302, and HB 531) belonging to six chemical categories [phenylethylamide (PEA), tryptamide, xenortide, xenocycloin, nematophin, and GameXPeptide] were found
(Supplementary Fig. 2). All these compounds exhibited median inhibitory concentration (IC50) of 58∼253 μM against cellular immune response of hemocytic nodulation. To support the specific
inhibitory activity of these selected compounds against Se-5HTR, competitive assay was performed between test compounds and 5-HT (Fig. 9). With a constant concentration of 5-HT, phagocytic
behavior of _S_. _exigua_ hemocytes gradually decreased with increasing amount of test compound (Fig. 9a). On the other hand, increase of 5-HT amount gradually decreased the phagocytotic
behavior using a constant amount of test compound (Fig. 9b). INFLUENCE OF PEA DERIVATIVES ON PHAGOCYTOSIS MEDIATED BY SE-5HTR These 10 selected compounds shared phenyl (or aromatic)
ethylamide backbone except xenocycloin (Supplementary Fig. 2). Based on PEA backbone, 45 derivatives were selected from a chemical bank and their inhibitory activities against the
phagocytotic behavior of _S_. _exigua_ hemocytes were tested (Fig. 10a). More than 66% (30 out of 45 compounds) of PEA derivatives exhibited significant (_P_ < 0.05) inhibition against
phagocytosis. Three PEA derivatives (Ph15, Ph17, Ph33) had inhibitory activities similar to a bacterial metabolite (HB 44), with IC50 at 1.8 ∼ 5.6 μM against phagocytosis (Fig. 10b). These
three PEA derivatives competitively inhibited the phagocytosis mediated by Se-5HTR (Fig. 10c). SYNTHESIS OF A POTENT INHIBITOR AND INHIBITORY EFFICACY AGAINST SE-5HTR Potent compounds from
45 PEA derivatives were analyzed for their structures and activities against phagocytosis mediated by Se-5HTR (Supplementary Fig. 3). They shared the PEA backbone. However, they had
different side chains at ‘X’ and ‘Y’ (Supplementary Fig. 3a). When different X substituents were compared for their inhibitory activities, methoxy was the most potent moiety (Supplementary
Fig. 3b). When different Y substituents were compared for their inhibitory activities with respect to identical X groups, hexahydropyrrolo[1,2-a]pyrazine-1,4-dione was the most potent moiety
(Supplementary Fig. 3c). This analysis allowed us to design a hypothetical compound containing methoxy in X and hexahydropyrrolo[1,2-a]pyrazine-1,4-dione at Y based on PEA backbone
(Supplementary Fig. 3d). The designed compound (‘PhX’) was chemically synthesized and in its inhibitory activity against phagocytosis was assessed compared with a specific 5-HT7 inhibitor,
SB269970, as a reference (Fig. 11). PhX was highly potent. It competitively inhibited cellular immune response with 5-HT. Its inhibitory activity was more potent (_t_ = 14.9; df = 32; _P_
< 0.0001) than SB269970. DISCUSSION 5-HT plays a crucial role in mediating cellular immune responses of _S_. _exigua_ by stimulating actin rearrangement45. Furthermore, it performs a
functional cross-talk with eicosanoid signaling via a small G protein Rac152. However, its signaling pathway leading to immune responses remains unclear due to the lack of its receptor
information in _S_. _exigua_. This study identified a 5-HT receptor and showed its physiological function in mediating immune responses. Se-5HTR attains seven transmembrane domains and
shares common molecular characters with other 5-HT receptors. Like other GPCRs, Se-5HTR contains the canonical seven transmembrane domains along with consensus glycosylation in the
N-terminus (Asn48 and Asn53)53. Additionally, its sequence contains a consensus aspartic acid residue in TM3 (Asp163) and a serine residue in TM5 (Ser247) to interact with functional groups
of biogenic monoamines54. At the intracellular border of TM3, the highly conserved Asp180-Arg181-Tyr182 motif is evident for a strong ionic interaction with Glu430 residue adjacent to the
intracellular end of TM6 that plays a crucial role in GPCR signal transduction55. The sequence contains consensus Phe444-X-X-X-Trp448-X-Pro450-X-Phe452 motif in TM6 which is unique to
biogenic monoamine GPCRs38. Additionally, Se-HTR contains two potential post-translational palmitoylation cysteine residues (Cys508 and Cys532)56 at the final intracellular region with a
PDZ-domain binding motif (Glu561-Ser562-Phe563-Leu564) at the C-terminus57. _Se-5HTR_ is expressed in all developmental stages. It is expressed in immune-associated tissues (hemocytes and
fat body), digestive (gut) tissues, and nervous (brain) tissues at larval stage. Three 5-HT receptors of _P_. _rapae_ larvae are all expressed, although their expression levels in tissues
are different. Subtypes 1A and 1B receptor are highly expressed in nervous tissues while subtype 7 receptor is mainly expressed in digestive tissue36. _Se-5HTR_ was also highly expressed in
the gut like 5-HT7 of _P_. _rapae_. Furthermore, our phylogenetic analysis of 5-HT receptors showed that these two insect 5-HT7s were closely related and clustered. Expression levels of
_Se-5HTR_ in hemocytes were similar to those in the brain. This suggests that Se-5HTR is associated with immune function as well as neurophysiological function. Indeed, bacterial or fungal
infection up-regulated the expression of _Se-5HTR_. The increase of _Se-5HTR_ expression might be explained by the up-regulation of _de novo_ biosynthesis of its ligand, 5-HT, via increase
in expression of biosynthetic genes as seen in hemocytes of _P_. _rapae_ larvae after immune challenge22. The presence of 5-HT7 receptor in hemocytes of _S_. _exigua_ and its physiological
function associated with immune responses were supported by its sensitivity to specific 5-HTR inhibitors. In response to 5-HT or bacterial challenge, _S_. _exigua_ larvae exhibited
significant increase of THC. This up-regulation of THC was explained by mobilization of sessile hemocytes to circulatory form by cytoskeletal rearrangement via a small G protein, Rac145,52.
This suggests that Se-5HTR can activate Rac1 to stimulate the hemocyte behavior. In vertebrates, activation of 5-HT7 receptor increases cAMP level via a trimeric G protein Gs and small G
proteins of Rho family including Cdc42, RhoA, and Rac1 via another trimeric G protein G1258. This suggests that immune challenge can induce biosynthesis and release of 5-HT which then binds
to Se-5HTR on hemocytes and activates Rac1 to stimulate hemocyte behaviors. In addition, the cAMP pathway triggered by Se-5HTR might activate Akt and ERK1/2 as seen in mammalian cancer
cells59 to facilitate actin rearrangement to form cellular shape change of hemocytes. These findings suggest that Se-5HTR plays a crucial role in hemocyte migration and cell shape change
during cellular immune responses. Indeed, RNAi of _Se-5HTR_ expression resulted in significant immunosuppression by exhibiting reduction in phagocytosis and nodulation. This was further
supported by _Se-5HTR_ knockout mutant generated by CRISPR-Cas9 technology. The mutant larvae suffered from poor phagocytotic activity even in the presence of 5-HT. Bacterial metabolites
derived from two entomopathogens, _Xenorhabdus_ and _Photorhabdus_, inhibited cellular immune responses mediated by Se-5HTR. Especially, six chemical groups (phenylethylamide, tryptamide,
xenortide, xenocycloin, nematophin, and GameXPeptide) highly inhibited both phagocytosis and nodulation, suggesting that they can inhibit Se-5HTR. This was supported by their competitive
inhibition with the ligand, 5-HT. Bacterial secondary metabolites may be synthesized and released in the bacterium-nematode complex in order to defend immune attack from target insect to
compete with other microbes occurring in the insect cadaver and facilitate host nematode development or bacterial quorum sensing47. It has been reported phenylethylamides and tryptamides
identified from _Xenorhabdus_ can act as quorum quenching activators by competitive binding to N-acylated homoserine lactone (AHL) receptor because AHL accumulation drives gene expression of
bioluminescence, virulence factor, and biofilm formation in bacteria60,61. Xenortides are linear peptides consisting of 2–8 amino acids synthesized from both _Xenorhabdus_ and
_Photorhabdus_62. They are synthesized in insect hosts during infection with putative role in inhibiting prophenoloxidase activation to suppress insect immunity63. Xenocycloins produced by
_X_. _bovienii_ are cytotoxic to hemocytes of _Galleria melonella_64. Nematophin is synthesized by condensation of α-keto acid and tryptamine in _X_. _nematophila_. It possesses a specific
antibacterial activity against _Staphylococcus aureus_65. GameXPeptides are cyclic pentapeptides widely synthesized in both _Xenorhabdus_ and _Photorhabdus_. However, their biological
functions remain unclear66. This current study showed that these six compound classes could inhibit cellular immune responses by competitive inhibition with 5-HT against Se-5HTR. A novel
phenylethylamide compound, PhX, was found to be highly inhibitory against Se-5HTR. Derivatives of phenylethylamide compound (HB 44) exhibited different inhibitory activities against cellular
immune responses mediated by Se-5HTR. Especially, PhX containing methoxy and hexahydropyrrolo[1,2-α]pyrazine-1,4-dione exhibited the highest inhibitory activity. 5-HT receptors have been
used for screening for potent insecticides with growth-inhibiting or larvicidal activities against _Pseudaletia separata_67. Thus, PhX can be a candidate for this application unless it shows
mammalian or non-target species toxicity68. METHODS INSECT REARING AND MICROBIAL CULTURE A laboratory strain of _S_. _exigua_ was originated from Welsh onion field (Andong, Korea) and
maintained for ∼20 years. Larvae of this laboratory strain were reared on an artificial diet69 at temperature of 25 ± 1 °C and relative humidity of 60 ± 10% with a photoperiod of 16:8 h
(L:D). Under these conditions, larvae had five instars (‘L1-L5’). Adults were reared with 10% sucrose solution. For immune challenge, _Escherichia coli_ Top10 (Invitrogen, Carlsbad, CA, USA)
was cultured in Luria-Bertani (LB) medium (BD Korea, Seoul, Korea) in a shaking incubator (200 rpm) at 37 °C overnight (16 h). CHEMICALS Serotonin hydrochloride was purchased from
Sigma-Aldrich Korea (Seoul, Korea). It was dissolved in distilled water. SB-269970 (a specific inhibitor to 5-HT receptor subtype 7, 5-HT7) was purchased from Cayman Chemical Company
(Korea). It was dissolved in desired concentrations with dimethyl sulfoxide (DMSO). Fluorescein isothiocyanate (FITC) [2-(6-hydroxy-3-oxo-3h-xanthen-9-yl)-5-isothiocyanatobenzoic acid] was
purchased from Sigma-Aldrich Korea. It was dissolved in DMSO to make a solution at 10 mg/mL. Anticoagulant buffer (ACB) was prepared using 98 mM NaOH, 186 mM NaCl, 17 mM Na2EDTA, and 41 mM
citric acid at pH 4.5. Phosphate-buffered saline (PBS) was prepared at pH 7.4 with 50 mM sodium phosphate and 0.7% NaCl. Tris-buffered saline (TBS) was prepared using 150 mM NaCl, 50 mM
Tris-HCl at pH 7.6. Hank’s balanced salt solution (HBSS) was prepared with the following compositions: 8 g NaCl, 400 mg KCl, 40 mg Na2HPO4, 60 mg KH2PO4, 1 g glucose, 140 mg CaCl2, 120 mg
MgSO4, and 350 mg NaHCO3 in 1,000 mL distilled H2O. BIOINFORMATICS TO SEARCH FOR 5-HT RECEPTOR AND SEQUENCE ANALYSIS _S_. _exigua_ 5-HT receptor (Se-5HTR) sequence was obtained from GenBank
by manual annotation. Briefly, a dopamine receptor sequence (AKR18180.1) of _Chilo suppressalis_ was used to screen a transcriptome (SRR1050532) of _S_. _exigua_. A blast contig
(GARL01017386.1) was analyzed for open reading frame (ORF) and the predicted amino acid sequence was used for analysis using BlastP program against GenBank (www.ncbi.nlm.nih.gov). After
confirming its high homologies (E value < 10−20) with other known insect 5HTRs, the resulting ORF sequence (=Se-5HTR) was deposited at NCBI-GenBank (accession number: MH025798). Sequence
alignment was established using Clustal Omega tool (https://www.ebi.ac.uk/Tools/msa/clustalo/) provided by European Bioinformatics Institution. Phylogenetic tree was generated with
Neighbor-joining method using Mega6 and ClustalW programs. Bootstrapping values were obtained with 1,000 repetitions to support branch and clustering. Protein domain was predicted using
InterPro tool (https://www.ebi.ac.uk/interpro/), pfam (http://pfam.xfam.org), and Prosite (http://prosite.expasy.org/). RNA EXTRACTION AND CDNA PREPARATION Using Trizol reagent (Invitrogen),
total RNAs were extracted from all developmental stages as well as larval tissues (hemocyte, midgut, fat body, and brain) of _S_. _exigua_ according to the instruction of the manufacturer.
Numbers of individuals used for RNA extraction for each individual developmental stage were as follows: ~500 eggs, ~20 larvae for L1-L2, ~10 larvae for L3, ~5 larvae for L4, one larva for
L5, one pupa, and one adult. L5 larvae were used for RNA extraction from different tissue samples. After extraction, total RNAs were resuspended in nuclease-free water and cDNAs were
synthesized from ~1 µg of RNAs using Maxime RT Premix (Intron Biotechnology, Seoul, Korea) according to the manufacturer’s instruction. EXPRESSION PATTERN OF SE-5HTR BY RT-QPCR A fragment of
_Se-5HTR_ was amplified with gene-specific primers (5′-CTT TAC CTT CGT GTC TTC TC-3′ and 5′-GGT GTC AGT CTT CTC ATT AC-3′). PCR was performed with 35 cycles of denaturation (94 °C, 1 min),
annealing (49 °C, 1 min), and extension (72 °C, 1 min). PCR products were subjected to agarose gel electrophoresis to visually confirm their amplifications. With the same gene-specific
primers used in RT-PCR, RT-qPCR was performed in a qPCR machine (CFX ConnectTM Real-Time PCR Detection System, Bio-Rad, Hercules, CA, USA) using SYBR Green Realtime PCR Master Mix (Toyobo,
Osaka, Japan) under a guideline of Bustin _et al_.70. The amplification used 40 cycles of 15 s at 95 °C, 30 s at 60 °C, and 45 s at 72 °C. After PCR reactions, melting curves from 60 to 95
°C were obtained to confirm unique PCR products. A ribosomal protein, RL32, gene was used as a control with primers of 5′-ATG CCC AAC ATT GGT TAC GG-3′ and 5′-TTC GTT CTC CTG GCT GCG GA-3′.
Each treatment was independently triplicated. Relative quantitative analysis method (2−ΔΔCT) was used to estimate mRNA expression levels of _Se-5HTR_. RNA INTERFERENCE (RNAI) Gene fragment
of _Se-5HTR_ was amplified from template DNA using gene-specific primers (5′-CTT TAC CTT CGT GTC TTC TC-3′ and 5′-GGT GTC AGT CTT CTC AT-3′) possessing T7 RNA polymerase promoter sequence
(5′-TAA TAC GAC TCA CTA TAG GGA GA-3′) at 5′ ends. PCR was performed with 5 cycles of denaturation (94 °C, 1 min), annealing (49 °C, 1 min), and extension (72 °C, 1 min) followed by 30
cycles of denaturation (94 °C, 1 min), annealing (60 °C, 1 min), and extension (72 °C, 1 min) to synthesize DNA template for dsRNA synthesis. Double-stranded RNA (dsRNA) against _Se-5HTR_
(‘dsSe-5HTR′) was synthesized using Megascript RNAi kit (Ambion, Austin, TX, USA) following the manufacturer’s instruction. The resulting dsRNA was blended with Metafectene PRO (Biontex,
Plannegg, Germany) at 1:1 (v:v) ratio and incubated at 25 °C for 30 min for liposome formation. Two microliters of the prepared mixture containing ~900 ng of dsRNA was injected twice into
_S_. _exigua_ larval hemocoel using a microsyringe (Hamilton, Reno, NV, USA) equipped with a 26-gauge needle. The first injection was at late L4 stage. It was repeated 12 h afterwards. RNAi
efficacy at 0, 24, and 48 h post-injection (PI) in reducing _Se-5HTR_ expression was determined by RT-qPCR. At 24 h PI, treated larvae were used for immune challenge experiments. Each
treatment was replicated thrice using 10 larvae for each replication. TOTAL HEMOCYTE COUNT (THC) Hemolymph was collected by cutting larval proleg and mixed with ACB (1:10, v/v). Hemocytes
were counted using a Neubauer hemocytometer (Superior Marienfeld, Lauda-Königshofen, Germany) under a phase contrast microscope (BX41, Olympus, Tokyo, Japan) at 100 × magnification.
Heat-killed (90 °C, 30 min) _Escherichia coli_ (5 × 105 cells/larva) and a test chemical (5-HT or SB-269970) were co-injected into hemocoel through abdominal proleg of L5 larvae in a volume
of 5 µL using a 10 µL micro-syringe (Hamilton) after surface-sterilization with 70% ethanol. After 4 h of incubation at 25 ± 2 °C, hemolymph of the insect was collected and assessed for THC.
HEMOCYTE-SPREADING ANALYSIS After hemolymph (~150 µL) was collected from five L5 larvae by cutting prolegs, it was mixed with three times volume of ice-cold ACB and incubated on ice for 30
min. ACB-treated hemolymph was then centrifuged at 800 × _g_ for 5 min at 4 °C. The resulting pellet was re-suspended in 500 µL of filter-sterilized TC-100 insect cell culture medium
(Welgene, Daegu, Korea). On a glass coverslip placed in a moist chamber, 10 µL of hemocyte suspension was applied and placed in a dark condition. Hemocytes were then fixed with 4%
paraformaldehyde (filter-sterilized) at 25 °C for 10 min and then washed thrice with filter-sterilized PBS. Cells were then permeabilized with 0.2% Triton-X dissolved in PBS at 25 °C for 2
min and washed with PBS. After that, hemocytes were blocked using 10% bovine serum albumin (BSA) dissolved in PBS at 25 °C for 10 min and washed again with PBS. Cells were then incubated
with FITC-tagged phalloidin in PBS for 60 min and washed thrice with PBS. Hemocytes nuclei were then stained by incubating with 4′,6-diamidino-2-phenylindole (DAPI, 1 μg/mL) (Thermo Fisher
Scientific, Rockford, IL, USA) dissolved in PBS and washed thrice with PBS. Hemocytes were then observed under a fluorescence microscope (DM2500, Leica, Wetzlar, Germany) at 400 ×
magnification. Hemocyte-spreading was dictated by appendages of F-actin outward the hemocyte cell boundary. To assess effect of _Se-5HTR_ RNAi on hemocyte-spreading, hemocytes were collected
at 24 h after injecting dsSe-5HTR (~900 ng/larva). In separate experiments, 5-HT and 5-HTR inhibitor SB-269970 were co-injected into dsRNA-treated larvae at ratios of 1:10 and 10:1 to check
their influence on hemocyte-spreading. At 6 h after co-injection, hemolymph was collected from the treated larva and hemocyte-spreading was observed using the above-mentioned method.
NODULATION ASSAY Overnight culture of _E_. _coli_ Top10 bacterial cells was washed with PBS and centrifuged at 1,120 × _g_ for 10 min. L5 larvae were used to assess hemocyte nodule formation
by immune-challenge with bacterial injection (~1.8 × 105 cells/larva) through the abdominal proleg into larval hemocoel using a microsyringe as previously described. To assess the effect of
_Se-5HTR_ RNAi on nodule formation, bacterial challenge was performed at 24 h after injecting dsSe-5HTR (~900 ng/larva). To check the influence of chemicals on nodule formation, 2 µg of
ketanserin and/or 10 µg of 5-HT was co-injected along with the bacterial suspension. In separate experiment, 1 µg of the bacterial secondary metabolite was co-injected with the bacterial
suspension to assess immune suppression activity of the test compound. After an incubation period of 8 h at 25 °C, treated insects were dissected under a stereo microscope (SZX9, Olympus,
Japan) to count melanized nodule numbers. Each treatment was triplicated independently using five insects for each replication. PHAGOCYTOSIS ASSAY Preparation of FITC-labeled bacterial cells
followed the method described by Harlow and Lane71. Briefly, _E_. _coli_ Top10 cells were cultured in 50 mL of Luria-Bertani broth (37 °C, 16 h). These bacterial cells were then harvested
by centrifuging 1 mL of the cultured broth at 1,120 × _g_ for 10 min at 4 °C. Bacterial cells (105 cells/mL) were washed twice with TBS and re-suspended in 1 mL of 0.1 M sodium bicarbonate
buffer (pH 9.0). In the suspension, 1 µL of 10 mg/mL FITC solution was added and immediately mixed. The mixture was then incubated in dark condition with end-over-end rotation at 25 °C for
30 min. After the incubation, FITC-tagged bacteria were harvested by centrifugation at 22,000 × _g_ for 20 min at 4 °C. Bacterial cells were then washed thrice with HBSS to remove unbound
dye and re-suspended in TBS. To assess _in vivo_ phagocytosis activity, 5 µL of FITC-tagged _E_. _coli_ suspension was injected into the hemocoel of L5 larva through the proleg. To assess
the effect of _Se-5HTR_ RNAi on phagocytosis activity, bacteria were injected at 24 h after injecting dsSe-5HTR (~900 ng/larva). To check the influence of a specific 5-HT7 receptor inhibitor
on phagocytosis, 2 µg of SB-269970 was co-injected with the tagged bacterial suspension. To determine the effect of secondary metabolite on phagocytosis, 1 µg of bacterial secondary
metabolite was co-injected with tagged bacterial suspension. After 15 min of incubation, treated larvae were surface-sterilized using 70% ethanol. With a pair of scissors, the proleg was cut
to collect hemolymph sample (~50 µL) in 150 µL of cold ACB with gentle shaking of the tube to mix hemolymph and ACB thoroughly. Hemocyte monolayers were made using 50 µL of hemocyte
suspension (~5 × 103 cells) and left in a moist chamber for 15 min for hemocytes to settle and attach to the glass surface. After the incubation period, monolayers were washed with TBS to
remove plasma. These monolayers were overlaid with 1% trypan blue dye solution to quench non-phagocytosed bacterial cells. After 10 min, monolayers were washed again with TBS and then fixed
with 1.5% glutaraldehyde solution to observe under a fluorescence microscope at 400× magnification. Hemocytes undergoing phagocytosis were counted from a total of 100 hemocytes observed from
different areas of each slide. Each observation was triplicated with three different slides. PREPARATION OF SE-5HTR KNOCKOUT MUTANT USING CRISPR-CAS9 A guide RNA sequence for CRISPR-Cas9
technology against _Se5HTR_ was designed by a CRISPR guide RNA analysis tool CHOPCHOP (https://chopchop.rc.fas.harvard.edu). Single-stranded guide RNA (sgRNA) was synthesized using a
PCR-based technique following the manufacturer’s protocol (Guide-it sgRNA _In Vitro_ Transcription Kit, Takara Bio USA, Mountain View, CA, USA). Briefly, a forward oligonucleotide (CGT GCG
CCA TCA TCG CGC TG) was mixed with Guide-it Scaffold template and performed PCR at 98 °C for 5 s, 33 cycles (98 °C for 10 s and 68 °C for 10 s) to generate sgRNA DNA template. After
confirming the single band at ~130 bp, _in vitro_ transcription reaction was performed to generate sgRNA. For injection to eggs, newly (<1 h) laid eggs was kept in desiccator for 10 min
and fixed onto a glass slide with double-sided tape. Injection needle was prepared by pulling siliconized 10 µL quartz microcapillaries using a horizontal micropipette puller (Narishige,
Tokyo, Japan) and beveled with a BV-10 beveller (Narishige). The fixed eggs were injected with 5 nL of a mixture containing 500 ng/µL of recombinant Cas9 nuclease and 50 ng/µL of
target-specific sgRNA using a micromanipulator (PN-30, Narishige) under a stereo microscope (SZX9, Olympus Corporation, Japan) equipped with a mechanical stage. Eggs were incubated at 27 °C
until hatching. To assess the mutation in the target DNA site, genomic DNA was extracted using 5% Chelex 100 resin (BioRad, Hercules, CA, USA) from hemolymph of L5 larvae. The PCR conditions
were as follows: 98 °C for 2 min, followed by 35 cycles of 94 °C for 30 s, 49 °C for 1 min, and 72 °C for 1 min, followed by a final extension period of 72 °C for 10 min. The PCR products
were then cloned into the pCR2.1 vector (ThermoFisher Scientific Korea, Seoul, Korea) and bidirectionally sequenced. PREPARATION OF ORGANIC EXTRACTS FROM BACTERIAL CULTURE BROTH _X_.
_nematophila_ K1 (Xn) and _P_. _temperata temperata_ ANU101 (Ptt) bacteria were cultured in TSB at 28 °C for 48 h. Culture broths were centrifuged at 12,500 × _g_ for 30 min and supernatants
were used for subsequent fractionation. To obtain ethyl acetate extract, the same volume (1 L) of ethyl acetate was mixed with the supernatant and separated into organic and aqueous
fractions. Ethyl acetate extract (‘EAX’) was dried using a rotary evaporator (Sunil Eyela, Seongnam, Korea) at 40 °C. The resulting extract (0.2 mg) was obtained from 1 L cultured broth and
resuspended with 5 mL of methanol. The aqueous phase was then combined with 1 L of butanol. Butanol extract (‘BX’) was also dried using the rotary evaporator at 40 °C and the resulting
extract (0.2 mg) was resuspended with 5 mL of methanol. SECONDARY BACTERIAL METABOLITES – BIOLOGICAL ACTIVITY AGAINST IMMUNE RESPONSES Secondary metabolites (37 samples, Supplementary Fig.
4) derived from _Xenorhabdus_ and _Photorhabdus_ cultures were from the Bode lab compound collection named ‘HB’ compounds. Their biological activities for suppressing _S_. _exigua_ immunity
were then determined. Individual chemicals were dissolved in DMSO, diluted into desired concentrations with DMSO, and stored at −20 °C. For hemocyte nodulation inhibition assay, overnight
culture of _E_. _coli_ bacterial cells was washed with PBS. Test compound (1 µg/larva) was injected into larval hemocoel along with the bacterial suspension (~1.8 × 105 cfu/larva) using a
microsyringe as previously described. Insects were then incubated at 25 °C for 8 h. After the incubation period, insects were dissected and nodule numbers were counted as described above.
Each treatment was triplicated independently using five insects for each replication. For phagocytosis inhibition assay, 5 µL of FITC-tagged _E_. _coli_ suspension along with 1 µg of test
compound was injected into L5 larval hemocoel. After 15 min of incubation period, hemocytes undergoing phagocytosis were counted as previously described. SECONDARY BACTERIAL METABOLITES –
COMPETITIVE ASSAY WITH 5-HT To determine effects of bacterial secondary metabolites on nodulation and phagocytosis, 10 potent chemicals were selected based on their common inhibitory
activity on both phagocytosis and nodulation and their median inhibition concentration (IC50) values were calculated. Percentages of phagocytosis against increasing concentrations of HB
chemicals were calculated and their IC50 values were calculated using Probit analysis (https://probitanalysis.wordpress.com). To assess a competitive inhibitory activity between test
compound and 5-HT, HB chemicals were injected in different doses (0, 0.01, 0.1, 1 and 10 µg/larva) along with a fixed 5-HT concentration (1 µg/larva) and FITC-tagged bacteria (500
cells/larva). In a separate experiment, different doses of 5-HT were injected into the larvae with a fixed HB compound content (1 µg/larva). At 15 min after bacterial injection, hemocytes
from treated larvae were collected in ACB and phagocytosis assay was performed as described above. CHEMICAL DERIVATIVES AND THEIR INHIBITORY ACTIVITIES AGAINST SE-5HTR Based on potent
phenylethylamide (PEA) HB compounds, 45 additional PEA samples (Supplementary Fig. 5) were obtained from Korea Chemical Bank of the Korea Research Institute of Chemical Technology (KRICT).
Derivative compounds were dissolved in DMSO, diluted into desired concentrations with DMSO, and stored at −20 °C. These PEA chemicals were then tested for their abilities to suppress nodule
formation and phagocytosis in _S_. _exigua_ as described above. For nodulation inhibition assay, each chemical (150 ng/larva) was injected into larval hemocoel along with bacterial
suspension (~1.8 × 105 cfu/larva). Each treatment was triplicated independently using five insects for each replication. For phagocytosis inhibition assay, 5 µL of FITC-tagged _E_. _coli_
suspension along with 150 ng of each chemical was injected into L5 larval hemocoel of _S_. _exigua_. After incubating for 15 min, hemocytes undergoing phagocytosis were counted using
previously described method. CHEMICAL SYNTHESIS OF PHX A potent chemical was designed as PhX ((_S_)-2-(1,4-dioxohexahydropyrrolo[1,2_-_α]pyrazin-2(1
_H_)-yl)-_N_-(4-methoxyphenethyl)acetamide) and chemically synthesized according to a method described in Supplementary Fig. 6. STATISTICAL ANALYSIS All studies were triplicated
independently. Results are expressed as mean ± standard error. Results were plotted using Sigma plot (Systat Software, San Jose, CA, USA). Means were compared by least squared difference
(LSD) test of one-way analysis of variance (ANOVA) using POC GLM of SAS program72 and discriminated at Type I error = 0.05. REFERENCES * Roshchina, V. V. New trends and perspectives in the
evolution of neurotransmitters in microbial, plant, and animal cells. _Adv. Exp. Med. Biol._ 874, 25–77 (2016). Article CAS PubMed Google Scholar * Lovenberg, W., Weissbach, H. &
Udenfriend, S. Aromatic L-amino acid decarboxylase. _J. Bio.l Chem._ 237, 89–93 (1962). CAS Google Scholar * Hufton, S. E., Jennings, I. G. & Cotton, R. G. Structure and function of
the aromatic amino acid hydroxylases. _Biochem. J._ 311, 353–66 (1995). Article CAS PubMed PubMed Central Google Scholar * Roberts, K. M. & Fitzpatrick, P. F. Mechanisms of
tryptophan and tyrosine hydroxylase. _IUBMB Life_ 65, 350–357 (2013). Article CAS PubMed PubMed Central Google Scholar * Zhang, X., Beaulieu, J. M., Sotnikova, T. D., Gainetdinov, R. R.
& Caron, M. G. Tryptophan hydroxylase-2 controls brain serotonin synthesis. _Science_ 305, 217 (2004). Article CAS PubMed Google Scholar * Gershon, M. D. & Tack, J. The
serotonin signaling system: from basic understanding to drug development for functional GI disorders. _Gastroenterology_ 132, 397–414 (2007). Article CAS PubMed Google Scholar *
Richtand, N. M. & McNamara, R. K. Serotonin and dopamine interactions in psychosis prevention. _Prog. Brain Res._ 172, 141–153 (2008). Article CAS PubMed Google Scholar * Monti, J.
M. Serotonin control of sleep-wake behavior. _Sleep Med. Rev._ 15, 269–281 (2011). Article PubMed Google Scholar * Švob Štrac, D., Pivac, N. & Mück-Šeler, D. The serotonergic system
and cognitive function. _Transl. Neurosci._ 7, 35–49 (2016). Article PubMed PubMed Central CAS Google Scholar * Seuwen, K. & Pouysségur, J. Serotonin as a growth factor. _Biochem.
Pharmacol._ 39, 985–990 (1990). Article CAS PubMed Google Scholar * Li, N., Wallén, N. H., Ladjevardi, M. & Hjemdahl, P. Effects of serotonin on platelet activation in whole blood.
_Blood Coagul. Fibrinolysis_ 8, 517–23 (1997). Article CAS PubMed Google Scholar * Hayashi, K. _et al_. Serotonin attenuates biotic stress and leads to lesion browning caused by a
hypersensitive response to _Magnaporthe oryzae_ penetration in rice. _Plant J._ 85, 46–56 (2016). Article CAS PubMed Google Scholar * Majeed, Z. R. _et al_. Modulatory action by the
serotonergic system: behavior and neurophysiology in _Drosophila melanogaster_. _Neural Plast._ 2016, 1–23 (2016). Article CAS Google Scholar * Huser, A. _et al_. Anatomy and behavioral
function of serotonin receptors in _Drosophila melanogaster_ larvae. _PLoS One_ 12, e0181865 (2017). Article PubMed PubMed Central CAS Google Scholar * Becnel, J., Johnson, O., Luo, J.,
Nässel, D. R. & Nichols, C. D. The serotonin 5-HT7Dro receptor is expressed in the brain of _Drosophila_, and is essential for normal courtship and mating. _PLoS One_ 6, e20800 (2011).
Article ADS CAS PubMed PubMed Central Google Scholar * Yuan, Q., Lin, F., Zheng, X. & Sehgal, A. _Serotonin modulates circadian entrainment in Drosophila. Neuron. 2005;_ 47,
115–127 (2005). CAS Google Scholar * Nichols, C. D. 5-HT2 receptors in _Drosophila_ are expressed in the brain and modulate aspects of circadian behaviors. _Dev. Neurobiol._ 67, 752–763
(2007). Article CAS PubMed Google Scholar * Yuan, Q., Joiner, W. J. & Sehgal, A. A sleep-promoting role for the _Drosophila_ serotonin receptor 1A. _Curr. Biol._ 16, 1051–1062
(2016). Article CAS Google Scholar * Neckameyer, W. S., Coleman, C. M., Eadie, S. & Goodwin, S. F. Compartmentalization of neuronal and peripheral serotonin synthesis in _Drosophila
melanogaster_. _Genes Brain Behav._ 6, 756–769 (2007). Article CAS PubMed Google Scholar * Dierick, H. A. & Greenspan, R. J. Serotonin and neuropeptide F have opposite modulatory
effects on fly aggression. _Nat. Genet._ 39, 678–682 (2007). Article CAS PubMed Google Scholar * Kaplan, D. D., Zimmermann, G., Suyama, K., Meyer, T. & Scott, M. P. A nucleostemin
family GTPase, NS3, acts in serotonergic neurons to regulate insulin signaling and control body size. _Genes Dev._ 22, 1877–1893 (2008). Article CAS PubMed PubMed Central Google Scholar
* Qi, Y. X. _et al_. Serotonin modulates insect hemocyte phagocytosis via two different serotonin receptors. _Elife_ 5, e12241 (2016). Article PubMed PubMed Central Google Scholar *
Python, F. & Stocker, R. F. Immunoreactivity against choline acetyltransferase, gamma-aminobutyric acid, histamine, octopamine, and serotonin in the larval chemosensory system of
_Drosophila melanogaster_. _J. Comp. Neurol._ 453, 157–167 (2016). Article CAS Google Scholar * Dasari, S. & Cooper, R. L. Direct influence of serotonin on the larval heart of
_Drosophila melanogaster_. _J. Comp. Physiol. B._ 176, 349–357 (2006). Article CAS PubMed Google Scholar * Rodriguez, M. V. G. & Campos, A. R. Role of serotonergic neurons in the
_Drosophila_ larval response to light. _BMC Neurosci._ 10, 66 (2009). Article CAS Google Scholar * Barnes, N. M. & Sharp, T. A review of central 5-HT receptors and their function.
_Neuropharmacology_ 38, 1083–1152 (1999). Article CAS PubMed Google Scholar * Nichols, D. E. & Nichols, C. D. Serotonin receptors. _Chem. Rev._ 108, 1614–1641 (2008). Article CAS
PubMed Google Scholar * Roth, B. L. The serotonin receptors: from molecular pharmacology to human therapeutics. _Humana Press_, _Totowa_, _New Jersey_ (2006). * Thompson, A. J. &
Lummis, S. C. R. 5-HT3 Receptors. _Curr. Pharm. Des._ 12, 3615–3630 (2006). Article CAS PubMed PubMed Central Google Scholar * Watanabe, T., Sadamoto, H. & Aonuma, H. Identification
and expression analysis of the genes involved in serotonin biosynthesis and transduction in the field cricket _Gryllus bimaculatus_. _Insect Mol. Biol._ 20, 619–635 (2011). Article CAS
PubMed Google Scholar * Ono, H. & Yoshikawa, H. Identification of amine receptors from a swallowtail butterfly, _Papilio xuthus_ L.: cloning and mRNA localization in foreleg
chemosensory organ for recognition of host plants. _Insect Biochem. Mol. Biol._ 34, 1247–1256 (2004). Article CAS PubMed Google Scholar * Vleugels, R., Lenaerts, C., Baumann, A., Vanden,
B. J. & Verlinden, H. Pharmacological characterization of a 5-HT1-type serotonin receptor in the red flour beetle, _Tribolium castaneum_. _PLoS One_ 8, e65052 (2013). Article ADS CAS
PubMed PubMed Central Google Scholar * Troppmann, B., Balfanz, S., Baumann, A. & Blenau, W. Inverse agonist and neutral antagonist actions of synthetic compounds at an insect 5-HT1
receptor. _Br. J. Pharmacol._ 159, 1450–1462 (2010). Article CAS PubMed PubMed Central Google Scholar * Guo, X., Ma, Z. & Kang, L. Serotonin enhances solitariness in phase
transition of the migratory locust. _Front. Behav. Neurosci._ 7, 129 (2013). Article PubMed PubMed Central CAS Google Scholar * Qi, Y. X. _et al_. Larvae of the small white butterfly,
_Pieris rapae_, express a novel serotonin receptor. _J. Neurochem._ 131, 767–777 (2014). Article CAS PubMed Google Scholar * Qi, Y. X. _et al_. Characterization of three serotonin
receptors from the small white butterfly, _Pieris rapae_. _Insect Biochem. Mol. Biol._ 87, 107–116 (2017). Article CAS PubMed Google Scholar * Thamm, M., Balfanz, S., Scheiner, R.,
Baumann, A. & Blenau, W. Characterization of the 5-HT1A receptor of the honeybee (_Apis mellifera_) and involvement of serotonin in phototactic behavior. _Cell Mol. Life Sci._ 67,
2467–2479 (2010). Article CAS PubMed Google Scholar * Schlenstedt, J., Balfanz, S., Baumann, A. & Blenau, W. Am5-HT7: molecular and pharmacological characterization of the first
serotonin receptor of the honeybee (_Apis mellifera_). _J. Neurochem._ 98, 1985–1998 (2006). Article CAS PubMed Google Scholar * Berridge, M. J. & Patel, N. Insect salivary glands -
stimulation of fluid secretion by 5-hydroxytryptamine and adenosine-3′, 5′-monophosphate. _Science_ 162, 462–463 (1968). Article ADS CAS PubMed Google Scholar * Berridge, M. J. The role
of 5-hydroxytryptamine and cyclic AMP in the control of fluid secretion by isolated salivary glands. _J. Exp. Biol._ 53, 171–186 (1970). CAS PubMed Google Scholar * Vanhoenacker, P.,
Haegeman, G. & Leysen, J. E. 5-HT7 receptors: current knowledge and future prospects. _Trends Pharmacol. Sci._ 21, 70–77 (2007). Article Google Scholar * Molaei, G. & Lange, A. B.
The association of serotonin with the alimentary canal of the African migratory locust, _Locusta migratoria_: distribution, physiology and pharmacological profile. _J. Insect Physiol._ 49,
1073–1182 (2003). Article CAS PubMed Google Scholar * Baines, D., DeSantis, T. & Downer, R. G. H. Octopamine and 5-hydroxytryptamine enhance the phagocytic and nodule formation
activities of cockroach (_Periplaneta americana_) haemocytes. _J. Insect Physiol._ 38, 905–914 (1992). Article CAS Google Scholar * Kim, K., Madanagapol, N., Lee, D. & Ki, Y.
Octopamine and 5-hydroxytryptamine mediate hemocytic phagocytosis and nodule formation via eicosanoids in the beet armyworm, _Spodoptera exigua_. _Arch. Insect Biochem. Physiol._ 90, 162–176
(2009). Article CAS Google Scholar * Kim, G. S. & Kim, Y. Up-regulation of circulating hemocyte population in response to bacterial challenge is mediated by octopamine and
5-hydroxytryptamine via Rac1 signal in _Spodoptera exigua_. _J. Insect Physiol._ 56, 559–566 (2010). Article CAS PubMed Google Scholar * Kim, Y., Ji, D., Cho, S. & Park, Y. Two
groups of entomopathogenic bacteria, _Photorhabdus_ and _Xenorhabdus_, share an inhibitory action against phospholipase A2 to induce host immunodepression. _J. Invertebr. Pathol._ 89,
258–264 (2005). Article CAS PubMed Google Scholar * Shi, Y. M. & Bode, H. B. Chemical language and warfare of bacterial natural products in bacteria-nematode-insect interactions.
_Nat. Prod. Rep._ 35, 309–335 (2018). Article CAS PubMed Google Scholar * Tobias, N. J., Shi, Y. M. & Bode, H. B. Refining the natural product repertoire in entomopathogenic
bacteria. _Trends Microbiol._ 26, 833–840 (2018). Article CAS PubMed Google Scholar * Barak, L. S. _et al_. A highly conserved tyrosine residue in G protein-coupled receptors is required
for agonist-mediated ß2-adrenergic receptor sequestration. _J. Biol. Chem._ 269, 2790–2795 (1994). CAS PubMed Google Scholar * Ji, T. H., Grossmann, M. & Ji, I. G protein-coupled
receptors. I. Diversity of receptor-ligand interactions. _J. Biol. Chem._ 273, 17299–17302 (1998). Article CAS PubMed Google Scholar * Huang, E. S. Construction of a sequence motif
characteristic of aminergic G protein-coupled receptors. _Protein Sci._ 12, 1360–1367 (2003). Article CAS PubMed PubMed Central Google Scholar * Park, J., Stanley, D. & Kim, Y. Rac1
mediates cytokine-stimulated hemocyte spreading via prostaglandin biosynthesis in the beet armyworm, _Spodoptera exigua_. _J. Insect Physiol._ 59, 682–689 (2013). Article CAS PubMed
Google Scholar * Venkatakrishnan, A. J. _et al_. Molecular signatures of G-protein-coupled receptors. _Nature_ 494, 185–194 (2013). Article ADS CAS PubMed Google Scholar * Ho, B. Y.,
Karschin, A., Branchek, T., Davidson, N. & Lester, H. A. The role of conserved aspartate and serine residues in ligand binding and in function of the 5-HT1A receptor: A site-directed
mutation study. _FEBS Lett._ 312, 259–262 (1992). Article CAS PubMed Google Scholar * Shapiro, D. A., Kristiansen, K., Weiner, D. M., Kroeze, W. K. & Roth, B. L. Evidence for a model
of agonist-induced activation of 5-hydroxytryptamine 2A serotonin receptors that involves the disruption of a strong ionic interaction between helices 3 and 6. _J. Biol. Chem._ 277,
11441–11449 (2002). Article CAS PubMed Google Scholar * Qanbar, R. & Bouvier, M. Role of palmitoylation/depalmitoylation reactions in G-protein-coupled receptor function. _Pharmacol.
Ther._ 97, 1–33 (2003). Article CAS PubMed Google Scholar * Romero, G., von Zastrow, M. & Friedman, P. A. Role of PDZ proteins in regulating trafficking, signaling, and function of
GPCRs: means, motif, and opportunity. _Adv. Pharmacol._ 62, 279–314 (2011). Article CAS PubMed PubMed Central Google Scholar * Guseva, D., Wirth, A. & Ponimaskin, E. Cellular
mechanisms of the 5-HT7 receptor-mediated signaling. _Front. Behav. Neurosci._ 8, 306 (2014). Article PubMed PubMed Central CAS Google Scholar * Choi, C. & Helfman, D. M. The
Ras-ERK pathway modulates cytoskeleton organization, cell motility and lung metastasis signature genes in MDA-MB-231 LM2. _Oncogene_ 33, 3668–3676 (2014). Article CAS PubMed Google
Scholar * Papenfort, K. & Bassler, B. L. Quorum sensing signal-response systems in Gram-negative bacteria. _Nat. Rev. Microbiol._ 14, 576–588 (2016). Article CAS PubMed PubMed
Central Google Scholar * Bode, E. _et al_. Biosynthesis and function of simple amides in _Xenorhabdus doucetiae_. _Environ. Microbiol._ 19, 4564–4575 (2017). Article CAS PubMed Google
Scholar * Reimer, D., Nollmann, F. I., Schultz, K., Kaiser, M. & Bode, H. B. Xenortide biosynthesis by entomopathogenic _Xenorhabdus nematophila_. _J. Nat. Prod._ 77, 1976–1980 (2014).
Article CAS PubMed Google Scholar * Bloudoff, K. & Schmeing, T. M. Structural and functional aspects of the nonribosomal peptide synthetase condensation domain superfamily:
discovery, dissection and diversity. _Biochim. Biophys. Acta_ 1865, 1587–1604 (2017). Article CAS Google Scholar * Proschak, A. _et al_. Biosynthesis of the insecticidal xenocyloins in
_Xenorhabdus bovienii_. _Chembiochem._ 15, 369–372 (2014). Article CAS PubMed Google Scholar * Li, J., Chen, G. & Webster, J. M. Nematophin, a novel antimicrobial substance produced
by _Xenorhabdus nematophilus_ (Enterobactereaceae). _Can. J. Microbiol._ 43, 770–773 (1997). Article CAS PubMed Google Scholar * Nollmann, F. I. _et al_. Insect-specific production of
new GameXPeptides in _Photorhabdus luminescens_ TTO1, widespread natural products in entomopathogenic bacteria. _Chembiochem._ 16, 205–208 (2015). Article CAS PubMed Google Scholar *
Cai, M. _et al_. Design and synthesis of novel insecticides based on the serotonergic ligand 1-[(4-Aminophenyl) ethyl]-4-[3-(trifluoromethyl) phenyl] piperazine (PAPP). _J. Agric. Food
Chem._ 58, 2624–2629 (2009). Article CAS Google Scholar * Verlinden, H., Vleugels, R. & Broeck, J. V. Serotonin, serotonin receptors and their actions in insects. _Neurotransmitter_
2, e314 (2015). Google Scholar * Shrestha, S., Stanley, D. & Kim, Y. PGE2 induces oenocytoid cell lysis via a G protein-coupled receptor in the beet armyworm, _Spodoptera exigua_. _J.
Insect Physiol._ 57, 1568–1576 (2011). Article CAS PubMed Google Scholar * Bustin, S. A. _et al_. The MIQE guidelines: minimum information for publication of quantitative real-time PCR
experiments. _Clin. Chem._ 55, 4 (2009). Article CAS Google Scholar * Harlow, E. & Lane, D. Labeling antibodies with fluorochromes. In: Using Antibodies. Cold spring harbor laboratory
press, New York, pp. 85–87 (1988). * SAS Institute. SAS/STAT user’s guide. SAS Institute, Inc., Cary, NC (1989). Download references ACKNOWLEDGEMENTS This work was supported by a grant (No.
2017R1A2133009815) of the National Research Foundation (NRF) funded by the Ministry of Science, ICT and Future Planning, Republic of Korea. This work was also supported by a project (No
SI1808, Development of crop protection agents leading the future market). The chemical library used in this research was provided by Korea Chemical Bank (http://www.chembank.org) of the
Korea Research Institute of Chemical Technology (KRICT). We appreciate Youngim Song for ordering and arranging materials and Malsook Cho for rearing insects. Work in the Bode lab was
supported by the LOEWE Center Translational Biodiversity Genomics funded by the state of Hesse, Germany. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Plant Medicals, College
of Life Sciences, Andong National University, Andong, 36729, Korea Ariful Hasan & Yonggyun Kim * Center for Eco-Friendly New Materials, Korea Research Institute of Chemicals Technology,
Yuseong, Daejeon, 34114, Korea Hyun-Suk Yeom & Jaewook Ryu * Department of Biosciences, Molecular Biotechnology, and Buchmann Institute for Molecular Life Sciences (BMLS),
Goethe-Universität Frankfurt am Main, Frankfurt, Germany Helge B. Bode Authors * Ariful Hasan View author publications You can also search for this author inPubMed Google Scholar * Hyun-Suk
Yeom View author publications You can also search for this author inPubMed Google Scholar * Jaewook Ryu View author publications You can also search for this author inPubMed Google Scholar *
Helge B. Bode View author publications You can also search for this author inPubMed Google Scholar * Yonggyun Kim View author publications You can also search for this author inPubMed
Google Scholar CONTRIBUTIONS A.H. and Y.K. performed the experiments and analyzed the data. Y.K. conceived and supervised the experiments. H.S.Y., J.R. and H.B. provided bacterial
metabolites and derivative compounds. A.H. and Y.K. wrote the paper with contributions of H.S.Y., J.R. and H.B. CORRESPONDING AUTHOR Correspondence to Yonggyun Kim. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps
and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the
source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative
Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Hasan, A., Yeom, HS., Ryu, J. _et al._ Phenylethylamides derived from bacterial
secondary metabolites specifically inhibit an insect serotonin receptor. _Sci Rep_ 9, 20358 (2019). https://doi.org/10.1038/s41598-019-56892-z Download citation * Received: 17 July 2019 *
Accepted: 16 December 2019 * Published: 30 December 2019 * DOI: https://doi.org/10.1038/s41598-019-56892-z SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative